Abstract
Background:
This study was performed to evaluate and compare the clinical and antimicrobial efficacy of subgingival irrigation with tetracycline and povidone-iodine as an adjunct to nonsurgical periodontal therapy.
Materials and Methods:
Twenty subjects with chronic moderate periodontitis were recruited in this split-mouth study with probing pocket depth of >3 and ≤5 mm and clinical attachment loss of 3-4 mm in relation to 16, 36, and 46. In each subject, three selected periodontal pockets were assigned to receive one out of three irrigants (1) sterile water (control) in 16; (2) tetracycline at 10 mg/ml in 36; (3) 2% povidone-iodine in 46, and these sites were designated as Group A, Group B, and Group C, respectively. Plaque score, gingival score, pocket probing depth, and clinical attachment level were evaluated before treatment and at 1 and 3 months posttreatment. Multiplex polymerase chain reaction was used to detect Porphyromonas gingivalis and Tannerella forsythensis which have been implicated as the major risk factors for periodontal disease. Subgingival plaque collected before treatment and at 1 and 3 months posttreatment. Data were analysed using ANOVA and repeated measure ANOVA. Results were considered significant if P < 0.05.
Results:
Clinical and microbiological parameters were reduced posttreatment, the reduction being significantly higher in Group B compared to Group C.
Conclusion:
It can be concluded that chemical and mechanical therapies were of slight benefit in the treatment of chronic moderate periodontitis, and there was an adjunctive effect of significance when scaling and root planing was combined with a single subgingival irrigation with tetracycline or povidone-iodine in lower concentration.
Keywords: Polymerase chain reaction, povidone-iodine, subgingival irrigation, tetracycline
INTRODUCTION
Periodontitis is an inflammatory disease of the supporting tissues of teeth caused by specific microorganisms or groups of specific microorganisms, resulting in progressive destruction of periodontal ligament and alveolar bone with increased probing depth formation, recession, or both.[1] The subgingival periodontal pocket of humans harbors more than 700 bacterial species. It is initiated due to colonization as subgingival biofilms by a group of Gram-negative anaerobes. The periodontal disease progresses as a result of the direct effects of bacterial virulence factors on host tissues as well as the self-damaging host responses to the colonizing bacteria.[2] While no single species has been implicated as the primary pathogen and the available evidence is consistent with a polymicrobial disease etiology, the red-complex bacteria consisting of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia have been strongly implicated in the onset of periodontitis.[2] Subgingival irrigation is a simple method to administer antibiotics directly into the periodontal pocket, thus localizing the effect of the chemotherapeutic agent. Current periodontal therapy aims at removing bacterial deposits from the tooth surface so as to achieve periodontal health. Scaling and root planing (SRP), in combination with optimal oral hygiene, has been shown to arrest periodontal destruction. However, the effectiveness of this conventional treatment is limited by the lack of accessibility to bacteria in the periodontal pocket, variation in the ability of the therapist to gain access to deep and tortuous pockets often results in substantial variation in the effectiveness of nonsurgical periodontal therapy.[3]
In such situations, antimicrobial agents are of great interest and may be valuable as adjuncts to mechanical therapy. However, development of antibiotic resistance, superinfections, and other systemic side effects such as gastrointestinal disturbances has been reported when antibiotics were given systemically. In view of this, the current study was performed to compare the clinical and antimicrobial efficacy of tetracycline hydrochloride (10 mg/ml) and 2% povidone-iodine as subgingival irrigants as an adjunct to nonsurgical periodontal therapy. Further, it was believed that if additional bacteria could be eliminated, a better outcome could be achieved. To the best of our knowledge, no studies have been done to compare the efficacy of these two commonly used irrigants in its lowest concentration and evaluated its antimicrobial action using polymerase chain reaction (PCR). Hence, this study was done to evaluate and compare the clinical and antimicrobial efficacy of these agents.
MATERIALS AND METHODS
Study design
This was a nonrandomized clinical trial with split-mouth design.
Study settings
The study was conducted in the Department of Periodontics, Amrita School of Dentistry, from November 2012 to March 2014. The study was approved by the Ethical Committee of the Amrita School of Dentistry, India.
Study participants
Eligibility criteria for participants
Inclusion criteria
The study participants were chronic moderate periodontitis[4] subjects in the age group of ≥35 and ≤55 years irrespective of their gender
Presence of minimum 20 number of natural teeth
Subjects should have probing periodontal pocket depth of ≥3 and ≤5 mm and clinical attachment loss of 3-4 mm in 16, 36, and 46.
Exclusion criteria
Subjects affected with systemic disorders or serious uncontrolled medical disorders
Subjects with history of antibiotic use within past 6 months
Subjects who are pregnant and lactating
Subjects who had undergone periodontal therapy in past 6 months
Subjects with history of allergy to tetracycline or povidone-iodine
Subjects with thyroid disorders
Subjects with aggressive periodontitis
Subjects who are immunocompromised
Subjects with artificial heart valves
Smoking (current and former smokers)
Alcoholism (current and former alcoholics).
Sample size estimation
The ideal sample size to ensure an adequate power for the study was calculated based on the results from a study by Stabholz et al.,[5] Krishna et al.,[6] and Hosaka et al.[7] Based on the calculation, it was decided that minimum 17 subjects per group were necessary for 80% power at 95% confidence interval (α = 0.05).
All participants were informed about the purpose of the study, potential benefits, and their possible side effects and those who declined to provide their written, informed consent were excluded from the study. The informed proforma was provided in both English and Malayalam (native language).
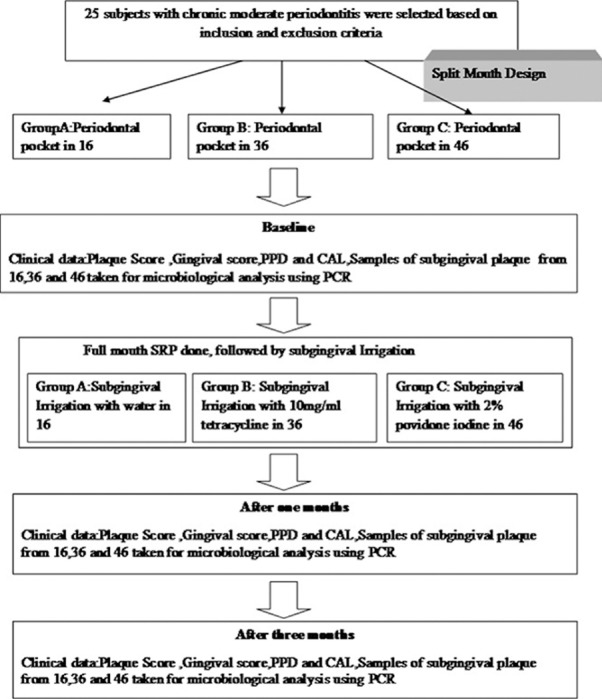
In the 25 subjects who were selected for the study, periodontal pocket in relation to 16 (first molar from the first quadrant) was selected for water irrigation, periodontal pocket in relation to 36 (first molar from the third quadrant) was selected for tetracycline irrigation, and periodontal pocket in relation to 46 (first molar from the fourth quadrant) was chosen for povidone-iodine irrigation and they were designated as Group A, Group B, and Group C, respectively [Figure 1].
Figure 1.
Flow chart of study design.
As we have done a split-mouth study, instead of gingival index and plaque index, we have taken into account, plaque score (PS)[8] and gingival score (GS)[9] for the designated area.
At baseline, clinical parameters such as PS,[8] GS,[9] probing pocket depth (PPD), and clinical attachment level (CAL) were recorded in 16, 36, and 46 and plaque samples were collected.
A single operator provided full mouth oral prophylaxis followed by oral hygiene instructions and nonsurgical periodontal therapy including SRP under local anesthesia to all subjects. After nonsurgical therapy, each tooth harboring an experimental site was subjected to 150 ml of (approximately 5 mm) continuous subgingival irrigation using an oral irrigator, Waterpik® water flosser (Waterpik Ultra Cordless® WP450). Periodontal pack was placed on each quadrant immediately after irrigation and was removed after 4 days. Then, these subjects were recalled after 1 month for evaluation of all clinical and microbiological parameters. At the end of 3 months, only 20 subjects completed the study. Five subjects were lost during follow-up due to their inconvenience. No treatment was provided for patients at the recall period. All clinical parameters evaluated at the baseline were reevaluated during recall visit after 1 and 3 months. Plaque samples were collected at baseline, 1 month, and at the end of 3 months for microbial analysis. PCR were employed for detecting P. gingivalis and T. forsythia from the plaque samples at Amrita Institute of Nano Sciences Laboratory.
Clinical measurements
Periodontal examination at baseline involved recording of clinical parameters such as PS,[8] GS,[9] PPD,[10] and CAL[10] were recorded at specific tooth of interest at baseline, 1 month, and after 3 months. PPD and CAL were measured using University of Michigan “O” probe with Williams markings at six sites per tooth (mesiobuccal/labial, mid-buccal/labial, distobuccal/labial, mesiolingual/palatal, mid-lingual/palatal, and distolingual/palatal) at 16, 36, and 46 using a customized acrylic stent as a reference to determine site and angle of measurement, ensuring reproducibility during examinations.
Plaque sample collection
Plaque samples were collected at baseline, 1 month, and at the end of 3 months.
After isolation of the site with cotton rolls, supragingival plaque was removed with sterile curettes and a sample of subgingival periodontal plaque was collected from the deepest periodontal pockets from 16, 36, and 46 before the commencement of periodontal therapy. Then, three paper points were successively inserted to the depth of the periodontal pocket for 10 s each to harvest subgingival plaque. The paper points were then removed, and the apical 3 mm of each point was immediately placed into a centrifuge tube containing 2 ml sterile saline (transport media) and stored at −20°C for microbial analysis.
Nonsurgical periodontal therapy (scaling and root planing)
A single operator provided full mouth oral prophylaxis followed by oral hygiene instructions; subgingival SRP under local anesthesia to all subjects in two sittings of 45 min duration. The outcome measures were done after 1 and 3 months.
Interventions
Twenty-five subjects were selected in this nonrandomized, clinical trial with split-mouth design who received nonsurgical therapy. In each subject, three selected periodontal pockets were assigned to receive one out of three irrigants (1) sterile water (control) in 16; (2) TTC at 10 mg/ml in 36 (3) povidone-iodine 2% in 46. At baseline, 1 and 3 months, clinical parameters such as PS, GS, PPD, and CAL were recorded in 16, 36, and 46 and plaque samples were collected.
Preparation of solution
TTC solutions were prepared by dissolving the content of three 500 mg TTC capsules into 150 ml distilled sterile water at approximately 60°C. Magnetic Stirrer was used for dissolving particles. Any capsule filler particles were filtered away.
Two percent of povidone-iodine (150 ml) was used for irrigation in Group C.
Subgingival irrigation
Subgingival irrigation in this study was done with an oral irrigator. Portable oral irrigator (Waterpik Ultra Cordless® WP450) was used in the study with 1200 pulsations/min and pressure range from 45 to 60 psi. Classic jet tip was used in this study. It has a 200 ml chamber attached to it in which 150 ml solution of each irrigants was filled. The device also has markings, I and II for pressure, of which pressure was set at first marking which is about 45 psi. For Group A, 150 ml sterile water was irrigated for 45 s, for Group B, 150 ml TTC 10 mg/ml solution was irrigated for a time period of 45 s, and for Group C, 150 ml of povidone-iodine was irrigated for 45 s. Excess irrigant was aspirated. Periodontal pack was placed after each irrigation so as to avoid contamination.
Polymerase chain reaction primers
The whole-genomic DNA extracts from plaque were used as templates in a PCR using the universal primers that target the 16S rRNA gene. The nucleotide sequences of the forward and reverse primers were P. gingivalis-specific forward primer (PgF), 5_-TGTAGATGACTGATGGTGAAAACC-3_and Tannerella forsythensis-specific forward primer (BfF), 5_TACAGGGGAATAAAATGA GATACG-3,_ and reverse primer 5_ACGTCATCCCC ACCTTCCTC-3.[11] The expected product length for this PCR was 197 bp, and this was compared with a 100 bp DNA molecular size marker. The primers were chosen for the detection of these putative pathogen-targeted specific regions of the 16S rRNA gene. (All primers were obtained from Sigma, Aldrich.).
PCR reaction mixture was prepared in sterile microfuge tubes as per requirement. The tubes were placed in the thermocycler. The appropriate program as mentioned below was selected to run the PCR. The reaction consisted of 35 amplification cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1.1 min. Initial dissociation of DNA was for 5 min at 95°C, and the final primer extension was for 10 min at 72°C.
After the termination of the run, the PCR product was loaded on agarose gel to analyze the product via electrophoresis as mentioned below.
Agarose gel electrophoresis
About 0.5 g of agarose powder was weighed in a conical flask. Fifty ml of 1X TAE was added (50X TAE buffer is diluted 50 times). The cast was prepared by sealing it with cellophane tape and was ensured leak proof. The agarose was heated in an oven until it dissolved completely in the buffer. The solution was poured into the gel cast and comb was placed until solidification. After solidification, the gel was immersed in an electrophoretic chamber filled with the gel running buffer. The sample was diluted with equal volumes of gel loading dye and loaded carefully into the well using a micropipette. The power pack was switched on, and the gel was run at 120-140 V until the dye front reached 1 cm above the end of the gel. The gel was removed from the electrophoretic chamber and immersed in EtBr solution for 15 min. Finally, analysis was carried out under ultraviolet light (302 nm) using a BIO-RAD gel documentation system. The pictures were captured and stored for data management.
Qualitative analysis of the bacteria in the sample was detected by multiplex PCR. A minimum of 100 bacterial cells are required for the amplification to be visualized as a band on the agarose gel.
Attrition loss
Twenty-five subjects with chronic periodontitis were selected based on inclusion and exclusion criteria. Baseline clinical parameters and microbiological samples were collected, and full-mouth SRP and subgingival irrigation were done in one sitting. But after 3 months, 5 subjects were lost during follow-up due to their inconvenience to report at the recall dates. So, only 20 subjects completed the study.
Enrollment, baseline measurements of clinical parameters, nonsurgical periodontal therapy, and subgingival irrigation were done by one investigator (KM). A masked outcome assessor (JC) performed outcome measurements of clinical parameters. PCR was done by the microbiologist.
Statistical analysis
Statistical tests were performed using software IBM-Statistical Package for Social Sciences (SPSS, version 20). Clinical parameters such as PS, GS, PPD, and CAL were calculated per group. Interpatient systemic differences (e.g., age, gender) are known to significantly confound the effect of periodontal treatment (independent variable). To limit these problems, we used a split-mouth design. Data derived from sites within the same mouth are correlated to some degree.
Intragroup comparison of parameters at baseline and at the end of 3 months recall interval was done using Paired t-test.
Intergroup comparison of clinical parameters such as PS, GS, PPD, and CAL was done using analysis of variance (ANOVA). The repeated measures ANOVA model complete block design, with the patients constituting the blocks, was used. When the results indicated that a significant difference existed between irrigation groups, post hoc multiple comparison tests (Bonferroni test and Dunnett's test) were used to determine which pairs were actually different. The results were considered statistically significant when P < 0.05.
Intergroup comparison of P. gingivalis and T. forsythia count was done using descriptive statistics.
RESULTS
Plaque score
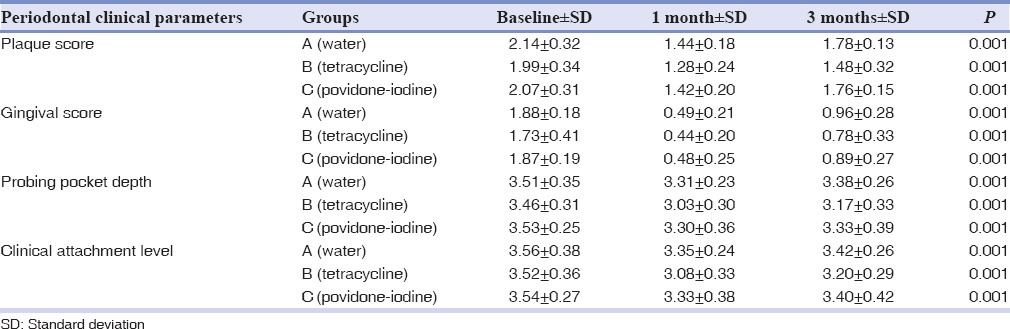
The mean PS of Group A, Group B, and Group C was given in Table 1. Intragroup comparison indicated that Group A, Group B, and Group C showed statistically significant difference in PS at baseline and during the recall visit at the end of 3 months (P < 0.05) [Table 1].
Table 1.
Intergroup comparison of clinical parameters
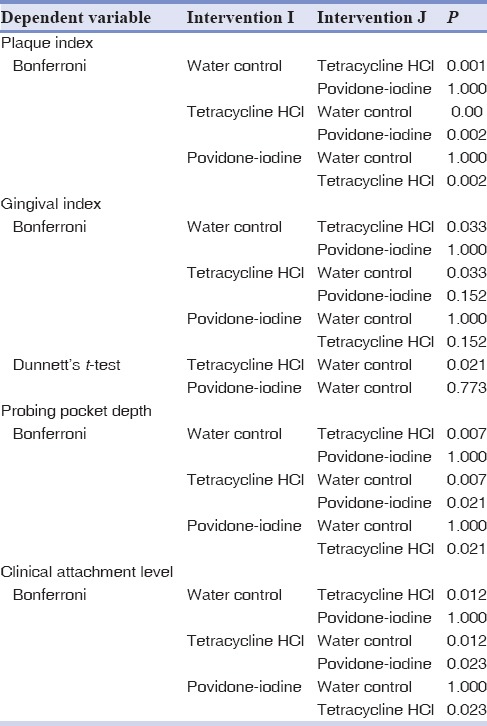
On intergroup comparison with Bonferroni test, there was statistically significant difference between Group A (water) and Group B (tetracycline) and also between Group B (tetracycline) and Group C (povidone-iodine), that is, P < 0.05. But, there was no statistically significant difference between Group A (water) and Group C (povidone-iodine), that is, P > 0.05. Here, Bonferroni test indicated that Group B had a statistically significant effect when compared to Group C [Table 2].
Table 2.
Intergroup comparison of clinical parameters
Gingival score
The mean GS of Group A, Group B, and Group C was given in Table 1. Intragroup comparison indicated that Group A, Group B, and Group C showed statistically significant difference in gingival score at baseline and during the recall visit at the end of 3 months, that is, P < 0.05 [Table 1].
On Intergroup comparison using Bonferroni test, there was statistically significant difference between Group A (water) and Group B (tetracycline), that is, P < 0.05. But, there was no statistically significant difference between Group A (water) and Group C (povidone-iodine) and also between Group B (tetracycline) and Group C (povidone-iodine), that is, (P > 0.05). So, to know the statistical difference between Group B and Group C, Dunnett's test was done between Group B (tetracycline) and Group C (povidone-iodine) with Group A (water) as control. This test indicated that Group B was statistically significant when compared to Group C (P < 0.05) [Table 2].
Probing pocket depth
The mean PPD of Group A, Group B, and Group C was given in Table 1. Intragroup comparison indicated that Group A, Group B, and Group C showed statistically significant difference in PPD at baseline and during the recall visit at the end of 3 months, that is, P < 0.05 [Table 1].
Intergroup comparison showed that there was statistically significant difference between Group A (water) and Group B (tetracycline), that is, (P < 0.05). Also, there was statistically significant difference between Group B (tetracycline) and Group C (povidone-iodine), that is, (P < 0.05), but there was no statistically significant difference between Group A (water) and Group C (povidone-iodine), that is, (P > 0.05). Here, Bonferroni test itself indicated that Group B was statistically significant when compared to Group C, so Dunnett's test was not needed [Table 2].
Clinical attachment level
The mean CAL of Group A, Group B, and Group C was given in Table 1. Intragroup comparison indicated that Group A, Group B, and Group C showed statistically significant difference in CAL at baseline and during the recall visits at the end of 3 months, that is, P < 0.05 [Table 1].
Intergroup comparison showed that there was statistically significant difference between Group A (water) and Group B (tetracycline), that is, (P < 0.05). Also, there was statistically significant difference between Group B (tetracycline) and Group C (povidone-iodine), that is, (P < 0.05), but there was no statistically significant difference between Group A (water) and Group C (povidone-iodine), that is, (P > 0.05). Here, Bonferroni test indicated that Group B was statistically significant when compared to Group C, so Dunnett's test was not needed [Table 2].
Porphyromonas gingivalis detection
Table 3 shows the number of subjects with presence/absence of P. gingivalis (presence/absence of band 197 bp in the PCR). Table shows the number of subjects with presence/absence of P. gingivalis (presence/absence of band 197 bp) in the PCR. A minimum of 100 bacterial cells are required for the amplification to be visualized as a band on the agarose gel.
Table 3.
Intergroup comparison of microbiological parameters
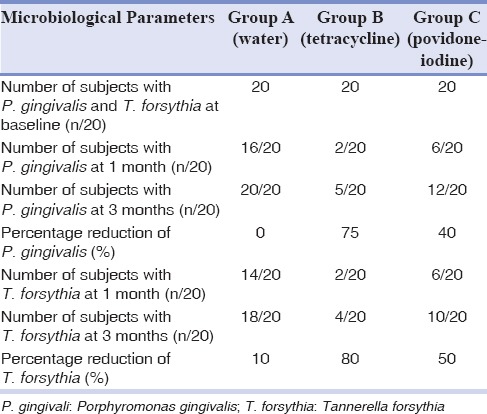
In Group A, at baseline, 20 subjects showed presence of P. gingivalis. During 1 month review, it was seen that number of subjects showing the presence of P. gingivalis reduced to 16. But at the end of 3 months, all 20 subjects showed the presence of P. gingivalis implying that there is 0% reduction in P. gingivalis levels from baseline to 3 months.
In Group B, at baseline, 20 subjects showed the presence of P. gingivalis. During 1 month review, it was seen that number of subjects showing the presence of P. gingivalis reduced to two subjects and at the end of 3 months, it became five subjects with the presence of P. gingivalis which implies that there is 75% reduction in P. gingivalis levels from baseline to 3 months.
In Group C, at baseline, 20 subjects showed the presence of P. gingivalis. During 1 month review, it was seen that number of subjects showing the presence of P. gingivalis reduced to 6. But at the end of 3 months, 12 subjects showed the presence of P. gingivalis implying that there is 40% reduction in P. gingivalis levels from baseline to 3 months.
Tannerella forsythia detection
Table 3 shows the number of subjects with presence/absence of T. forsythia (presence/absence of band 745 bp in the PCR). A minimum of 100 bacterial cells are required for the amplification to be visualized as a band on the agarose gel.
In Group A, at baseline, 20 subjects showed the presence of T. forsythia. During 1 month review, it was seen that number of subjects showing the presence of T. forsythia reduced to 14. But at the end of 3 months, all 18 subjects showed the presence of P. gingivalis implying that there is 10% reduction in P. gingivalis levels from baseline to 3 months.
In Group B, at baseline, 20 subjects showed the presence of T. forsythia. During 1 month review, it was seen that number of subjects showing the presence of T. forsythia reduced to two subjects and at the end of 3 months, it became four subjects with the presence of P. gingivalis which implies that there is 80% reduction in T. forsythia levels from baseline to 3 months.
In Group C, at baseline, 20 subjects showed the presence of T. forsythia. During 1 month review, it was seen that number of subjects showing the presence of T. forsythia reduced to 6. But at the end of 3 months, 10 subjects showed the presence of P. gingivalis implying that there is 50% reduction in T. forsythia levels from baseline to 3 months.
DISCUSSION
Periodontitis, infection of the tooth-supporting tissues, results from the accumulation of pathogenic bacterial plaque at and below the gingival margin. The composition of the dental plaque plays a central role in the etiology of periodontitis.[12] This study was a nonrandomized clinical trial with split-mouth design. The split-mouth design has the advantage of eliminating intersubject variables. This study aimed to compare the clinical and antimicrobial efficacy of tetracycline (10 mg/ml) and 2% povidone-iodine subgingival irrigation as an adjunct to nonsurgical periodontal therapy.
In this study, Group A (water) showed significant improvement in all the clinical parameters such as PS, GS, PPD, and CAL after SRP along with subgingival irrigation with water after 3 months. This may be due to the disruption of bacterial plaque in the periodontal pockets due to SRP and subgingival irrigation.
The results are in accordance with Jolkovsky et al.[13] and Flemmig et al.[14] who found an improvement of the gingival index after 3 months regardless of the irrigant used. The implication of the present study and other studies is that the physiologic flushing of the pocket itself may comprise the primary therapeutic effect of irrigation, regardless of the irrigant used. There could be different reasons for improvement in Group A that is one reason could be that supragingival irrigation alters the population of key pathogens, reducing gingival inflammation. Another reason could be that the water pulsation may alter the specific host-microbial interaction in the subgingival environment. There is also the possibility that the beneficial action of an oral irrigator is at least partly because of the removal of loosely adherent soft deposits interfering with plaque maturation and stimulation of the immune response. Other explanations could be a mechanical stimulation of the gingiva or a combination of the above-mentioned factors. Furthermore, irrigation may reduce the thickness of the plaque, which may not be easily detectable using two-dimensional scoring systems.[13]
PCR done in Group A implied a result of 0% reduction when baseline value was compared to 3 months.
From this study, it was clear that subgingival irrigation with water alone neither prevent plaque accumulation nor reduced bacterial count. Even if the clinical parameters showed improvement, P. gingivalis count was not reduced which shows water could not remove this tissue invading organism effectively. The clinical improvements may have been due to the nonsurgical periodontal therapy.
Most of the published studies show that P. gingivalis and T. forsythia occurs at periodontal pockets of 4-5 mm and this may be due to higher levels of anaerobiosis at deeper sites, difference in subgingival temperature, and requirement for hemin or other substances, thereby providing a more conductive environment for the growth of fastidious and anaerobic microorganisms. This reaffirms the finding that T. forsythia, alone or in combination with P. gingivalis, may be involved in the process of tissue destruction such as pocket deepening or active attachment loss. The studies by Haffajee et al.,[15] Takamatsu et al.,[16] and Wadhwani et al.[17] showed nonsurgical periodontal therapy had effectively reduced these pathogens from the periodontal pocket. And also, there were clinical improvements in their study which can be compared with our study.
Multiplex PCR employed in our study qualitatively analyzed the bacterial count. That is, it detected the presence/absence of bacteria. A minimum of 100 cells are required for a band to be formed. Following SRP with water irrigation, the band was detected in the PCR analysis. However, this result needs to be assessed with caution since the presence of the band only suggests that bacterial count did not decrease below 100 cells. But, there was definitive improvement in clinical parameters suggesting that there has been a significant bacterial load reduction from baseline to 3 months.
In this study, Group B (tetracycline irrigation) showed significant improvement in clinical parameters such as PS, GS, PPD, and CAL in 3 months after adjunctive subgingival irrigation with tetracycline along with nonsurgical periodontal therapy. This may be due to the disruption of bacterial plaque in the periodontal pockets due to SRP and antimicrobial action of tetracycline subgingival irrigation. According to Silverstein et al.,[18] tetracycline irrigation with a waterpik has been reported to produce gingival crevicular fluid tetracycline levels higher than those seen with systemic administration of antibiotic. In addition to this fact, this drug has demonstrated to be effective against the periodontopathogenic microbiota, inhibiting collagenase as well as increasing fibroblast attachment to dental structure when associated with fibronectin. Also, the main advantage of irrigation of periodontal pockets with tetracycline-HCl over systemic administration appears to be the localized concentration of the drug at the sites of disease activity, with minimal effects on the microflora present in other areas. The amount of drug delivered often creates sulcular medication concentrations exceeding the equivalent of 1 mg/ml. This level is considered bactericidal for the majority of bacteria that exhibit resistance to systemically delivered concentrations. Thus, the control of disease activity in deep periodontal pockets brought about by local delivery of the drug may be attributable to the improvement in the clinical parameters.
Our study is not in agreement with Stabholz et al.[5] and Krishna et al.[6] who evaluated the results of a single episode of irrigation with tetracycline 10 mg/ml and 50 mg/ml in the absence of SRP in the experimental sites. According to their study, the amount of antimicrobial activity retained is proportional to the concentration of tetracycline HCl used for irrigation, so 10 mg/ml tetracycline was not sufficient to bring significant clinical results. They have done SRP 1 month prior to the study initiation, exempting the study sites. But in our study, we used a low dose of tetracycline preparation 10 mg/ml which was delivered using Water pik at low pressure immediately after nonsurgical periodontal therapy. This might have allowed a deeper penetration of the irrigants into the periodontal pockets. The results we got were lower PS, GS, lower pocket depth, greater attachment gain, and also 75% reduction of P. gingivalis and 80% reduction of T. forsythia. This study showed that even if the concentration of tetracycline is low, when it is combined with subgingival irrigation and nonsurgical therapy, it could bring significant improvement in the periodontal treatment outcome. This divergence in the results could be associated with the methodology used with regard to the drug, such as concentration, exposure time on radicular surface, and local temperature where the solution was prepared, which could interfere in its dissolution. In our study, the tetracycline solution was prepared at approximately 60°C, which allows complete dissolution of the drug. Other reasons could be the different levels of disease activity or disease severity present at the time of treatment and could account for the lack of agreement between these studies.
There are many other reasons for the clinical improvement in Group B. It can be due to the use of tetracycline that may be attributed to its effects on periodontal regeneration. Other studies have demonstrated multiple beneficial properties of tetracycline unrelated to its antimicrobial properties. Tetracycline HCI has been shown to etch and/or remove the root surface smear layer and cause surface demineralization, to delay pellicle and plaque formation, and to exhibit anticollagenase activity as well as to inhibit, in vitro, parathyroid hormone-induced bone resorption, and human neutrophil functions. It is apparent, therefore, that the effects of tetracycline may be multifactorial through modulation of both the subgingival microflora and mechanisms of tissue destruction.[19] Also, in vitro studies have shown tetracycline can enhance soft tissue attachment to root surfaces, dentin root surface demineralization by low pH tetracycline increases adsorption of fibronectin, and an extracellular matrix glycoprotein. The adsorbed or bound fibronectin enhances fibroblast attachment and growth, while suppressing epithelial cell attachment and growth.[20] All these factors might have led to a better periodontal health in Group B compared to Group A.
PCR in Group B showed that there was 75% reduction of P. gingivalis and 80% reduction of T. forsythia when compared to baseline values which indicated that tetracycline was effective in eliminating these microbes from periodontal pockets.
A growing body of literature[21] showed that P. gingivalis and T. forsythia was sensitive to tetracycline. So in this study, a minimum concentration of tetracycline 10 mg/ml was used for subgingival irrigation as an adjunct to SRP. This study showed if nonsurgical therapy was succeeded by tetracycline irrigation, it allows the drug to reach deep into the pockets. So, even if the concentration of tetracycline is low, if it is used as an adjunct to SRP, it can bring significant improvement in clinical and microbiological parameters.
In this study, Group C (povidone-iodine) showed significant improvement in all the clinical parameters such as PS, GS, PPD, and CAL after nonsurgical therapy along with subgingival irrigation with povidone-iodine; this may be due to the disruption of bacterial plaque in the periodontal pockets due to SRP and antiseptic and bacteriostatic action of povidone-iodine as a subgingival irrigant.
This study, as well as the previous reports by Nakagawa et al.[22] and Hoang et al.[23] suggests that povidone-iodine with low concentrations would be effective when biofilm bacteria are disrupted by SRP.
Without mechanical disruption, higher concentrations are necessary to exert its effect on biofilm. However, there are some concerns involved in the use of povidone-iodine in high concentrations or long-term use.[24] Povidone-iodine gargle solution has been known to cause yellowish discoloration of the teeth of people using this solution for more than 6 months, although this discoloration is reversible after weeks of discontinuation of its use. Other potential adverse events include allergy or hypersensitivity to the solution and its component products and hyperthyroidism due to its systemic absorption. Therefore, it may be advisable to use povidone-iodine with the lowest concentration that would yield a maximum effect against periodontal biofilm.
Other studies which reported significant results were by Rosling et al.,[25] Forabosco et al.,[26] and Krόck et al.,[27] but they had used 10% povidone-iodine which is slightly of higher concentration.
PCR in Group C showed that there is 40% reduction of P. gingivalis and 50% reduction of T. forsythia when baseline values are compared at 3 months. This shows though povidone-iodine was better than water in eliminating the bacteria in the pockets, the results were not as satisfying as the use of tetracycline.
Hosaka et al.[7,28] used 2% povidone-iodine and checked for the reduction of P. gingivalis and concluded that 3 s application of 2% PVP-I would be effective in suppressing both P. gingivalis and Fusobacterium nucleatum in dual-species biofilm, and this provides clinical implication for the control of subgingival biofilm. In their study, they have done only microbial analysis without evaluation of any periodontal parameters. But, our study has evaluated periodontal parameters and the presence of P. gingivalis and T. forsythia. SRP with subgingival irrigation of 2% povidone-iodine was done, and results showed that all the periodontal parameters improved with this low concentration of povidone-iodine.
Our results suggest that subgingival irrigation with 2% povidone-iodine was effective in restoring the gingival health by showing improvement in all the clinical parameters. It also resulted in the effective elimination of P. gingivalis and T. forsythia in vivo. So, results indicate even if the concentration of povidone-iodine is as low as 2%, if it is used as an adjunct to nonsurgical periodontal therapy, it can bring significant improvement in restoring periodontal health.
In the intergroup evaluation, the PS, GS, PPD, and CAL were compared among Group A (water), Group B (tetracycline), and Group C (povidone-iodine). The intergroup comparison using Dunnett's test indicated Group B (tetracycline) had significant improvement in all the clinical parameters such as PS, GS, PPD, and CAL. It may be due to its antimicrobial activity against the pathogenic microflora, it has also been demonstrated that tetracycline inhibits collagenase and the osteoclastic function, stimulates osteoblastic bone formation, regulates angiogenesis, and when associated with fibronectin, it increases the fibroblast insertion over the radicular structure.[29]
The intergroup comparison of P. gingivalis also showed 75% reduction in Group B (TTC) and 40% reduction in Group C (povidone-iodine) at the end of 3 months when compared to baseline. The intergroup comparison of T. forsythia showed similar values as that of P. gingivalis. The tetracycline group showed 80% reduction whereas povidone-iodine group showed 50% reduction at the end of 3 months when compared to baseline.
Farias et al.[30] explained the strong association of T. forsythia and P. gingivalis pathogens in the pathogenesis of periodontitis, as these bacteria cause tissue loss and severe alveolar bone resorption. Moreover, the simultaneous presence of these bacteria in deep sites suggests a symbiotic relationship between these virulent species, favoring, in this way, a further progression of periodontal disease.
The nonsurgical therapy itself must have eliminated these pathogens. The subgingival irrigation would have given an added benefit of eliminating bacteria left behind in the pocket.[15,16] The intragroup comparison showed that both groups, tetracycline and povidone-iodine, resulted in significant improvement in periodontal health after 3 months when subgingival irrigation was used as an adjunct to nonsurgical periodontal therapy.
Microbiological results showed a reduction in P. gingivalis and T. forsythia levels with tetracycline showing a better result when compared to povidone-iodine.
The intergroup comparison showed Group B (tetracycline) was slightly superior to Group C (povidone-iodine) in improving the periodontal health.
At the end of 3 months after nonsurgical periodontal therapy, irrespective of the irrigants, all groups showed significant improvement in their periodontal health, even though complete elimination of periodontal pockets and complete gain in clinical attachment could not be achieved. This may be due to difference in morphology of each tooth, multifactorial etiology of periodontitis where tissue invading organisms can recolonize[28] or reinfect not only from periodontal pockets, but also from other oropharyngeal habitats (mucous membranes, tongue, tonsils, and saliva).
The intragroup comparisons of the clinical parameters and microbial analysis of bacterial cells at baseline, 1 month, and at 3 months recall interval showed that all the subgingival irrigants, when used as an adjunct with nonsurgical periodontal therapy, were effective in improving the periodontal health. Clinically, there was a reduction in inflammation, mean PPDs, and a mean gain of attachment, in all groups. The intergroup comparison showed Group B (tetracycline) was slightly superior to povidone-iodine in improving the periodontal health.
Microbiologically, there is more reduction in P. gingivalis and T. forsythia with tetracycline followed by povidone-iodine after 3 months. Significant clinical and microbiological improvement occurred in the group with tetracycline irrigation when compared to povidone-iodine, though both the results were comparable and noteworthy in short-term. Finally, on detection of microbes in the periodontal pockets using PCR, we arrived at the conclusion that though the clinical parameters improved irrespective of the irrigants, water could not remove P. gingivalis and T. forsythia effectively from periodontal pockets whereas tetracycline and povidone-iodine were effective in partially removing P. gingivalis and T. forsythia from the periodontal pockets.
CONCLUSION
Within the limitations of this study, the following may be concluded regarding the effect of single subgingival irrigation with tetracycline and povidone-iodine:
Both tetracycline and povidone-iodine appear to have nearly comparable effects, with tetracycline subgingival irrigation displaying slightly superior effect in comparison to povidone-iodine, when used as subgingival irrigants as an adjunct to nonsurgical periodontal therapy
When antimicrobial efficacy of tetracycline was compared with povidone-iodine, tetracycline was found to be much more effective than povidone-iodine against P. gingivalis and T. forsythia, when used as subgingival irrigants as adjuncts to nonsurgical periodontal therapy.
Nevertheless, the results presented herein do not suggest that subgingival irrigation with tetracycline/povidone-iodine can replace nonsurgical periodontal therapy. The results suggest that subgingival irrigation with antimicrobials may be valuable adjuncts to nonsurgical periodontal therapy.
Financial support and sponsorship
The authors thank COLGATE PALMOLIVE INDIA PVT LTD for their financial support to complete this study.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Hinrichs JE, Novak MJ. Classification of diseases and conditions affecting the periodontium. In: Carranza FA, editor. Carranza's Clinical Periodontology. 11th ed. New Delhi: Reed Elsevier India Private Limited; 2012. p. 41. [Google Scholar]
- 2.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 3.Suchetha A, Garg A, Lakshmi P, Sapna N, Mundinamane DB, Apoorva SM. Povidone iodine vs tetracycline fibers - To analyse the therapeutic effect. Journal of Oral health and community Dentistry. 2014;8(1):24–9. [Google Scholar]
- 4.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Stabholz A, Nicholas AA, Zimmerman GJ, Wikesjö UM. Clinical and antimicrobial effects of a single episode of subgingival irrigation with tetracycline HCl or chlorhexidine in deep periodontal pockets. J Clin Periodontol. 1998;25:794–800. doi: 10.1111/j.1600-051x.1998.tb02372.x. [DOI] [PubMed] [Google Scholar]
- 6.Krishna MK, Ravindran SK, Vivekanandan G, Navasivayam A, Thiagarajan R, Mohan R. Effects of a single episode of subgingival irrigation with tetracycline HCl or chlorhexidine: A clinical and microbiological study. J Indian Soc Periodontol. 2011;15:245–9. doi: 10.4103/0972-124X.85668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosaka Y, Saito A, Maeda R, Fukaya C, Morikawa S, Makino A, et al. Antibacterial activity of povidone-iodine against an artificial biofilm of Porphyromonas gingivalis and Fusobacterium nucleatum. Arch Oral Biol. 2012;57:364–8. doi: 10.1016/j.archoralbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Soben P. Essentials of Public Health Dentistry Community Dentistry. 5th ed. 2013. p. 322. [Google Scholar]
- 9.Soben P. Essentials of Public Health Dentistry Community Dentistry. 5th ed. 2013. p. 326. [Google Scholar]
- 10.Takei HH, Carranza FA. Clinical diagnosis. In: Carranza FA, editor. Carranza's Clinical Periodontology. 11th ed. New Delhi: Reed Elsevier India Private Limited; 2012. p. 353. [Google Scholar]
- 11.Gafan GP, Lucas VS, Roberts GJ, Petrie A, Wilson M, Spratt DA. Prevalence of periodontal pathogens in dental plaque of children. J Clin Microbiol. 2004;42:4141–6. doi: 10.1128/JCM.42.9.4141-4146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paju S, Pussinen PJ, Suominen-Taipale L, Hyvönen M, Knuuttila M, Könönen E. Detection of multiple pathogenic species in saliva is associated with periodontal infection in adults. J Clin Microbiol. 2009;47:235–8. doi: 10.1128/JCM.01824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jolkovsky DL, Waki MY, Newman MG, Otomo-Corgel J, Madison M, Flemmig TF, et al. Clinical and microbiological effects of subgingival and gingival marginal irrigation with chlorhexidine gluconate. J Periodontol. 1990;61:663–9. doi: 10.1902/jop.1990.61.11.663. [DOI] [PubMed] [Google Scholar]
- 14.Flemmig TF, Epp B, Funkenhauser Z, Newman MG, Kornman KS, Haubitz I, et al. Adjunctive supragingival irrigation with acetylsalicylic acid in periodontal supportive therapy. J Clin Periodontol. 1995;22:427–33. doi: 10.1111/j.1600-051x.1995.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 15.Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324–34. doi: 10.1111/j.1600-051x.1997.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 16.Takamatsu N, Yano K, He T, Umeda M, Ishikawa I. Effect of initial periodontal therapy on the frequency of detecting Bacteroides forsythus, Porphyromonas gingivalis, and Actinobacillus actinomycetemcomitans. J Periodontol. 1999;70:574–80. doi: 10.1902/jop.1999.70.6.574. [DOI] [PubMed] [Google Scholar]
- 17.Wadhwani RB, Chaudhary MS, Tharani DA, Chandak SA. Effect of scaling and root planing on detection of Tannerella forsythia in chronic periodontitis. J Oral Dis. 2013;2013:e383746. [Google Scholar]
- 18.Silverstein L, Bissada N, Manouchehr-Pour M, Greenwell H. Clinical and microbiologic effects of local tetracycline irrigation on periodontitis. J Periodontol. 1988;59:301–5. doi: 10.1902/jop.1988.59.5.301. [DOI] [PubMed] [Google Scholar]
- 19.Trombelli L, Scabbia A, Carotta V, Scapoli C, Calura G. Clinical effect of subgingival tetracycline irrigation and tetracycline-loaded fiber application in the treatment of adult periodontitis. Quintessence Int. 1996;27:19–25. [PubMed] [Google Scholar]
- 20.Drisko CH. Non-surgical pocket therapy: Pharmacotherapeutics. Ann Periodontol. 1996;1:491–566. doi: 10.1902/annals.1996.1.1.491. [DOI] [PubMed] [Google Scholar]
- 21.Tanner AC, Izard J. Tannerella forsythia, a periodontal pathogen entering the genomic era. Periodontol 2000. 2005;42:88–113. doi: 10.1111/j.1600-0757.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Saito A, Hosaka Y, Yamada S, Tsunoda M, Sato T, et al. Bactericidal effects on subgingival bacteria of irrigation with a povidone-iodine solution (Neojodin) Bull Tokyo Dent Coll. 1990;31:199–203. [PubMed] [Google Scholar]
- 23.Hoang T, Jorgensen MG, Keim RG, Pattison AM, Slots J. Povidone-iodine as a periodontal pocket disinfectant. J Periodontal Res. 2003;38:311–7. doi: 10.1034/j.1600-0765.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- 24.Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res. 2002;37:389–98. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosling B, Hellström MK, Ramberg P, Socransky SS, Lindhe J. The use of PVP-iodine as an adjunct to non-surgical treatment of chronic periodontitis. J Clin Periodontol. 2001;28:1023–31. doi: 10.1034/j.1600-051x.2001.281106.x. [DOI] [PubMed] [Google Scholar]
- 26.Forabosco A, Galetti R, Spinato S, Colao P, Casolari C. A comparative study of a surgical method and scaling and root planing using the odontoson. J Clin Periodontol. 1996;23:611–4. doi: 10.1111/j.1600-051x.1996.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 27.Krück C, Eick S, Knöfler GU, Purschwitz RE, Jentsch HF. Clinical and microbiologic results 12 months after scaling and root planing with different irrigation solutions in patients with moderate chronic periodontitis: A pilot randomized trial. J Periodontol. 2012;83:312–20. doi: 10.1902/jop.2011.110044. [DOI] [PubMed] [Google Scholar]
- 28.Quirynen M, De Soete M, Boschmans G, Pauwels M, Coucke W, Teughels W, et al. Benefit of “one-stage full-mouth disinfection” is explained by disinfection and root planing within 24 hours: A randomized controlled trial. J Clin Periodontol. 2006;33:639–47. doi: 10.1111/j.1600-051X.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes LA, Martins TM, Almeida JM, Nagata MJ, Theodoro LH, Garcia VG, et al. Experimental periodontal disease treatment by subgingival irrigation with tetracycline hydrochloride in rats. J Appl Oral Sci. 2010;18:635–40. doi: 10.1590/S1678-77572010000600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farias BC, Souza PR, Ferreira B, Melo RS, Machado FB, Gusmão ES, et al. Occurrence of periodontal pathogens among patients with chronic periodontitis. Braz J Microbiol. 2012;43:909–16. doi: 10.1590/S1517-83822012000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]