Abstract
Purpose:
To determine the efficacy of lung volume recruitment maneuver (LVRM) with high frequency oscillatory ventilation (HFOV) on oxygenation, hemodynamic alteration, and clinical outcomes when compared to conventional mechanical ventilation (CV) in children with severe acute respiratory distress syndrome (ARDS).
Materials:
We performed a randomized controlled trial and enrolled pediatric patients who were diagnosed to have severe ARDS upon pediatric intensive care unit (PICU) admission. LVRM protocol combined with HFOV or conventional mechanical ventilation was used. Baseline characteristic data, oxygenation, hemodynamic parameters, and clinical outcomes were recorded.
Results:
Eighteen children with severe ARDS were enrolled in our study. The primary cause of ARDS was pneumonia (91.7%). Their mean age was 47.7 ± 61.2 (m) and body weight was 25.3 ± 27.1 (kg). Their initial pediatric risk of mortality score 3 and pediatric logistic organ dysfunction were 12 ± 9.2 and 15.9 ± 12.8, respectively. The initial mean oxygen index was 24.5 ± 10.4, and mean PaO2/FiO2 was 80.6 ± 25. There was no difference in oxygen parameters at baseline the between two groups. There was a significant increase in PaO2/FiO2 (119.2 ± 41.1, 49.6 ± 30.6, P = 0.01*) response after 1 h of LVRM with HFOV compare to CV. Hemodynamic and serious complications were not significantly affected after LVRM. The overall PICU mortality of our severe ARDS at 28 days was 16.7%. Three patients in CV with LVRM group failed to wean oxygen requirement and were cross-over to HFOV group.
Conclusions:
HFOV combined with LVRM in severe pediatric ARDS had superior oxygenation and tended to have better clinical effect over CV. There is no significant effect on hemodynamic parameters. Moreover, no serious complication was noted.
Keywords: Conventional ventilation, high frequency oscillatory ventilation, lung volume recruitment maneuver, oxygenation
Introduction
Acute respiratory distress syndrome (ARDS) is a leading cause of morbidity and mortality in intensive care unit (ICU) worldwide. Mechanical ventilation is a cornerstone in management of ARDS patients. Low tidal volume combined with adequate positive end-expiratory pressure (PEEP) has been shown to reduce mortality.[1,2] An open lung or lung volume recruitment maneuver (LVRM) is a procedure to recruit collapsed alveoli. It may be achieved by a brief raising of transpulmonary pressure to higher levels than that achieved during normal ventilation.[3,4] It has been recommended as a useful tool to re-open collapsed lung regions, promoting homogeneity within the lungs, and eventually improve oxygenation. Recent review including our previous work had shown that using high frequency oscillatory ventilation (HFOV) was better, less barotraumas and unlikely to cause harm compared to conventional ventilation (CV).[5,6,7] It is characterized by the rapid delivery of small tidal volume of gas combined with high mean airway pressure (mPaw). Thus, it may be an ideal model for lung protective strategy.
Although several experimental studies have shown a positive effect of LVRM on oxygenation, clinical studies are currently controversies. Two recent randomized control clinical trials in adult ARDS have failed to show benefits of its use compared to CV.[8,9] Too high intrathoracic pressures applied during LVRM to expand the collapsed lung unit may cause further barotraumas as well as biological sequelae such as cytokines upregulation, and translocation may have clinical deterioration occurring following LVRM.[10,11] In addition, suboptimal technique, inadequate sedation, and muscle relaxant or uncontrol of fluid balance could be responsible for this failure.[12,13] Thus, it was our desire to study the clinical benefits of using LVRM with HFOV compared with CV with LVRM in children with severe ARDS.
Materials and Methods
Design
This was a prospective, randomized control trial. Our study was registered as a control–clinical trial (ISRCTN No. 64665281).
Study population
Eighteen children (>1 month and <15 years of age) with diagnosis of severe ARDS (PaO2/FiO2 <100) by Berlin definition who were admitted to our pediatric ICU (PICU) with no exclusion criteria were enrolled to our study during July 2012–December 2013. The PICU is a 10-bedded unit in the tertiary care referral center. They were enrolled by blocked randomization before start of the study. Nine children were randomly to CV with LVRM, and another nine children were allocated to HFOV with open lung technique group.
HFOV protocol used in this study was approved by our Institutional Review Board. Informed consents were obtained from the parents prior to their evaluation for HFOV therapy. Before randomization to the treatment arm, all patients were received CV with the FiO2 of 1, the median PEEP of 12 cm H2O, fluid resuscitation to keep high central venous pressure (CVP) (range between 8 and 12 mmHg) and were mostly on either inotropics or vasopressors at the time of LVRM with either CV or HFOV. All patients were deeply sedated and paralyzed.
Patients were diagnosed as ARDS by Berlin definition [14] and met the following entry criteria: (1) Respiratory failure not fully explained by cardiac failure or fluid overload, (2) chest X-ray findings of new infiltrates consistent with acute pulmonary parenchymal disease, and (3) full face-mask, bi-level ventilation or continuous positive airway pressure >5 cm H2O. Our exclusion criteria included the following: (1) Evidence/suspect of congestive heart failure, or (2) evidence of left atrial hypertension, or (3) Severe irreversible neurological injury or intractable shock, or (4) the underlying disease was deemed irreversible or ARDS >48 h, and (5) Preexisting air leak syndrome (e.g., pneumothorax or pneumomediastinum) or preexisting cystic lung disease.
Oxygenation index (OI) = mPaw × FiO2 × 100/PaO2
Ventilator strategy
We used either a SensorMedics (3100A/B) oscillator (VIASyS, USA) with a rapid high lung volume recruitment protocol as described previously.[15] In brief, this strategy aims to recruit and stabilize the majority of collapsed alveoli. Starting mPaw at 30 cm H2O (or used 35 cm H2O for subjects body weight [BW] >35 kg), the continuous distending pressure was sustained for 20 s (or 30 s for subjects BW >35 kg). Then, the piston started together with gradually weaned down mPaw to the target level (+5–8 cm H2O above previous mPaw of CV), and other ventilator settings were adjusted accordingly base on clinical response. The initial pressure amplitude was set at 3 × mPaw of continuous mandatory ventilation (CMV), and frequency was set according to age. The fraction of inspired oxygen (FiO2) was gradually reduced stepwise to keep SpO2 above 92%. The LVRM procedure was repeated if SpO2 was below 95% with FiO2 of 1. After 1 h, the initial arterial blood gas was obtained and ventilator settings were adjusted accordingly. Hypotension was defined as a 25% decreased in baseline mean arterial pressure (MAP).
Nine children were enrolled to CV with lung volume recruitment by using either Servo I or Bennett 840 ventilators as per protocol. LVRM protocol combined with HFOV or CV were used in all the studies patients (use of 15–20 cm H2O of PEEP, driving pressure of 20 cm H2O, with 2 min decremental PEEP titrate down in each step to get the best compliance, and then set + 2 cm H2O above that level, finally wean down positive inspiratory pressure to get 6–8 cc/kg of tidal volume). Baseline clinical characteristic data, oxygenation, hemodynamic parameters, and clinical outcomes were recorded during the procedure and at 1, 4, 12, 24, and 48 h after LVRM and were analyzed at the end of the study.
Statistics
All data are presented as means ± standard deviation or median (95% confidence interval) if not normally distributed. They were compared by using nonparametric Wilcoxon signed rank test. A Friedman repeated measures analysis was used for multiple comparisons. A P < 0.05 was considered statistically significant. It was performed by using SPSS version 13 (SPSS; Chicago, IL, USA).
Results
Twenty-three children with severe ARDS (8 female, 15 male) were recruited to our study, and five were excluded due to our exclusion criteria. Eighteen (nine in each group) followed our LVRM protocol [Table 1]. Their baseline demographic, clinical characteristics are listed and there is no statistically significant compared between HFOV and CV group [Tables 1 and 2]. Their age was 47.7 ± 61.2 (month) and BW was 25.3 ± 27.1 (kg).
Table 1.
Demonstrate baseline, clinical characteristics in children enrolled in this study (n=18)

Table 2.
Compare baseline clinical characteristics between high frequency oscillatory ventilation group and conventional ventilation group. There is no statistical significant between two groups at baseline
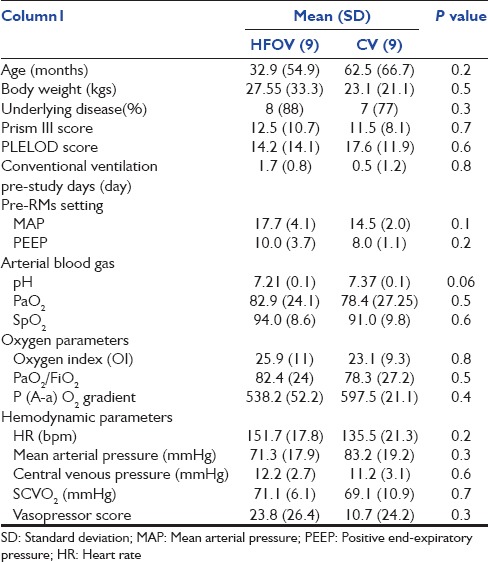
Basic hemodynamic responses during lung recruitment maneuver
Change of heart rate, mean arterial pressure, and central venous pressure during lung recruitment maneuver
Before the LVRM intervention, all patients were stabilized (with fluid resuscitation and inotropes per protocol). All of them were received vasopressors based on their hemodynamic conditions. The MAP was somewhat lower at 5–10 min after LVRM but no statistically significant. Heart rate (HR) tended to lower at 60 min compare to baseline (140.4 ± 15.9 beat/min, 132.3 ± 18.5 beat/min, P = 0.1). There was no significant difference in HR, CVP, or MAP compared between both groups at baseline [Figure 1].
Figure 1.
Demonstrate delta change in VS (heart rate, mean blood pressure, central venous pressure) after lung volume recruitment (n = 18)
Oxygenation responses after lung volume recruitment maneuvers
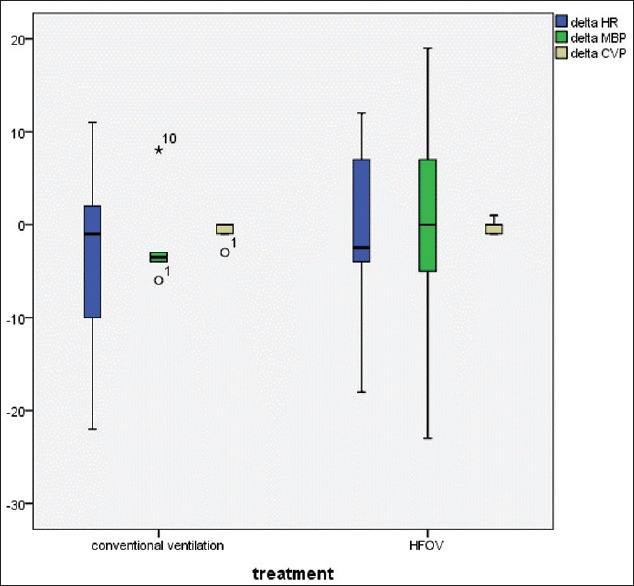
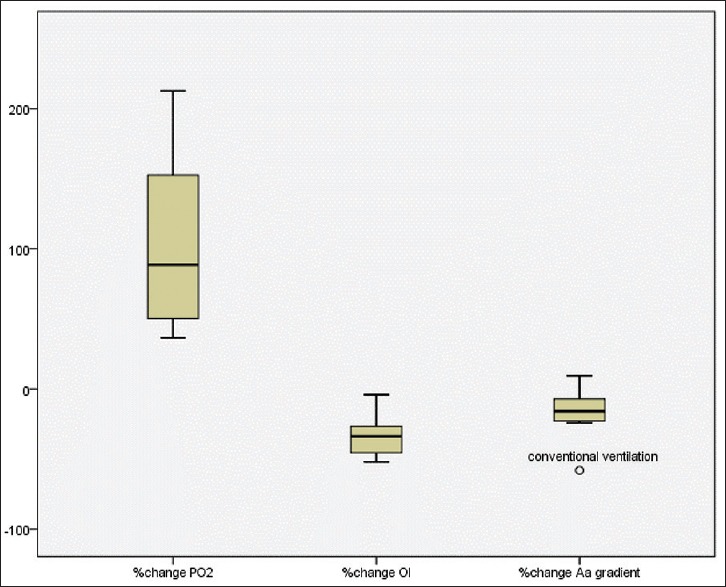
All of the enrolled patients followed with the LVRM protocol at the initiation of HFOV or CV. Our primary outcome demonstrated that most of the patients in both groups were response to LVRM after 1 h (Figure 2, 12/18 [66%]). Furthermore, 7/9 (77%) of HFOV group and 5/9 (55%) of CV group were significantly increase in PaO2/FiO2 following 1 h of lung recruitment. HFOV group had better PaO2/FiO2 response compared to CV with LVRM (138.5 ± 49.7, 69 ± 56.8%, P < 0.01), [Figure 3]. In addition, 6/9 (66%) children in CV group were failed to wean oxygen requirement lower than FiO2 of 0.6 after LVRM and had to switch to HFOV mode at 6 h after enrollment (two patients had refractory hypoxemia and 1 patient had refractory hypercapnia). There was no significant difference in PaO2/FiO2 ratio at 4 h compared between both groups [Figure 4]. There was no significant change in PCO2 after 1 h, 4 h, 6 h, 12 h, and 24 h after LVRM.
Figure 2.
Demonstrate percentage change of PaO2, percentage change of oxygenation index and alveolar-arterial gradient after 1 h of lung volume recruitment maneuver (n = 18)
Figure 3.
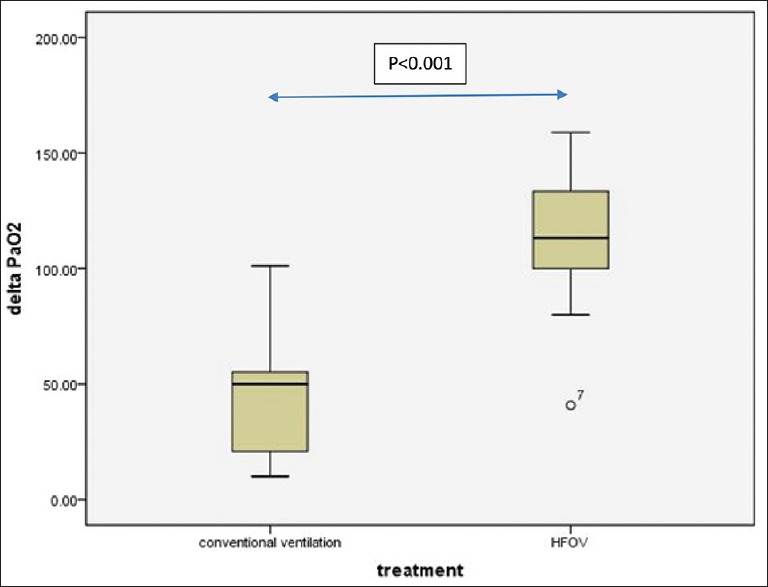
Demonstrate significant increase of PaO2 after 1 h of lung volume recruitment maneuver compare between high frequency oscillatory ventilation and conventional ventilation (P < 0.001, n = 9 each group)
Figure 4.
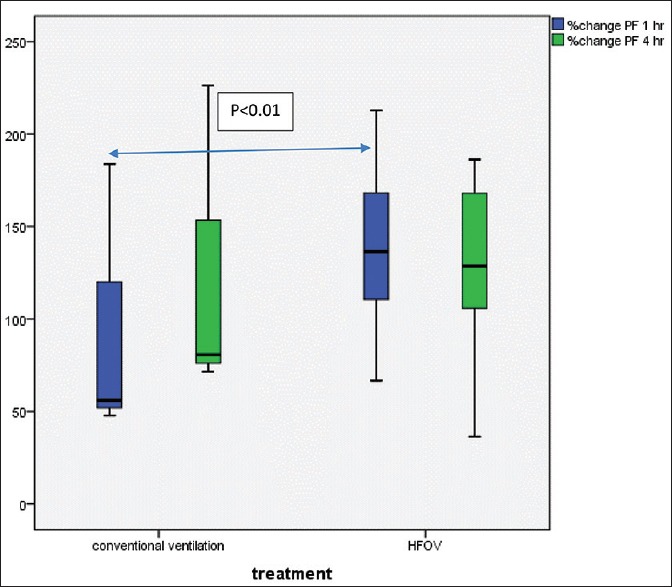
Significant increase of PaO2/FiO2 after 1 h of lung volume recruitment maneuver compare between high frequency oscillatory ventilation and conventional ventilation (P < 0.01, n = 9 each group)
Transition from high frequency oscillatory ventilation to conventional ventilation
Eleven patients (91%) (8/9 from HFOV and 3/9 cross-over from CV) were able to switch back from HFOV to a CV according to our transitional criteria. OI was significantly decreased at 24 h in patients who were able to switch back to CV compared to ones who were not (17.8 ± 7.2, 29.8 ± 29.9, P = 0.007) [Figure 5].
Figure 5.
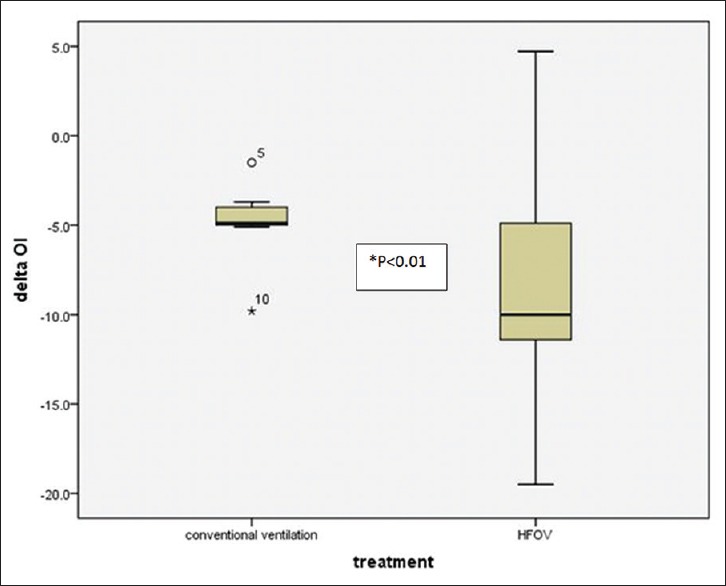
Demonstrate significant reduction in oxygenation index after 24 h of lung volume recruitment maneuver compare between high frequency oscillatory ventilation and conventional ventilation group (P < 0.01)
Complications and outcomes
There was no serious complication following LVRM procedure from both HFOV and CV. Most of our patients tolerated the study protocol well. No significant hemodynamic disturbance was observed. Two patients were expired (one from each group). Our PICU mortality rate of severe ARDS was 11% (2/18). The cause of death was multiple organ failures. Patients were on HFOV for a median of 6 days and had 15 ± 3.5 total days on ventilator. No patient was withdrawn from the protocol.
Discussion
CV with optimum PEEP combined with low tidal volume is currently a ventilator strategy that is, widely accepted as a standard therapy for ARDS. HFOV is an alternative mode of mechanical ventilator that helps to improve oxygenation. A recent consensus management of pediatric ARDS is recommended to use HFOV with incremental LVRM in severe form. It is, however, different than our technique that used decremental LVRM. Our previous study demonstrated applying decremental lung volume recruitment together with HFOV is effective and well-tolerated in pediatric ARDS. Recent publication investigated in adult ARDS failed to demonstrate benefit of using HFOV compared to CV. Nevertheless, there were lots of pitfalls regarding these particular trials and required further study. At present, there are very few studies involving LVRM in children, and there is none compared HFOV with CV in the same trial. Our study is the first prospective randomized control trial in severe Pediatric ARDS investigated clinical outcomes compared between HFOV and CV with decremental LVRM. We found a rapid and significant improvement in gas exchange evidence by improving PaO2/FiO2 (138 ± 49.7, 69 ± 56.8%, P < 0.05) and significant reduced oxygen requirement at 1 h after LVRM compared to CV. It is also in agreement with recent report from neonate and some adult studies.[14,16,17] There was no significant difference in basic hemodynamic data compare between HFOV and CV following LVRM.
Ferguson et al. was the first to explore the benefit of using HFOV combined with decremental LVRM protocol in adult ARDS.[16] They showed that using combination of HFOV with LVRM resulted in rapid and sustained improvement of oxygenation. Tingay et al. and De Jaegere et al. also demonstrated significant improvement in oxygenation by utilizing HFOV with LVRM in neonatal RDS whereas using different techniques.[17,18] Recent large multicenter clinical trials in adult ARDS has failed to demonstrate benefit of using HFOV over CV with LVRM protocol. This may explain the difference in physiologic alteration during HFOV in adult patients with ARDS or suboptimal technique of using HFOV. In general, patients who are on HFOV would require high dose of sedation and often need paralysis to ensure synchronize in ventilation. Both of these particular trials used muscle relaxant in only 50% of their patients. This common practice could make hemodynamic together with oxygenation in these patients more fluctuation and difficult to control. It also would lead to increase requirement of fluid bolus and vasopressors. The fixed oxygenation/mPaw table is also troublesome. In addition, more positive fluid balance is well known for increasing morbidity and mortality in patients with ARDS.
An open lung approach with higher levels of PEEP together with lung volume recruitment with protective ventilation strategy in CV should increase the end-expiratory lung volume through alveolar recruitment to avoid cyclic alveolar derecruitment. There were several reports of clinical responses following LVRM in ARDS patients,[19,20] but there was very few in pediatrics. Moreover, its benefit and clinical efficacy have not been compared with HFOV in children with severe ARDS. Another recent publication strongly demonstrated the efficacy of HFOV over CV in management of extremely premature newborn with RDS by showing those who received HFOV had superior lung function at 11–14 years of age. Our ARDS mortality in this trial is at 11% (2/18) which is better compared to recent report from ESPNIC group (17%).[21] Three (3/9, 33%) patients in CV group had to cross-over to HFOV group and could eventually extubated. Thus, our study confirmed the clinical benefit of using HFOV in neonatal or children with severe respiratory failure.[22] However, this study had some limitations such as our small sample size and represented only one center.
Conclusions
This study showed the oxygenation benefit of using HFOV over CV with LVRM in severe pediatric ARDS. There is no significant effect on hemodynamic parameters. Furthermore, no serious complication was noted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P, Jr, Wiener CM, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999;27:1492–8. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky AS. Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med. 1999;25:1297–301. doi: 10.1007/s001340051061. [DOI] [PubMed] [Google Scholar]
- 4.Lachmann B. Open up the lung and keep the lung open. Intensive Care Med. 1992;18:319–21. doi: 10.1007/BF01694358. [DOI] [PubMed] [Google Scholar]
- 5.Samransamruajkit R, Prapphal N, Deelodegenavong J, Poovorawan Y. Plasma soluble intercellular adhesion molecule-1 (sICAM-1) in pediatric ARDS during high frequency oscillatory ventilation: A predictor of mortality. Asian Pac J Allergy Immunol. 2005;23:181–8. [PubMed] [Google Scholar]
- 6.Arnold JH, Truog RD, Thompson JE, Fackler JC. High-frequency oscillatory ventilation in pediatric respiratory failure. Crit Care Med. 1993;21:272–8. doi: 10.1097/00003246-199302000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Sud S, Sud M, Friedrich JO, Meade MO, Ferguson ND, Wunsch H, et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): Systematic review and meta-analysis. BMJ. 2010;340:c2327. doi: 10.1136/bmj.c2327. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 9.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–13. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 10.Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med. 2004;32:168–74. doi: 10.1097/01.CCM.0000104203.20830.AE. [DOI] [PubMed] [Google Scholar]
- 11.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–14. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 12.Hager DN. High-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Curr Opin Anaesthesiol. 2012;25:17–23. doi: 10.1097/ACO.0b013e32834ea57b. [DOI] [PubMed] [Google Scholar]
- 13.Goffi A, Ferguson ND. High-frequency oscillatory ventilation for early acute respiratory distress syndrome in adults. Curr Opin Crit Care. 2014;20:77–85. doi: 10.1097/MCC.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 14.Samransamruajkit R, Jiraratanawong K, Siritantiwat S, Chottanapan S, Deelodejanawong J, Sritippayawan S, et al. Potent inflammatory cytokine response following lung volume recruitment maneuvers with HFOV in pediatric acute respiratory distress syndrome. Asian Pac J Allergy Immunol. 2012;30:197–203. [PubMed] [Google Scholar]
- 15.Khemani RG, Smith LS, Zimmerman JJ, Erickson S. Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S23–40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson ND, Chiche JD, Kacmarek RM, Hallett DC, Mehta S, Findlay GP, et al. Combining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: The treatment with oscillation and an open lung strategy (TOOLS) trial pilot study. Crit Care Med. 2005;33:479–86. doi: 10.1097/01.ccm.0000155785.23200.9e. [DOI] [PubMed] [Google Scholar]
- 17.Tingay DG, Mills JF, Morley CJ, Pellicano A, Dargaville PA. The deflation limb of the pressure-volume relationship in infants during high-frequency ventilation. Am J Respir Crit Care Med. 2006;173:414–20. doi: 10.1164/rccm.200502-299OC. [DOI] [PubMed] [Google Scholar]
- 18.De Jaegere A, van Veenendaal MB, Michiels A, van Kaam AH. Lung recruitment using oxygenation during open lung high-frequency ventilation in preterm infants. Am J Respir Crit Care Med. 2006;174:639–45. doi: 10.1164/rccm.200603-351OC. [DOI] [PubMed] [Google Scholar]
- 19.Halbertsma FJ, van der Hoeven JG. Lung recruitment during mechanical positive pressure ventilation in the PICU: What can be learned from the literature? Anaesthesia. 2005;60:779–90. doi: 10.1111/j.1365-2044.2005.04187.x. [DOI] [PubMed] [Google Scholar]
- 20.Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, et al. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology. 2002;96:795–802. doi: 10.1097/00000542-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 21.De Luca D, Piastra M, Chidini G, Tissieres P, Calderini E, Essouri S, et al. The use of the Berlin definition for acute respiratory distress syndrome during infancy and early childhood: Multicenter evaluation and expert consensus. Intensive Care Med. 2013;39:2083–91. doi: 10.1007/s00134-013-3110-x. [DOI] [PubMed] [Google Scholar]
- 22.Zivanovic S, Peacock J, Alcazar-Paris M, Lo JW, Lunt A, Marlow N, et al. Late outcomes of a randomized trial of high-frequency oscillation in neonates. N Engl J Med. 2014;370:1121–30. doi: 10.1056/NEJMoa1309220. [DOI] [PMC free article] [PubMed] [Google Scholar]


