Abstract
BACKGROUND:
The advantages of laparoscopic adrenalectomy (LA) over open adrenalectomy are undeniable. Nevertheless, carbon dioxide (CO2) pneumoperitoneum may have an unfavourable effect on the local immune response. The aim of this study was to compare changes in the systemic inflammation and immune response in the early post-operative (p.o.) period after LA performed with standard and low-pressure CO2 pneumoperitoneum.
MATERIALS AND METHODS:
We studied, in a prospective randomised study, 51 patients consecutively with documented adrenal lesion who had undergone a LA: 26 using standard-pressure (12-14 mmHg) and 25 using low-pressure (6-8 mmHg) pneumoperitoneum. White blood cells (WBC), peripheral lymphocyte subpopulation, human leucocyte antigen-DR (HLA-DR), neutrophil elastase, interleukin (IL)-6 and IL-1, and C-reactive protein (CRP) were investigated.
RESULTS:
Significantly higher concentrations of neutrophil elastase, IL-6 and IL-1 and CRP were detected p.o. in the standard-pressure group of patients in comparison with the low-pressure group (P < 0.05). A statistically significant change in HLA-DR expression was recorded p.o. at 24 h, as a reduction of this antigen expressed on the monocyte surface in patients from the standard group; no changes were noted in low-pressure group patients (P < 0.05).
CONCLUSIONS:
This study demonstrated that reducing the pressure of the pneumoperitoneum to 6-8 mmHg during LA reduced p.o. inflammatory response and averted p.o. immunosuppression.
Keywords: Immune response, laparoscopic adrenalectomy (LA), standard-pressure/low-pressure pneumoperitoneum
INTRODUCTION
In 1991, Clayman first described the technique of laparoscopic nephrectomy. This report was the first demonstration that retroperitoneal organs could be removed via minimally invasive approach.[1] In 1992, Gagner reported three cases of laparoscopic adrenalectomy (LA) performed by the lateral approach with the patient in lateral decubitus position (lateral flank approach).[2] Later, several reports appeared on adrenalectomies performed via this minimally invasive technique by the flank (or lateral), anterior, or posterior approach.[3,4,5,6,7,8] Its advantages over open adrenalectomy, such as lower surgical stress, shorter hospitalisation period and quicker recovery, are undeniable. As the technique has improved, the number of implications for laparoscopy has grown and the number of contradictions decreased.[9,10,11,12,13,14,15]
Nevertheless, laparoscopy is not devoid of disadvantages, mostly because of carbon dioxide (CO2) pneumoperitoneum used during standard-technique laparoscopic procedures. The CO2 and higher abdominal pressure adversely affect the patient's homeostasis, causing significant changes in cardiovascular and respiratory systems, decreasing perfusion in abdominal organs and blood flow in the inferior vena cava, and causing increased risk of thrombotic disease.[16] It has been also suggested by some authors that CO2 pneumoperitoneum may have an unfavourable effect on the local immune response.[17,18,19] Peritoneal macrophages seem to produce fewer cytokines, and their intrinsic function (phagocytosis) diminishes in the presence of CO2.[20,21] Many studies have evaluated the effect of the degree of surgical trauma and different anaesthetic agents on immune response.[17,18,22,23,24,25,26] There have, however, been only a few studies conducted that compared standard-pressure and low-pressure CO2 pneumoperitoneum laparoscopy with respect to immune response.[27,28] Moreover, we could not find any effect of various degrees of intra-abdominal pressure during LA. The aim of this prospective randomised study was to compare changes in the systemic inflammation and immune response in the early post-operative (p.o.) period after LA performed with standard-pressure and low-pressure CO2 pneumoperitoneum.
MATERIALS AND METHODS
From January 2011 to May 2015, we studied, in a prospective randomised manner, 51 patients consecutively (35 women, 16 men; mean age 66 years) with benign adrenal lesions who underwent a LA after exhaustive endocrinological and imaging assessment. Twenty-seven patients with non-secreting adenoma, 21 with aldosterone-producing adenoma and 3 with pheochromocytoma were considered eligible for adrenalectomy. Lesion size ranged 4.5-11 cm.
Criteria for exclusion were: Cortisol-producing adenoma; immune system disorders; haematologic disorders; anticoagulant treatment; current or recent (6 months) thromboembolic disorders; renal, hepatic, rheumatic, or vascular disorders; pregnancy; recent (6 months) surgery; current or recent (3 years) malignancy; chronic inflammatory disease; and marked obesity (body mass index >36 kg/m2). We also excluded patients taking corticosteroid drugs or others drugs, which might affect their immunological responses.
Randomisation to intervention was stratified by the study centre, and the patients were randomised in blocks. Computer-generated codes were maintained in sequentially numbered opaque envelopes. The randomisation envelopes were opened in the operating department after induction of anaesthesia by the anaesthesiologist.
Patients were randomly assigned to two groups.
Twenty-six patients (17 women, 9 men; mean age 66.2 years) [Table 1] underwent standard LA (left adrenalectomy:17 patients; right adrenalectomy: 9 patients) (Group 1).
Table 1.
Characteristics of patients
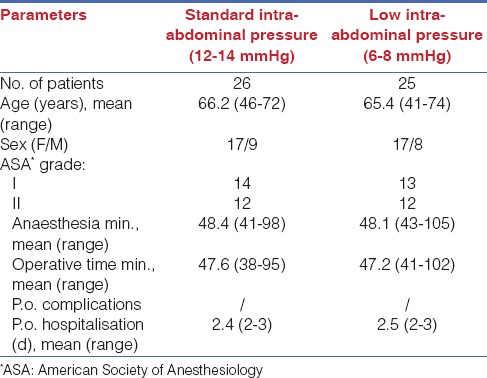
The remaining 25 patients (17 women, 8 men; mean age 65.4 years) [Table 1] were operated on using a low-pressure CO2 pneumoperitoneum laparoscopy technique (left adrenalectomy:15 patients; right adrenalectomy: 10 patients) (Group 2).
The LA was performed by the same team of surgeons in each case, using the same surgical technique (lateral flank approach) and three or four trocars. CO2 pneumoperitoneum was established with open laparoscopy. In the standard laparoscopy group, intra-abdominal pressure of 12-14 mmHg was maintained throughout the procedure, whereas in patients from the second group, pressure of 6-8 mmHg was applied to perform surgery. There were no differences in total dose (volume) of insufflated CO2 between the two groups.
The study protocol was approved by the Ethics Committee of the Faculty of Medicine of the University of L’Aquila, and informed consent was obtained from every patient. The patients were classified as Grade I or II, according to the American Society of Anesthesiologists (ASA) grading system.[29]
Anaesthesia was obtained in both groups using the same procedure. Preanaesthesia was accomplished using atropine (0.01 mg/kg) plus promethazine (0.5 mg/kg); for induction, sodium thiopental (5 mg/kg) and atracurium (0.5 mg/kg) were used; and tracheal intubation and assisted ventilation were done using nitrogen dioxide (NO2)/oxygen (O2) 2:1. After intubation, anaesthesia was maintained with O2 in air, sevoflurane, and remifentanil (0.25 µg/kg/min).
No patients were converted to an open surgery. As presented in Table 1, age, sex, ASA grades, time of anaesthesia and operation, and p.o. hospitalisation stay were comparable in the two groups.
Venous blood samples were taken from all patients before the surgery, and 6 h, 24 h and 48 h from the beginning of the operation. Serum concentrations of interleukin (IL)-1 and IL-6 were measured at 0 min, 30 min, 60 min, 90 min, 120 min and 180 min and at 4 h, 6 h, 12 h, 24 h and 48 h p.o.
All samples were tested for total white blood cell (WBC) count and WBC population (neutrophils, total lymphocytes), T-helper lymphocytes (CD4), T-suppressor lymphocytes (CD8), natural killer lymphocytes (CD16 and CD56), pan B-cell antigen (CD20), T cell receptor γ/δ and the T-helper/T-suppressor ratio (CD4/CD8).
Human leucocyte antigen-DR (HLA-DR) of peripheral monocytes was measured by the cytofluorimetric method. All blood samples (10 mL) were collected with ethylenediaminetetraacetic acid (EDTA) (0.5 mL). A monoclonal antibody for the HLA-DR antigen conjugated with fluorescein isothiocyanate (10 μL) was added. Whole blood (100 μL) from each patient was then used, and the tubes were stirred with vortex and stored at 4°C for 30 min. Lysing solution (2 mL) was added to each sample. All samples were stirred and then incubated for 15 min at room temperature. Finally, an orthocytofluorimeter was used for the assay.
Elastase concentration was determined photometrically, using an immune-activation immunoassay (Merck, Damstadt, Germany), as a complex with α1-proteinase inhibitor, according to the method described by Hafner et al.[30]
The plasma concentration of C-reactive protein (CRP) was measured using a competitive CRP enzyme-linked immunosorbent assay (ELISA) kit.
Serum concentrations of IL-1 and IL-6 were measured using a quantitative “sandwich” ELISA kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's description (range: IL-1 β 3.9-250 pg/mL-1; IL-6 3.13-300 pg/mL-1). Samples of serum (100 μL) were dispensed into wells of 96-well microtiter plates, which had been coated with the relevant monoclonal cytokine antibody. After incubation for 2 h at room temperature, unbound proteins were washed away from the wells, to which subsequently an enzyme-linked antibody was added, directed against the relevant cytokine for another 2 h at room temperature. After further rinsing to remove the unbound antibody, a substrate solution was added to each well and the mixture were incubated for 20 min at 37°C. The reaction was terminated with the addition of a stop solution. Adsorbance was determined by using an ELISA plate reading at 450 nm. Serial dilution of the relevant recombinant cytokine provided the standard curve. Assays were performed on duplicate samples. Samples were diluted appropriately with the diluent provided in the kit, if the levels of neat samples were beyond the linear measuring range.
Statistical analysis
A statistical analysis was performed using Student's t-test, and P values of <0.05 were considered to be significant. The values were expressed as the mean and standard deviation (SD). Areas under the curves [Figures 3–5] in the two groups were compared using the Mann-Whitney U test. The magnitude of changes in each metabolic variable (areas under the curve) were compared by the Pearson correlation coefficient (r). An α-adjustment according to the Holm-Bonferroni method was applied when appropriate.
Figure 3.
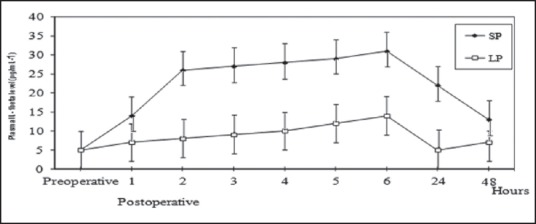
IL-1 β levels in standard-pressure (SP) versus low-pressure (LP) pneumoperitoneum laparoscopy. There were significant differences between the two groups: r > 0.78, P < 0.05 (Mann-Whitney U tests and Pearson correlation coefficient)
Figure 5.
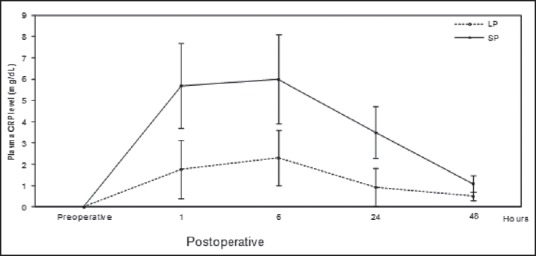
Plasma concentration (mean ± SD) of CRP in standard-pressure (SP) versus low-pressure (LP) pneumoperitoneum laparoscopy. There were significant differences between the two groups: r > 0.71, P < 0.05 (Mann-Whitney U tests and Pearson correlation coefficient)
RESULTS
P.o. hospitalisation, time of anaesthesia, and operation were comparable in the two groups [Table 1]. No complications were present during the surgeries or in the p.o. period. During the p.o. period, no differences in the biochemical or haematological parameters were found.
Median body temperature and WBC were similar in the two groups on the first 2 days of p.o. care. other wbc types (neutrophils-lymphocites-monocytes-eosinophils and basophilis) show no significant variation. With respect to lymphocyte subpopulation, there were no differences between the two groups of patients before and after operation.
A statistically significant change in HLA-DR expression was recorded p.o. at 6 h and 24 h, as a reduction of this antigen expressed on monocyte surfaces in patients from the standard group when compared with the low-pressure CO2 group [Figure 1] (P < 0.05). In the standard group, HLA-DR expression returned to normal values within 2 days after operation. Finally, the ages of the patients did not affect HLA-DR expression in either group.
Figure 1.
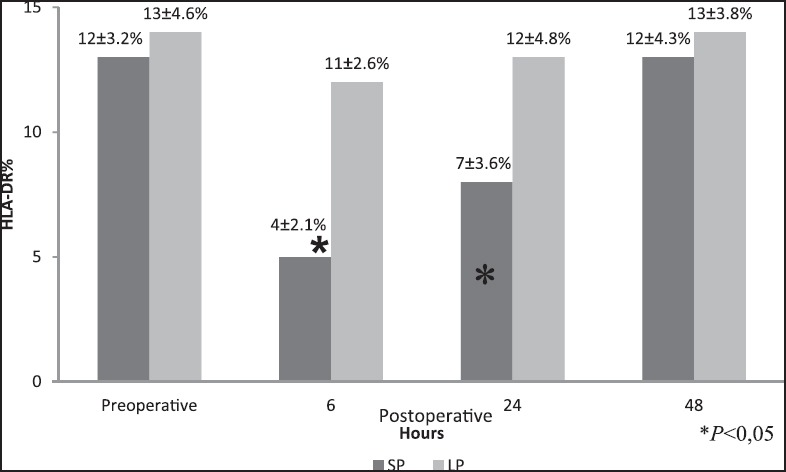
HLA-DR expression (mean ± SD) in the standard-pressure (SP) versus low-pressure (LP) pneumoperitoneum laparoscopy. *P < 0.05 (6 h and 24 h)
A statistically significant change in plasma elastase concentration was recorded p.o. at 6 h and 24 h, as there was an increase of this neutral proteinase in patients from standard group when compared with the low-pressure CO2 group [Figure 2] [P < 0.05). In the standard group, the plasma elastase concentration returned to normal values within 48 h after operation. Finally, the ages of patients did not affect neutrophil elastase concentration in either group.
Figure 2.
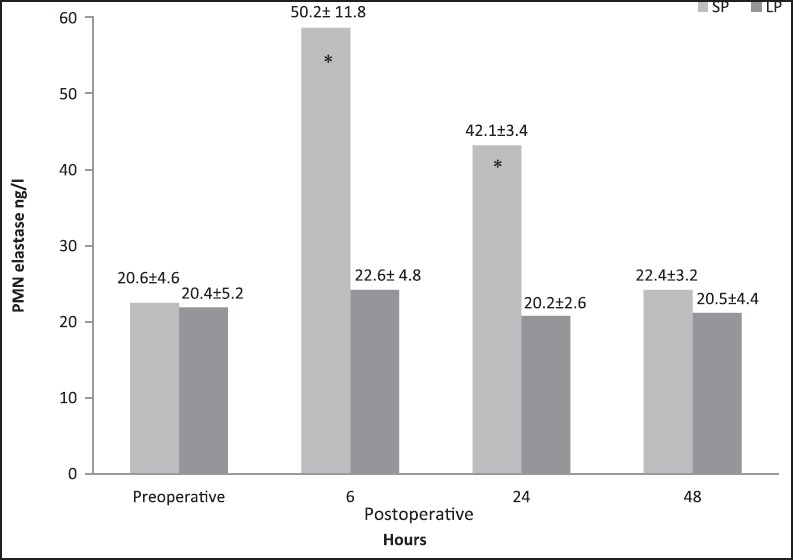
Plasma elastase concentration (mean ± SD) in the standard-pressure (SP) versus low-pressure (LP) pneumoperitoneum laparoscopy *P < 0.05 (6 h and 24 h), *P < 0.05
Before the operation, the serum levels of IL-1 and IL-6 showed no significant difference between the two groups. Figures 3 and 4 show the chronological changes in the serum level of IL-1 and IL-6 after surgery. In the standard group, the serum IL-1 and IL-6 levels began to significantly increase as early as 1 h from the beginning of the operation, revealing a peak at the sixth hour (approximately 4 h after the operation) and, thereafter, declining to p.o. levels by 48 h. In contrast, in the low-pressure CO2 group patients, the increase in serum IL-1 and IL-6 was delayed, and the peak values were significantly lower than these in the standard group (r > 0.78, P < 0.05).
Figure 4.
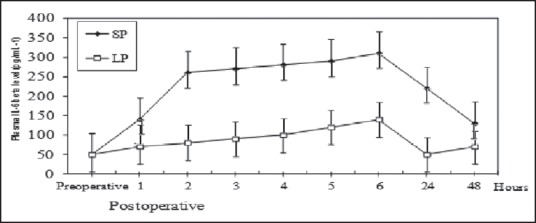
IL-6 levels in standard-pressure (SP) versus low-pressure (LP) pneumoperitoneum laparoscopy. There were significant differences between the two groups: r > 0.78, P < 0.05 (Mann-Whitney U tests and Pearson correlation coefficient)
The mean values of the serum CRP on p.o. days were also lower in the low-pressure CO2 group when compared with those in the standard group (r > 0.71, P < 0.05) [Figure 5]. In this case, CRP concentration returned to normal values within 48 h after operation.
Follow-up after 1 month, 6 months and 12 months, a routine procedure for all patients who underwent LA at our hospital, showed three simple infections (Grade I) in standard group wounds.
DISCUSSION
LA is nowadays considered the treatment of choice for patients with secreting and non-secreting adrenal lesions.[11,12,13] When compared with open adrenalectomy, LA presents several advantages, such as reduced p.o. pain, prompt p.o. bowel activity (6-24 h after operation), reduced hospitalisation (1-2 days), earlier return to work, better aesthetic results and reduced p.o. infections.[11,12,13]
Although laparoscopy is ‘minimally invasive’, systemic immune response is still invariably activated.[23] Overall, responses to surgery in general are reflected in terms of cytokine function and cellular messenger systems — although cytokine levels do not directly reflect immune status, they give us a framework to understand systemic immunity in terms of underlying immune activation. Because alterations are proportional to the extent of the injury, the physiological response to minimally invasive surgery may, intuitively, be different than those for traditional open surgery. The acute phase protein response appears to be one example.[31] The cytokines IL-1, tumor necrosis factors (TNF) and IL-6 are known to be major mediators of acute phase response.[32] IL-6 plays a central role in the acute phase of inflammation seen after surgery.[33,34] IL-6 induces the production of acute phase protein, such as CRP in hepatocytes,[31] and also causes fever.[35] It has been reported that surgical stress causes the serum IL-6 level to increase and that the extent of such an increase is closely correlated to the subsequent increase in serum CRP levels.[34] CRP rises approximately 4-12 h after surgery and peaks at 24-72 h. Subsequently, CRP remains elevated for approximately 2 weeks.[34]
The inflammatory and immune response has been studied in several clinical trials after laparoscopic procedures for benign[23,25,36,37,38,39,40] and neoplastic disease.[41,42,43] The advantages of laparoscopy, commonly reported in trials assessing laparoscopic surgery,[24] are believed to be secondary to less trauma to the abdominal wall with the laparoscopic approach, which results in a lower inflammatory response.[40,41,42,43]
CO2 is the insufflation gas of choice in laparoscopy. It is preferred over air insufflation, which affects the systemic and peritoneal response to a larger degree than CO2.[44] The usage of CO2 has some important advantages. It is transparent, non-inflammable, and well dissolvable in blood. There are, however, some disadvantages associated with its usage. The increased intra-abdominal pressure increases the absorption of CO2, causing hypercapnia and acidosis, which has to be avoided by hyperventilation.[45] It also pushes the diaphragm upwards, decreasing the pulmonary compliance,[45,46] and increases the peak airway pressure.[46,47] Pneumoperitoneum also increases systemic vascular resistance[47,48] and pulmonary vascular resistance.[47] CO2 pneumoperitoneum also predisposes the patient to cardiac arrhythmias.[49] During the early phase of pneumoperitoneum, there is a reduction in the cardiac output[46,47] by decreasing the venous return.[50] Although these cardiorespiratory changes may be tolerated by healthy adults with adequate cardiopulmonary reserve, people with cardiopulmonary disease may not be able to tolerate these cardiopulmonary changes. Abdominal wall lift, using a special device, e.g., Laparolift)[49] or Laparo-tensor,[46] introduced through a port in the abdominal wall has been applied to decrease the cardiopulmonary changes.[51] Helium insufflation is an alternative to CO2 insufflation,[52] and has been reported to have little or no effect on pulmonary function in pigs.[53] However, concerns about the solubility of helium in the blood and hence the risk of gas embolism have precluded its routine use in humans.[52]
Randomised clinical trials have shown that using a lower pressure of pneumoperitoneum decreases the cardiac changes,[54] the number of people complaining of shoulder-tip pain,[55] the intensity of pain,[56] and the analgesic requirement.[55,56,57,58,59,60,61,62]
Low intra-abdominal pressure may prevent mortality due to CO2 embolism.[63] Moreover, Schwarte et al.[64] found that increasing intra-abdominal pressure decreased gastric mucosal O2 saturation. The European Association for Endoscopic Surgery (EAES) guidelines[50] also recommend use of the lowest intra-abdominal pressure rather than a routine pressure (14 mmHg) to allow adequate exposure of the operative field. In a study conducted by Torres et al.,[27] (laparoscopic cholecystectomy performed with standard and low-pressure pneumoperitoneum), no differences were observed between the groups with regard to IL-6, IL-8 and IL-10 levels. Different results were presented by Basgul et al.,[28] who revealed IL-6 p.o. levels to increase less in patients operated on with the implementation of low-pressure pneumoperitoneum.
A Cochrane review of Gurusamy et al.[65] has demonstrated that low-pressure pnemoperitoneum is effective in decreasing pain after laparoscopic cholecystectomy, whereas is the incompleteness of the data that is associated with the risk of bias that makes it impossible to come to any conclusion about the safety of low-pressure pneumoperithoneum.
The aim of the study by Sood et al.[66] was to see if a decreased intraoperative intra-abdominal pressure during LA would affect the serum haemodynamic variables and the serum levels of catecholamines. They conclude that a low intra-abdominal pressure of 8-10 mmHg causes less catecholamine release and fewer haemodynamic fluctuations.
Therefore, although there are several studies of intra-abdominal pressure and immune functions,[16,27,28,46,56,57,67] we could not find any effect of various degrees of intra-abdominal pressure during LA. We aimed to show this effect by measuring the level of serum ILs and preferred to detect IL-1 is one of the early systemic immune events after surgery,[17] and IL-6, which is one of the mediators of acute phase response.
In our study IL-1, IL-6 and CRP showed a significant increase perioperatively in the standard intra-abdominal pressure group, whereas the increase in IL-1, IL-6 and CRP was lower in the low intra-abdominal pressure group.
Neutrophil elastase [polymorphonuclear (PMN)-elastase] is a neutral proteinase (30 kD) consisting of 218 amino acids, present mainly in the azurophilic granules of segmented granulocytes. Its function is to contribute to tissue repair after trauma, inflammation or necrosis, and it can also cause, by non-specific proteolysis, tissue injuries and breakdown of regulatory proteins, thus sustaining the inflammatory process.[68,69] Ninety percent of the circulating elastase is bound to an α1-proteinase inhibitor complex. The remaining 10% is bound to α2-macroglobulin, another elastase inhibitor. During surgical procedures there is a massive release of elastase from the neutrophils,[70] along with other proteinases. Therefore, the measurement of the elastase-α1-proteinase inhibitor complex might be a useful indicator of the degree of surgical trauma. In our study, in the standard group, we observed a p.o. increase of plasma elastase concentration. In the low-pressure CO2 group, the patients showed no significant difference in the activity of leucocytes of elastase considering the preoperative and p.o. values.
Moreover, in the standard group, we observed a p.o. decrease of the HLA-DR of peripheral monocytes. Patients who underwent LA using a low-pressure CO2 showed no significant difference in the activity of HLA-DR expression considering the preoperative and p.o. values. Previous studies have demonstrated the crucial role of this antigen in assessing the activity of the immune system.[71] The HLA-DR antigen expression on monocytes has an important role in antigen presentation to lymphocytes, particularly T-helper lymphocytes.[71] In fact, these cells require both HLA-DR and exogenic antigens on the macrophage surface to initiate proliferation. Moreover, studies have shown that HLA-DR is related to surgical trauma and that the occurrence of p.o. sepsis is strongly correlated with lower expression of the HLA-DR of peripheral monocytes.[23] Because HLA-DR expression is not significantly affected by age, sex, or race, this antigen can be considered of crucial significance in the p.o. monitoring of surgical patients.[71]
Does the difference in immune functioning between standard-pressure and low-pressure pneumoperitoneum influence clinical outcome? Publications on the subject of immune alterations after standard and low pneumoperitoneum are few and little is known about this difference in p.o. clinical outcomes.[65] Sparse information is available on immune function and clinical outcome. Because there still are too few data, no direct correlation could be found between clinical outcome and immunologic changes after standard pneumoperitoneum as compared with low pneumoperitoneum.
The results obtained in our study did not reveal significant differences between the studied operative procedures with regard to clinical data. Nevertheless, these observations might be a result of the type of the surgical procedure that was chosen for this study. LA is a short and relatively less traumatic procedure. However, the influence of CO2 pneumoperitoneum might have been strong enough to induce significant cytokine and HLA-DR concentration changes. The lower concentrations of IL-1, IL-6, CPR and neutrophil elastase, and the increased levels of HLA-DR observed in the population operated on with low-pressure pneumoperitoneum may suggest that this technique is more favourable with regard to systemic inflammation and immune response. During LA, the parameter returns to near-baseline levels by 48 h as this intervention causes a moderate surgical trauma. Even though there is a need for additional studies to prove beneficial effects of low-pressure pneumoperitoneum on inflammatory and immunologic factor concentrations, our observations they are of certain importance in choosing operative technique in case of cancer patients. On the basis of the results obtained by other authors, we think that there is a need for studies comparing changes in the concentration of cytokine factors during and after laparoscopic oncology operations performed with standard-pressure and low-pressure pneumoperitoneum, as cancer patients may show different cytokine responses to the two techniques with different pressures. In contrast, laparoscopic-assisted colorectal resection for cancer requires an incision substantially larger than that typically required for other advanced laparoscopic procedures, such as LA. Further, with respect to colectomy, there is a wide variation in the size of the incision needed to extract the specimen and facilitate the anastomosis, dependent on body habitus, the size of the specimen, and the surgeon.[19] Other variables that may impact the results of cytokine studies include blood transfusions and elevated (≥14 mmHg) and persistent (≥3 h) intra-abdominal pressure as a result of intraperitoneal insufflations. In these cases, the parameters tested return to near-baseline levels on the order of 3 or 6 days.[19,42] Laparoscopic-assisted colorectal resection for cancer, performed with low-pressure pneumoperitoneum, may induce the release of minor acute phase response mediators with the return to near-baseline levels by 48-72 h. The clinical importance of better-preserved immune function p.o. has yet to be proven. Certainly, when one compares operative morbidity and cancer recurrence rates in healthy immunocompetent patients to those in patients who are immunosuppressed to begin with, the latter group shows significantly worse results than the former.[72,73]
Furthermore, as surgeons continue to perform increasingly longer, more complex operations with extensive operative dissection and internal tissue manipulation, the degree of surgical insult attributable to the size of the incision(s) becomes relatively less important. As the magnitude of the operation begins to outweigh the magnitude of the incision, the predominant difference between laparoscopic and open surgery become the unique physiology of CO2 pneumoperitoneum.
The main criticism of low-pressure pneumoperitoneum is about its ability to provide adequate surgical exposure and hence, its safety. The nearly equal operating times and the lack of difference in the morbidity or conversion to open LA suggest that we had adequate view. Future trials must include surgeons’ satisfaction as one of the outcomes. Sandhu et al.[62] affirm that if there is evidence that the operative field is not feasible in the low-pressure group, there should be no hesitation in increasing the pressure to 14 mmHg, as in their series. In this study, conversion to high-pressure pneumoperitoneum was required in 2 of 70 cases; both of these patients were rather obese. For this reason, we have excluded patients with marked obesity (body mass index >36 kg/m2).
Even though there is a need for more studies to prove beneficial effects of low-pressure pneumoperitoneum on the concentration of inflammatory and immunologic factors, our observation could be of some importance in choosing operative technique, perhaps not so much for the surgical treatment of benign diseases but certainly when treating cancer patients. In these cases, p.o. immunosuppression is indicated among the factors responsible not only for p.o. infections but also for tumour spread and metastasis.[74] In contrast, surgery is almost always the ultimate therapeutic procedure in oncology. Therefore, it is important to avoid in these patients, often already presenting immunologic depression, all the conditions that could further reduce the p.o. immune response.
CONCLUSION
In conclusion, this study demonstrated that reducing the pressure of the pneumoperitoneum to 6-8 mmHg is feasible during LA. Reduced insufflation pressures can also lead to reduction in p.o. inflammatory response and, possibly, avert p.o. immunosuppression. The low-pressure technique could be employed in the majority of patients subjected to LA with reasonable safety by an experienced surgeon. We need detailed studies concerning the effects of various degrees of intra-abdominal pressure on systemic inflammation and immune response in laparoscopic surgeries, especially during and after laparoscopic oncology operations.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Clayman RV, Kavoussi LR, Soper NJ, Dierks SM, Meretyk S, Darcy MD, et al. Laparoscopic nephrectomy. 1991. J Urol. 2002;167:862–3. [PubMed] [Google Scholar]
- 2.Gagner M, Lacroix A, Prinz RA, Bolté E, Albala D, Potvin C, et al. Early experience with laparoscopic approach for adrenalectomy. Surgery. 1993;114:1120–5. [PubMed] [Google Scholar]
- 3.Bonjer HJ, van der Harst E, Steyerberg EW, de Herder WW, Kazemier G, Mohammedamin RS, et al. Retroperitoneal adrenalectomy: Open or endoscopic? World J Surg. 1998;22:1246–9. doi: 10.1007/s002689900553. [DOI] [PubMed] [Google Scholar]
- 4.De Cannière L, Michel L, Hamoir E, Hubens G, Meurisse M, Squifflet JP, et al. Multicentric experience of the Belgian Group for Endoscopic Surgery (BGES) with endoscopic adrenalectomy. Surg Endosc. 1997;11:1065–7. doi: 10.1007/s004649900530. [DOI] [PubMed] [Google Scholar]
- 5.Demeure MJ, Jordan M, Zeihen M, Wilson SD. Endoscopic retroperitoneal right adrenalectomy with the patient in the lateral decubitus position. Surg Laparosc Endosc. 1997;7:307–9. [PubMed] [Google Scholar]
- 6.Fernández-Cruz L, Saenz A, Taura P, Benarroch G, Astudillo E, Sabater L. Retroperitoneal approach in laparoscopic adrenalectomy: Is it advantageous? Surg Endosc. 1999;13:86–90. doi: 10.1007/s004649900907. [DOI] [PubMed] [Google Scholar]
- 7.Filipponi S, Guerrieri M, Arnaldi G, Giovagnetti M, Masini AM, Lezoche E, et al. Laparoscopic adrenalectomy: A report on 50 operations. Eur J Endocrinol. 1998;138:548–53. doi: 10.1530/eje.0.1380548. [DOI] [PubMed] [Google Scholar]
- 8.Lezoche E, Guerrieri M, Paganini AM, Feliciotti F, Zenobi P, Antognini F, et al. Laparoscopic adrenalectomy by the anterior transperitoneal approach: Results of 108 operations in unselected cases. Surg Endosc. 2000;14:920–5. doi: 10.1007/s004640000204. [DOI] [PubMed] [Google Scholar]
- 9.Bjornsson B, Birgisson G, Oddsdottir M. Laparoscopic adrenalectomies: A nationwide single-surgeon experience. Surg Endosc. 2008;22:622–6. doi: 10.1007/s00464-007-9729-3. [DOI] [PubMed] [Google Scholar]
- 10.Guerrieri M, Patrizi A, Rimini M, Romiti C, Baldarelli M, Campagnacci R. Laparoscopic adrenalectomy approaches: A 15-year experience in the search for a tailored procedure. Minerva Chir. 2010;65:601–7. [PubMed] [Google Scholar]
- 11.Liao CH, Chen J, Chueh SC, Tu YP, Chen SC, Yuan RH. Effectiveness of transperitoneal and trans-retroperitoneal laparoscopic adrenalectomy versus open adrenalectomy. J Formos Med Assoc. 2001;100:186–91. [PubMed] [Google Scholar]
- 12.Nehs MA, Ruan DT. Minimally invasive adrenal surgery: An update. Curr Opin Endocrinol Diabetes Obes. 2011;18:193–7. doi: 10.1097/MED.0b013e32834693bf. [DOI] [PubMed] [Google Scholar]
- 13.Greco F, Hoda MR, Rassweiler J, Fahlenkamp D, Neisius DA, Kutta A, et al. Laparoscopic adrenalectomy in urological centres - the experience of the German Laparoscopic Working Group. BJU Int. 2011;108:1646–51. doi: 10.1111/j.1464-410X.2010.10038.x. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen PH, Keller JE, Novitsky YW, Heniford BT, Kercher KW. Laparoscopic approach to adrenalectomy: Review of perioperative outcomes in a single center. Am Surg. 2011;77:592–6. [PubMed] [Google Scholar]
- 15.Ramacciato G, Nigri GR, Petrucciani N, Di Santo V, Piccoli M, Buniva P, et al. Minimally invasive adrenalectomy: A multicenter comparison of transperitoneal and retroperitoneal approaches. Am Surg. 2011;77:409–16. [PubMed] [Google Scholar]
- 16.Nguyen NT, Wolfe BM. The physiologic effects of pneumoperitoneum in the morbidly obese. Ann Surg. 2005;241:219–26. doi: 10.1097/01.sla.0000151791.93571.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: A review. Surg Endosc. 2004;18:1022–8. doi: 10.1007/s00464-003-9169-7. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Watson DI. Effect of laparoscopy on immune function. Br J Surg. 2001;88:1296–306. doi: 10.1046/j.0007-1323.2001.01860.x. [DOI] [PubMed] [Google Scholar]
- 19.Sylla P, Kirman I, Whelan RL. Immunological advantages of advanced laparoscopy. Surg Clin North Am. 2005;85:1–18. doi: 10.1016/j.suc.2004.09.005. vii. [DOI] [PubMed] [Google Scholar]
- 20.Hajri A, Mutter D, Wack S, Bastien C, Gury JF, Marescaux J, et al. Dual effect of laparoscopy on cell-mediated immunity. Eur Surg Res. 2000;32:261–6. doi: 10.1159/000008773. [DOI] [PubMed] [Google Scholar]
- 21.West MA, Baker J, Bellingham J. Kinetics of decreased LPS-stimulated cytokine release by macrophages exposed to CO2. J Surg Res. 1996;63:269–74. doi: 10.1006/jsre.1996.0259. [DOI] [PubMed] [Google Scholar]
- 22.Helmy SA, Wahby MA, El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia. 1999;54:733–8. doi: 10.1046/j.1365-2044.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- 23.Carlei F, Schietroma M, Cianca G, Risetti A, Mattucci S, Ngome Enang G, et al. Effects of laparoscopic and conventional (open) cholecystectomy on human leukocyte antigen-DR expression in peripheral blood monocytes: Correlations with immunologic status. World J Surg. 1999;23:18–22. doi: 10.1007/s002689900559. [DOI] [PubMed] [Google Scholar]
- 24.Schietroma M, Carlei F, Cappelli S, Amicucci G. Intestinal permeability and systemic endotoxaemia after laparotomic or laparoscopic cholecystectomy. Ann Surg. 2006;243:359–63. doi: 10.1097/01.sla.0000201455.89037.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schietroma M, Carlei F, Rossi M, Mattucci S, Gullà N, Lezoche E. Neutrophil-elastase in patients undergoing open versus laparoscopic cholecystectomy. Surgery. 2001;130:898. doi: 10.1067/msy.2001.117374. [DOI] [PubMed] [Google Scholar]
- 26.Visser BC, Parks RW, Garden OJ. Open cholecystectomy in the laparoendoscopic era. Am J Surg. 2008;195:108–14. doi: 10.1016/j.amjsurg.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Torres K, Torres A, Stas´kiewicz GJ, Chros´cicki A, Los´ T, Maciejewski R. A comparative study of angiogenic and cytokine responses after laparoscopic cholecystectomy performed with standard- and low-pressure pneumoperitoneum. Surg Endosc. 2009;23:2117–23. doi: 10.1007/s00464-008-0234-0. [DOI] [PubMed] [Google Scholar]
- 28.Basgul E, Bahadir B, Celiker V, Karagoz AH, Hamaloglu E, Aypar U. Effects of low and high intra-abdominal pressure on immune response in laparoscopic cholecystectomy. Saudi Med J. 2004;25:1888–91. [PubMed] [Google Scholar]
- 29.American Society of Anesthesiologists. New classification of physiology status. Anesthesiologists. 1963;24:111. [Google Scholar]
- 30.Hafner G, Dreher M, Lütgehaus M, Ehrenthal W, Heubner A, Swars H, et al. Determination of human granulocyte elastase by the immunoactivation method on the Hitachi 717 automated analyser. Eur J Clin Chem Clin Biochem. 1991;29:179–83. doi: 10.1515/cclm.1991.29.3.179. [DOI] [PubMed] [Google Scholar]
- 31.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte- stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987;84:7251–5. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perlmutter DH, Dinarello CA, Punsal PI, Colten HR. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986;78:1349–54. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond) 1990;79:161–5. doi: 10.1042/cs0790161. [DOI] [PubMed] [Google Scholar]
- 34.Ohzato H, Yoshizaki K, Nishimoto N, Ogata A, Tagoh H, Monden M, et al. Interleukin-6 as a new indicator of inflammatory status: Detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery. 1992;111:201–9. [PubMed] [Google Scholar]
- 35.Helle M, Brakenhoff JP, De Groot ER, Aarden LA. Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988;18:957–9. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- 36.Sietses C, Wiezer MJ, Eijsbouts QA, Beelen RH, van Leeuwen PA, von Blomberg BM, et al. A prospective randomized study of the systemic immune response after laparoscopic and conventional Nissen fundoplication. Surgery. 1999;126:5–9. doi: 10.1067/msy.1999.98702. [DOI] [PubMed] [Google Scholar]
- 37.Collet D, Vitale GC, Reynolds M, Klar E, Cheadle WG. Peritoneal host defenses are less impaired by laparoscopy than by open operation. Surg Endosc. 1995;9:1059–64. doi: 10.1007/BF00188987. [DOI] [PubMed] [Google Scholar]
- 38.Perttilä J, Salo M, Ovaska J, Grönroos J, Lavonius M, Katila A, et al. Immune response after laparoscopic and conventional Nissen fundoplication. Eur J Surg. 1999;165:21–8. doi: 10.1080/110241599750007469. [DOI] [PubMed] [Google Scholar]
- 39.Maruszynski M, Pojda Z. Interleukin-6 (IL-6) levels in the monitoring of surgical trauma. A comparison of serum IL-6concentrations in patients treated by cholecystectomy via laparotomy or laparoscopy. Surg Endosc. 1995;9:882–5. doi: 10.1007/BF00768883. [DOI] [PubMed] [Google Scholar]
- 40.Peters MJ, Mukhtar A, Yunus RM, Khan S, Pappalardo J, Memon B, et al. Meta-analysis of randomized clinical trials comparing open and laparoscopic anti-reflux surgery. Am J Gastroenterol. 2009;104:1548–62. doi: 10.1038/ajg.2009.176. quiz 1547. [DOI] [PubMed] [Google Scholar]
- 41.Goldfarb M, Brower S, Schwaitzberg SD. Minimally invasive surgery and cancer: Controversies part 1. Surg Endosc. 2010;24:304–34. doi: 10.1007/s00464-009-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng CS, Whelan RL, Lacy AM, Yim AP. Is minimal access surgery for cancer associated with immunologic benefits? World J Surg. 2005;29:975–81. doi: 10.1007/s00268-005-0029-6. [DOI] [PubMed] [Google Scholar]
- 43.Han SA, Lee WJ, Park CM, Yun SH, Chun HK. Comparison of immunologic outcomes of laparoscopic vs open approaches in clinical stage III colorectal cancer. Int J Colorectal Dis. 2010;25:631–8. doi: 10.1007/s00384-010-0882-0. [DOI] [PubMed] [Google Scholar]
- 44.Watson RW, Redmond HP, McCarthy J, Burke PE, Bouchier-Hayes D. Exposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in a murine model. Br J Surg. 1995;82:1060–5. doi: 10.1002/bjs.1800820820. [DOI] [PubMed] [Google Scholar]
- 45.Henny CP, Hofland J. Laparoscopic surgery: Pitfalls due to anesthesia, positioning, and pneumoperitoneum. Surg Endosc. 2005;19:1163–71. doi: 10.1007/s00464-004-2250-z. [DOI] [PubMed] [Google Scholar]
- 46.Alijani A, Hanna GB, Cuschieri A. Abdominal wall lift versus positive-pressure capnoperitoneum for laparoscopic cholecystectomy: Randomized controlled trial. Ann Surg. 2004;239:388–94. doi: 10.1097/01.sla.0000114226.31773.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galizia G, Prizio G, Lieto E, Castellano P, Pelosio L, Imperatore V, et al. Hemodynamic and pulmonary changes during open, carbon dioxide pneumoperitoneum and abdominal wall-lifting cholecystectomy. A prospective, randomized study. Surg Endosc. 2001;15:477–83. doi: 10.1007/s004640000343. [DOI] [PubMed] [Google Scholar]
- 48.Mertens zur Borg IR, Lim A, Verbrugge SJ, IJzermans JN, Klein J. Effect of intraabdominal pressure elevation and positioning on hemodynamic responses during carbon dioxide pneumoperitoneum for laparoscopic donor nephrectomy: A prospective controlled clinical study. Surg Endosc. 2004;18:919–23. doi: 10.1007/s00464-003-8817-2. [DOI] [PubMed] [Google Scholar]
- 49.Egawa H, Morita M, Yamaguchi S, Nagao M, Iwasaki T, Hamaguchi S, et al. Comparison between intraperitoneal CO2 insufflation and abdominal wall lift on QT dispersion and rate-corrected QT dispersion during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2006;16:78–81. doi: 10.1097/00129689-200604000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, et al. The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endos. 2002;16:1121–43. doi: 10.1007/s00464-001-9166-7. [DOI] [PubMed] [Google Scholar]
- 51.Gurusamy KS, Samraj K, Davidson BR. Abdominal lift for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006574.pub2. CD006574. [DOI] [PubMed] [Google Scholar]
- 52.Neuhaus SJ, Watson DI, Ellis T, Lafullarde T, Jamieson GG, Russell WJ. Metabolic and immunologic consequences of laparoscopy with helium or carbon dioxide insufflation: A randomized clinical study. ANZ J Surg. 2001;71:447–52. doi: 10.1046/j.1440-1622.2001.02170.x. [DOI] [PubMed] [Google Scholar]
- 53.Junghans T, Böhm B, Gründel K, Schwenk W. Effects of pneumoperitoneum with carbon dioxide, argon, or helium on hemodynamic and respiratory function. Arch Surg. 1997;132:272–8. doi: 10.1001/archsurg.1997.01430270058012. [DOI] [PubMed] [Google Scholar]
- 54.Dexter SP, Vucevic M, Gibson J, McMahon MJ. Hemodynamic consequences of high- and low-pressure capnoperitoneum during laparoscopic cholecystectomy. Surg Endosc. 1999;13:376–81. doi: 10.1007/s004649900993. [DOI] [PubMed] [Google Scholar]
- 55.Sarli L, Costi R, Sansebastiano G, Trivelli M, Roncoroni L. Prospective randomized trial of low-pressure pneumoperitoneum for reduction of shoulder-tip pain following laparoscopy. Br J Surg. 2000;87:1161–5. doi: 10.1046/j.1365-2168.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 56.Wallace DH, Serpell MG, Baxter JN, O’Dwyer PJ. Randomized trial of different insufflation pressure for laparoscopic cholecystectomy. Br J Surg. 1997;84:455–8. [PubMed] [Google Scholar]
- 57.Barczyn´ski M, Herman RM. Low-pressure pneumoperitoneum combined with intraperitoneal saline washout for reduction of pain after laparoscopic cholecystectomy: A prospective randomized study. Surg Endosc. 2004;18:1368–73. doi: 10.1007/s00464-003-9299-y. [DOI] [PubMed] [Google Scholar]
- 58.Davides D, Birbas K, Vezakis A, McMahon MJ. Routine low-pressure pneumoperitoneum during laparoscopic cholecystectomy. Surg Endosc. 1999;13:887–9. doi: 10.1007/s004649901126. [DOI] [PubMed] [Google Scholar]
- 59.Barczyn´ski M, Herman RM. Influence of different pressures of pneumoperitoneum on the autonomic system function during laparoscopy. Folia Med Cracov. 2002;43:51–8. [PubMed] [Google Scholar]
- 60.Perrakis E, Vezakis A, Velimezis G, Savanis G, Deverakis S, Antoniades J, et al. Randomized comparison between different insufflation pressures for laparoscopic cholecistectomy. Surg Laparosc Endosc Percutan Tech. 2003;13:245–9. doi: 10.1097/00129689-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Barczynski M, Herman RM. A prospective randomized trial on comparison of low-pressure (LP) and standard-pressure (SP) pneumoperitoneum for laparoscopic cholecystectomy. Surg Endosc. 2003;17:533–8. doi: 10.1007/s00464-002-9121-2. [DOI] [PubMed] [Google Scholar]
- 62.Sandhu T, Yamada S, Ariyakachon V, Chakrabandhu T, Chongruksut W, Ko-iam W. Low-pressure pneumoperitoneum versus standard pneumoperitoneum in laparoscopic cholecystectomy, a prospective randomized trial. Surg Endosc. 2009;23:1044–7. doi: 10.1007/s00464-008-0119-2. [DOI] [PubMed] [Google Scholar]
- 63.Beebe DS, Zhu S, Kumar MV, Komanduri V, Reichert JA, Belani KG. The effect of insufflation pressure on CO(2) pneumoperitoneum and embolism in piglets. Anesth Analg. 2002;94:1182–7. doi: 10.1097/00000539-200205000-00024. table of contents. [DOI] [PubMed] [Google Scholar]
- 64.Schwarte LA, Scheeren TW, Lorenz C, De Bruyne F, Fournell A. Moderate increase intraabdominal pressure attenuates gastric mucosal oxygen saturation in patients undergoing laparoscopy. Anesthesiology. 2004;100:1081–7. doi: 10.1097/00000542-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 65.Gurusamy KS, Samray K, Davidson BR. Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD006930.pub2. CD006930. [DOI] [PubMed] [Google Scholar]
- 66.Sood J, Jayaraman L, Kumra VP, Chowbey PK. Laparoscopic approach to pheochromocytoma: Is a lower intraabdominal pressure helpful? Anesth Analg. 2006;102:637–41. doi: 10.1213/01.ane.0000184816.00346.65. [DOI] [PubMed] [Google Scholar]
- 67.Kim WW, Jeon HM, Park SC, Lee SK, Chun SW, Kim EK. Comparison of immune preservation between CO2 pneumoperitoneum and gasless abdominal lift laparoscopy. JSLS. 2002;6:11–5. [PMC free article] [PubMed] [Google Scholar]
- 68.Biffl WL, Moore EE, Zallen G, Johnson JL, Gabriel J, Offner PJ, et al. Neutrophils are primed for cytotoxicity and resist apoptosis in injured patients at risk for multiple organ failure. Surgery. 1999;126:198–202. [PubMed] [Google Scholar]
- 69.Partrick DA, Moore EE, Offner PJ, Barnett CC, Barkin M, Silliman CC. Nitric oxide attenuates platelet-activating factor priming for elastase release in human neutrophils via a cyclic guanosine monophosphate-dependent pathway. Surgery. 1997;122:196–203. doi: 10.1016/s0039-6060(97)90009-x. [DOI] [PubMed] [Google Scholar]
- 70.Borregaard N. The human neutrophil. Function and dysfunction. Eur J Haematol. 1988;41:401–13. doi: 10.1111/j.1600-0609.1988.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 71.Neefjes JJ, Ploegh HL. Intracellular transport of MHC class II molecules. Immunol Today. 1992;13:179–84. doi: 10.1016/0167-5699(92)90123-O. [DOI] [PubMed] [Google Scholar]
- 72.Eilber FR, Morton DL. Impaired immunologic reactivity and recurrence following cancer surgery. Cancer. 1970;25:362–7. doi: 10.1002/1097-0142(197002)25:2<362::aid-cncr2820250213>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 73.Pietsch JB, Meakins JL, MacLean LD. The delayed hypersensitivity response: Application in clinical surgery. Surgery. 1977;82:349–55. [PubMed] [Google Scholar]
- 74.Lundy J, Lovett EJ, 3rd, Wolinsky SM, Conran P. Immune impairment and metastatic tumor growth: The need for an immunorestorative drug as an adjunct to surgery. Cancer. 1979;43:945–51. doi: 10.1002/1097-0142(197903)43:3<945::aid-cncr2820430324>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]


