Keywords: nerve regeneration, ginsenoside Rg1, neurite outgrowth, Aβ25–35, hippocampal neurons, Akt, MAPK, apoptosis, growth associated protein-43, Hoechst 33258 staining, PD98059, API-2, neural regeneration
Abstract
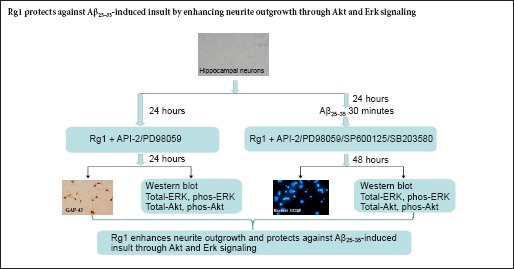
Ginsenoside Rg1 (Rg1) has anti-aging and anti-neurodegenerative effects. However, the mechanisms underlying these actions remain unclear. The aim of the present study was to determine whether Rg1 affects hippocampal survival and neurite outgrowth in vitro after exposure to amyloid-beta peptide fragment 25–35 (Aβ25–35), and to explore whether the extracellular signal-regulated kinase (ERK) and Akt signaling pathways are involved in these biological processes. We cultured hippocampal neurons from newborn rats for 24 hours, then added Rg1 to the medium for another 24 hours, with or without pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) family or Akt signaling pathways for a further 24 hours. We then immunostained the neurons for growth associated protein-43, and measured neurite length. In a separate experiment, we exposed cultured hippocampal neurons to Aβ25–35 for 30 minutes, before adding Rg1 for 48 hours, with or without Akt or MAPK inhibitors, and assessed neuronal survival using Hoechst 33258 staining, and phosphorylation of ERK1/2 and Akt by western blot analysis. Rg1 induced neurite outgrowth, and this effect was blocked by API-2 (Akt inhibitor) and PD98059 (MAPK/ERK kinase inhibitor), but not by SP600125 or SB203580 (inhibitors of c-Jun N-terminal kinase and p38 MAPK, respectively). Consistent with this effect, Rg1 upregulated the phosphorylation of Akt and ERK1/2; these effects were reversed by API-2 and PD98059, respectively. In addition, Rg1 significantly reversed Aβ25–35-induced apoptosis; this effect was blocked by API-2 and PD98059, but not by SP600125 or SB203580. Finally, Rg1 significantly reversed the Aβ25–35-induced decrease in Akt and ERK1/2 phosphorylation, but API-2 prevented this reversal. Our results indicate that Rg1 enhances neurite outgrowth and protects against Aβ25–35-induced damage, and that its mechanism may involve the activation of Akt and ERK1/2 signaling.
Introduction
The prevalence of neurodegenerative diseases such as Alzheimer's disease (AD) is increasing, owing to an aging world population. However, despite considerable research efforts, the pathogenic mechanisms of AD remain poorly understood, and the effectiveness of currently available clinical treatments is limited. Ginsenoside Rg1 (Rg1), the major pharmacologically active ingredient of ginseng, crosses the blood-brain barrier and has anti-aging and anti-neurodegenerative effects (Cheng et al., 2005). Amyloid beta (Aβ), a 39–43 amino acid β-sheet peptide, is a key constituent of amyloid plaques and contributes to cognitive, neuronal and synaptic malfunctioning in AD. Notably, Rg1 reduces the level of Aβ in the brains of aged and transgenic AD mice, as well as in PC12 cells in vitro (Chen et al., 2006; Wang and Du, 2009; Shi et al., 2010). These studies indicate that Rg1 modulates the generation of Aβ, which may contribute to its enhancement of cognitive performance in vivo. Aβ25–35 is a short Aβ fragment with large β-sheet fibrils, which possesses the same neurotoxicity as the full-length peptide (Iverson et al., 1995). It was recently reported that Rg1 prevents Aβ25–35-induced apoptosis in cultured hippocampal neurons by upregulating the ratio of the apoptotic regulators Bcl-2/Bax (Gong et al., 2011). However, its mechanism of action needs further exploration.
Neurite growth is an important process in neuronal development, synapse formation, and regeneration. There is great clinical interest in neurite growth, and the process can be used to assess neurotrophic properties of pharmaceuticals (Mitchell et al., 2007). It has been reported that Rg1 promotes neurite outgrowth in PC12 cells (Rudakewich et al., 2001). In contrast, Radad et al (2004a) showed that Rg1 did not promote neurite outgrowth in mesencephalic dopaminergic cells. Furthermore, the same group reported that Rg1 reversed the reduction in neurite length and number induced by glutamate or 1-methyl-4-phenylpyridinium (MPP+) (Radad et al., 2004a, b). Neurite outgrowth provides the morphological basis for synaptogenesis, the foundation of learning and memory. Whether Rg1 has the ability to affect neurite growth in neurons in the hippocampus, the learning and memory center of the brain, has not yet been investigated. Previous studies have shown that Rg1 increases the number of synapses and the density of synaptophysin, which provides the morphological basis for Rg1-induced facilitation of learning and memory (Mook-Jung et al., 2001).
Mitogen-activated protein kinases (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling are involved in many physiological and pathological processes. The MAPK family includes extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK (Roux et al., 2004), and is phosphorylated by MAPK/ERK kinase (MEK). The Akt signaling pathway is well-known for its role in cell survival and anti-apoptosis (Kennedy et al., 1999; Brunet et al., 2001). ERK/MAPK signaling has been implicated in hippocampal synaptic plasticity and hippocampus-dependent memory formation (Sweatt et al., 2004). Conversely, JNK and p38 MAPK are activated by a variety of stress signals and are implicated in the induction of apoptotic cell death (Mielke et al., 2000).
Here, we investigated the effect of Rg1 on neurite outgrowth and survival of hippocampal neurons after exposure to Aβ25–35 in vitro, and explored Akt and MAPK signaling in these biological processes.
Materials and Methods
Chemicals and reagents
Rg1 (purity 98%, lot No. 110703-200424) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Neurobasal Medium, B27 supplements and GlutaMAX were from Gibco BRL (Gaithersburg, MD, USA). Bovine serum albumin (BSA), HEPES, poly-L-lysine solution, DNase I, soybean trypsin inhibitor, and bovine trypsin were all from Sigma-Aldrich (St. Louis, MO, USA). Mouse anti-rat growth associated protein-43 (GAP-43) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Peroxidase-conjugated rabbit anti-mouse IgG was from Beyotime Institute of Biotechnology (Haimen, China). Rabbit anti-rat ERK1/2 and rabbit anti-rat phospho-ERK1/2 (Thr202/Tyr204), rat anti-human Akt (Ser473), rat anti-human phospho-Akt (Ser473), and horseradish peroxidase-conjugated goat anti-rabbit or anti-rat IgG were purchased from Cell Signaling Technology (Beverly, MA, USA). API-2, PD98059, SP600125 and SB203580 were purchased from Tocris Cookson, Inc. (Ballwin, MO, USA). BCA protein assay and enhanced chemiluminescence (ECL) kits were from Beyotime. Synthetic Aβ25–35 and insulin-like growth factor-1 (IGF-1) were obtained from AnaSpec (Fremont, CA, USA) and ProSpec (Rehovot, Israel), respectively.
Rg1 was dissolved in 0.9% NaCl to 2 mM and stored as a stock solution at −80°C. PD98059, API-2, SP600125 and SB203580 were dissolved in dimethyl sulfoxide to 25 mM and stored at −80°C. IGF-1 was dissolved in sterile ionized water to 100 μg/mL, or as a 3 mM stock solution, and stored at −20°C. Aβ25–35 was dissolved in distilled water to make a 2 mM stock solution; aliquots were stored at −20°C for more than 24 hours and thawed at 37°C for 3 days to induce aggregation prior to use.
Animals
Ninety newborn Sprague-Dawley rats, 45 females and 45 males, aged less than 24 hours and weighing approximately 2 g, were purchased from the Shanghai Institute of the Chinese Academy of Sciences (license No. SCXK (Hu) 2007-0003). All animal procedures were performed under a protocol reviewed and approved by the Institutional Ethical Committee of Tongji University School of Medicine, China.
Primary culture of hippocampal neurons
Primary cultured hippocampal neurons were prepared from the rat neonates as described previously (Banker et al., 1977). Briefly, the hippocampi were dissected out and trypsinized (0.25% bovine trypsin) for 9–10 minutes at 37°C. The cells were plated in Neurobasal™ Medium with 2% B27 supplement, 10 μL/mL penicillin-streptomycin, 1% GlutaMAX, 0.4% BSA and 20 mM HEPES at 37°C in a humidified 5% CO2 incubator. Neurons were characterized using phase contrast microscopy (Nikon Corporation, Tokyo, Japan) and immunocytochemistry for GAP-43. Neuronal cultures were over 90% pure.
Neurite outgrowth of hippocampal neurons in vitro
Primary hippocampal neurons were plated at a density of 1 × 104/cm2, onto coverslips pre-coated with 10 mg/mL poly-L-lysine, in 24-well plates for 24 hours for GAP-43 immunostaining. The medium was then refreshed and 50 μM Rg1 was added, with or without the following drugs: 10 μM API-2; 10 μM PD98059; 5, 10 or 15 μM SP600125; 5, 10 or 15 μM SB203580. After a 24 hour incubation, the cells were fixed in 4% paraformaldehyde for 30 minutes at room temperature, and incubated with blocking solution (5% BSA) for 30 minutes at 37°C in a moisture chamber to block nonspecific binding. The cells were then incubated overnight at 4°C with mouse anti-rat GAP-43 antibody (dilution 1:1,000), followed by peroxidase-conjugated rabbit anti-mouse IgG (dilution 1:100) at 37°C for 2 hours. Finally, the cells were incubated with avidin-biotin complex (dilution 1:100) at 37°C for 1.5 hours. Diaminobenzidine (Sigma, St. Louis, MO, USA) was used as a chromogen for light microscopy. A negative control was carried out using the same procedures without primary antibody.
An investigator blinded to the cell treatment analyzed neurite outgrowth in cell cultures by counting the number of neuronal cell bodies (n = 100 per well) and measuring neurite length. Digitized images of cells were taken at 20× magnification using a digital camera connected to a microscope (Nikon, NY, USA). The ratio between neurite length and number of cell bodies was used to calculate the average neurite length, using Image Tool software (Pro Plus V 6.0) (Media Cybernetics, Inc, Silver Spring, MD, USA). The experiment was replicated five times.
For western blot analysis, cells were seeded at a density of 2 × 105/cm2 in 60 mm diameter flasks pre-coated with 10 mg/mL poly-L-lysine. After 24 hours in culture, the cell pellets were collected for western blot analysis. This experiment was performed in triplicate.
Hippocampal neurons exposed to Aβ25–35 in vitro
After 24 hours in culture, the cells were divided into groups: (1) Normal control group: cells cultured for a further 48 hours with no treatment; (2) Aβ group: cells exposed to 20 μM Aβ25–35 for 48 hours; (3) Aβ + treatment group: cells exposed to 20 μM Aβ25–35 for 48 hours, with the following reagents and inhibitors added after 30 minutes: IGF-1 (50 ng/mL; positive control); Rg1 (50 μM); or Rg1 (50 μM) with either PD98059 (10 μM), API-2 (10 μM), SB203580 (5, 10 or 15 μM) or SP600125 (5, 10 or 15 μM). The inhibitors were added immediately after Rg1 treatment. All cells were cultured for 48 hours.
For western blot analysis, cells were plated at a density of 2 × 105/cm2 in 60 mm diameter flasks. After 24 hours, the cells were exposed to Aβ25–35, Rg1, and inhibitors as described above. After 48 hours, cell precipitations were collected for western blot analysis. The experiment was performed in triplicate.
Hoechst 33258 staining
After 48 hours in culture, the cells were fixed in 4% paraformaldehyde for 30 minutes at room temperature, and Hoechst 33258 was added to the medium for 15 minutes at 37°C. Images were obtained using an inverted fluorescence microscope (Nikon). Viable cells were identified by round nuclei with pale blue fluorescence, and apoptotic cells were characterized by condensation and fragmentation of nuclei. A researcher blinded to the cell treatment counted apoptotic and viable cells (n = 200 per well) and calculated the percentage of apoptotic cells (number of apoptotic neurons/[number of surviving + apoptotic neurons]). The experiment was replicated five times.
Western blot analysis
The cells were washed in ice-cold phosphate buffered saline and lysed in radioimmunoprecipitation assay buffer for 30 minutes at 4°C. Cell lysates were centrifuged at 18,514 × g (reactive centrifugal force) for 30 minutes at 4°C, and protein concentration was determined using a BCA protein assay kit. Total protein (40 μg) was dissolved in sample buffer and boiled for 5 minutes prior to loading onto polyacrylamide gels. The concentrations of the separation and stacking gels were 12% and 5%, respectively. Proteins were then transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween-20, and then incubated with primary antibodies against phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, phospho-Akt (Ser473) and Akt (all 1:1,000 dilution) overnight at 4°C. Finally, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-rat IgG (dilution 1:3,000) for 1 hour at room temperature and visualized using an ECL kit. Proteins were quantified by densitometric analysis of the bands.
Statistical analysis
Data are expressed as the mean ± SEM and were analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance followed by Newman-Keuls post hoc tests was carried out to assess the differences between the relevant control and each experimental condition. Statistical significance was set at P < 0.05.
Results
Rg1 promoted neurite outgrowth of cultured hippocampal neurons, and was inhibited by PD98059 and API-2 but not SP600125 or SB203580
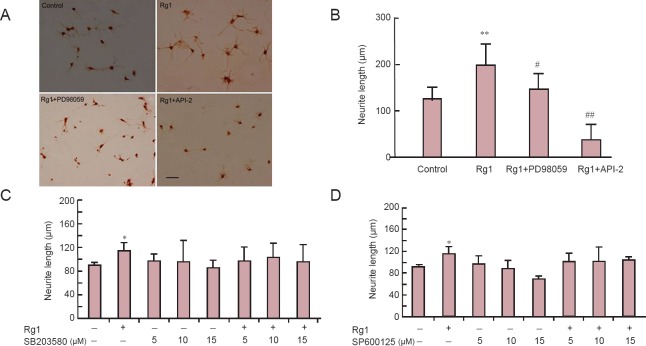
We assessed the neurotrophic effects of Rg1 on hippocampal neurons in vitro by measuring neurite outgrowth. Intact cell bodies and moderate neurite outgrowth were observed in control cells (Figure 1A). Cells incubated with Rg1 for 24 hours showed larger cell bodies with more numerous and longer neurites. Cells treated with Rg1 had significantly greater neurite outgrowth than control cells (Figure 1B).
Figure 1.
Ginsenoside Rg1 (Rg1) promoted neurite outgrowth of cultured hippocampal neurons via ERK1/2 and PI3K/Akt signaling.
(A) Hippocampal neurons were observed by immunostaining for growth associated protein-43 after treatment with 50 μM Rg1 in the presence or absence of 10 μM API-2 (Akt inhibitor) or 10 μM PD98059 (MEK inhibitor) for 24 hours. (B–D) Neurite length was measured in primary cultures after treatment with Rg1 in the presence or absence of PD98059 or API-2 (B), or various concentrations of SB203580 (p38MAPK inhibitor; C) or SP600125 (JNK inhibitor; D) for 24 hours. Data are the mean ± SEM of five individual experiments. *P < 0.05, **P < 0.01, vs. control group (untreated cells); #P < 0.05, ##P < 0.01, vs. Rg1 (one-way analysis of variance followed by Newman-Keuls post hoc test). Scale bar in A: 200 μm.
To further explore the intracellular signaling mechanisms underlying Rg1-promoted neurite outgrowth, MAPK and Akt signaling pathway inhibitors were applied. Incubation with Rg1 plus the MEK inhibitor PD98059 (10 μM) resulted in malnourished cells with small cell bodies and rough membrane surfaces compared with Rg1 alone (Figure 1A; Rg1 + PD98059). Rg1 with the Akt inhibitor API-2 (10 μM) resulted in fewer viable cells, with shorter and fewer neurites, than Rg1 alone (Figure 1A; Rg1+API-2). In contrast, addition of different concentrations of SP600125 (p38 MAPK inhibitor) or SB203580 (JNK inhibitor) to Rg1-treated cells did not affect the survival or morphology of the cells compared with Rg1 alone. Rg1-induced neurite outgrowth was significantly reversed by PD98059 and API-2, but was not affected by SP600125 or SB203580. These results indicate that the ERK1/2 and Akt signaling pathways, but not the JNK or p38 MAPK pathways, are involved in Rg1-mediated neurotrophic effects in cultured hippocampal neurons.
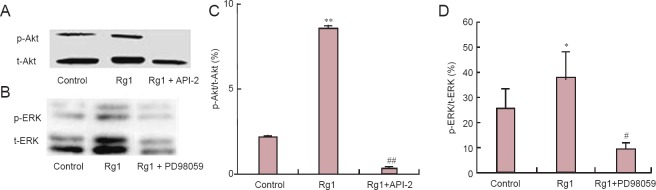
Rg1 activated ERK1/2 and Akt phosphorylation in cultured hippocampal neurons
Phosphorylation levels of ERK1/2 and Akt were determined by western blot analysis to further investigate the role of ERK1/2 and Akt signaling in Rg1-mediated neurotrophic effects. Cells treated with Rg1 for 24 hours showed significantly greater phosphorylation of Akt (Figure 2A, C) and ERK1/2 (Figure 2B, D) than untreated cells. These effects were blocked by PD98059 and API-2. Together with the effects of PD98059 and API-2 described above (Figure 1A–D), these results strongly indicate that ERK1/2 and Akt signaling is involved in Rg1-induced hippocampal neurite outgrowth.
Figure 2.
Rg1 increased phosphorylation of Akt and ERK in cultured hippocampal neurons.
Primary hippocampal neurons were cultured for 24 hours and then treated with 50 μM Rg1 in the presence or absence of 10 μM API-2 (Akt inhib-itor) or 10 μM PD98059 (MEK inhibitor) for 24 hours. (A, B) Western blot analysis of total (t-Akt) and phosphorylated Akt (p-Akt; A) and total (t-ERK) and phosphorylated ERK (p-ERK; B). (C, D) Phosphorylation levels of Akt (C) and ERK (D). Data are the mean ± SEM of three indi-vidual experiments. *P < 0.05, **P < 0.01, vs. control group; #P < 0.05, ##P < 0.01, vs. Rg1 alone (one-way analysis of variance followed by New-man-Keuls post hoc test)
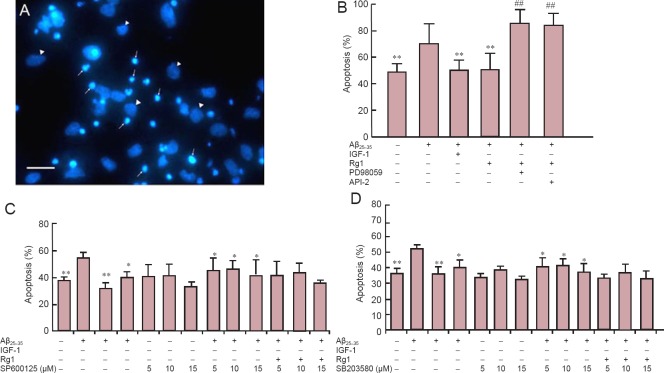
Rg1 protected against Aβ25–35-induced neurotoxicity, and was inhibited by PD98059 and API-2, but not SP600125 or SB203580
After 24 hours in culture, cells were exposed to Aβ25–35, followed by Rg1 in the presence or absence of Akt, ERK, JNK and p38 MAPK inhibitors for a further 48 hours. In untreated control cells, long and numerous neurites were observed under an inverted phase contrast microscope. Conversely, cells exposed to Aβ25–35 for 48 hours were fewer in number and showed fragmented and floating cell debris. Hoechst 33258 staining was used to classify viable and apoptotic cells. Viable cells were identified by regular, round nuclei with pale blue fluorescence, and apoptotic cells were condensed and fragmented (Figure 3A). Aβ25–35-exposed cells showed a significantly greater degree of apoptosis than untreated cells. The positive control, IGF-1, significantly reduced Aβ25–35-induced apoptosis (Figure 3B), as did post-treatment with Rg1, and this effect was blocked by co-incubation with API-2 and with PD98059 (Figure 3B). In contrast, SP600125 and SB203580 did not significantly alter the anti-apoptotic effect of Rg1 (Figure 3C, D). These results indicate that Rg1 treatment after Aβ25–35 exposure prevents apoptosis in hippocampal neurons, and the underlying mechanisms may involve the Akt and ERK1/2 pathways, but not JNK or p38 MAPK.
Figure 3.
Rg1 post-treatment protected against Aβ25–35-induced neurotoxicity via ERK1/2 and Akt signaling in hippocampal neurons.
Primary hippocampal neurons were cultured for 24 hours, exposed to Aβ25–35 for 30 minutes, and Rg1 with or without API-2 (10 μM, Akt inhibitor) or PD98059 (10 μM, MEK inhibitor), SP600125 (5, 10 or 15 μM, p38 MAPK inhibitor), or SB203580 (5, 10 or 15 μM, JNK inhibitor) was added for another 48 hours. Insulin-like growth factor-1 (IGF-1) was added to the culture as a positive control. Normal controls were not exposed to Aβ25–35. (A) Hoechst 33258 staining: apoptotic (arrows) and viable (arrowheads) cells. (B–D) Apoptosis of hippocampal neurons in Rg1 with API-2 or PD98059 (B), SP600125 (C) or SB203580 (D). Data are the mean ± SEM of five individual experiments. *P < 0.05, **P < 0.01, vs. Aβ25–35; ##P < 0.01, vs. Aβ25–35 + Rg1 (one-way analysis of variance followed by Newman-Keuls post hoc test). Scale bar in A: 200 μm.
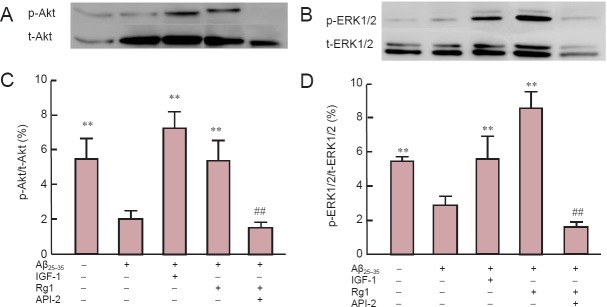
Rg1 treatment reversed Aβ25–35-induced reduction of ERK1/2 and Akt phosphorylation
To further investigate the possibility that the Akt and ERK1/2 signaling pathways are involved in the neuroprotective effects of Rg1 in hippocampal neurons after Aβ25–35 exposure, phosphorylation levels of Akt and ERK1/2 were measured by western blot analysis (Figure 4). Aβ25–35 exposure for 48 hours significantly inhibited phosphorylation of Akt and ERK1/2 compared with that observed in untreated control cells. IGF-1 significantly reversed Aβ25–35-induced reduction of Akt and ERK1/2 phosphorylation to levels observed in untreated cells, and cells treated with Rg1 also showed greater levels of Akt and ERK1/2 phosphorylation after Aβ25–35 exposure than cells exposed to Aβ25–35 alone. In contrast, API-2 blocked Rg1-induced Akt and ERK1/2 phosphorylation. These results strongly suggest that Aβ25–35 exposure inhibits phosphorylation of Akt and ERK1/2 in hippocampal neurons, resulting in neurotoxicity, and the protective effects of Rg1 against this neurotoxicity may be mediated by the ERK1/2 and Akt signaling pathways.
Figure 4.
Rg1 post-treatment reversed Aβ25–35-induced reduction of ERK1/2 and Akt phosphorylation in hippocampal neurons.
Primary hippocampal neurons were cultured for 24 hours, exposed to 20 μM Aβ25–35 for 30 minutes, and Rg1 with or without 10 μM API-2 was added for another 48 hours. In addition, insulin-like growth factor-1 (IGF-1) was added to the culture as a positive control. Normal control cells were not exposed to Aβ25–35. (A, B) Western blot analysis of total (t-Akt) and phosphorylated Akt (p-Akt) (A) and total (t-ERK) and phosphorated ERK (p-ERK). (C, D) Phosphorylation levels of Akt (C) and ERK (D). Data are the mean ± SEM of three individual experiments. **P < 0.01, vs. Aβ25–35; ##P < 0.01, vs. Rg1 + Aβ25–35 (oneway analysis of variance followed by Newman-Keuls post hoc test).
Discussion
In the present study, we have demonstrated that Rg1 promotes neurite outgrowth in cultured hippocampal neurons and protects against Aβ25–35-induced damage, and that these effects are mediated by the Akt and ERK1/2 signaling pathways.
Neurite growth is an important event in neuronal development, synapse formation, and neural regeneration. The promotion of neurite outgrowth by Rg1 suggests that this molecule possesses neurotrophic and anti-aging properties (Rausch et al., 2006). However, contrary to our results, Radad et al (2004a) reported that Rg1 did not promote neurite outgrowth in mesencephalic dopaminergic cells. This discrepancy may result from the different types of neurons studied. Since it has been demonstrated that Rg1 may facilitate learning and memory, ginseng shows potential as a preventative therapy for aging-related diseases.
When we exposed cells to API-2 with Rg1, a large amount of degeneration was observed, and the cells remaining were smaller and had shorter neurites than those incubated with Rg1 alone. However, compared with another active ingredient of ginseng, Rb1, which, when combined with API-2, resulted in a large number of fragmented cells, Rg1 may provide a greater level of resistance against Akt signaling inhibition. These results are in agreement with the widely accepted view that Akt signaling is related to neuronal survival (Kennedy et al., 1999; Brunet et al., 2001). In contrast, PD98059 blocked Rg1-induced neurite outgrowth, but did not significantly affect the number of cells in the medium compared to Rg1 alone. Interestingly, neither SP600125 nor SB203580 affected neurite outgrowth. These results suggest that signaling by ERK1/2, but not p38 MAPK or JNK, is involved in Rg1-induced neurite outgrowth. In addition, western blot analysis further demonstrated that Rg1 treatment upregulated phosphorylation of ERK1/2 and Akt compared to that in normal untreated cells, and these effects were reversed by PD98059 and API-2. Taken together, these results confirm that Akt and ERK1/2 signaling may be involved in Rg1-induced neurite outgrowth in cultured hippocampal neurons. However, how Rg1 affects Akt and ERK1/2 signaling is uncertain. Rg1 is a functional ligand of the glucocorticoid receptor, and exerts steroid hormone-like activity (Lee et al., 1997). Leung et al. (2007) reported that the neuroprotective effects of Rg1 on primary nigral neurons against rotenone toxicity could be abolished by RU486, an antagonist at both the glucocorticoid (GR) and progesterone receptors, and suggested that Rg1 exerted neuroprotective effects via a GR-dependent mechanism. Recently, Rg1 has been reported to promote non-amyloidogenic cleavage of APP via estrogen receptor signaling and to ameliorate Aβ25–35-induced cortical neuronal apoptosis at least in part by two complementary estrogen receptor α- and GR-dependent downstream pathways (Shi et al., 2012; Wu et al., 2012). Further investigations are necessary to determine whether Rg1 crosses the cellular membrane and binds to cytoplasmic molecules, or acts on membrane receptors that trigger a signaling cascade.
Previous reports have shown that Rg1 pretreatment improves the viability of cells injured by Aβ, reduces the levels of intracellular Aβ1-42, and attenuates the activation of caspase-3 and apoptosis in vitro (Ji et al., 2006; Wei et al., 2008; Choi et al., 2010). Those studies focused mainly on Rg1 pretreatment and used cortical neurons, suggesting its potential role for preventing AD. However, post-treatment is more clinically relevant, and the hippocampus is a key structure for learning and memory. It is also the site where senile plaques are formed in AD. Here, we have demonstrated that Aβ25–35 exposure for 48 hours induces apoptosis of hippocampal neurons, and that Rg1 post-treatment for 48 hours significantly reverses this. In vivo studies have also confirmed that administration of ginsenosides including Rg1 can reduce cerebral Aβ generation in transgenic AD mice (Chen et al., 2006) as well as aging mice (Shi et al., 2010). Taken together, these data highlight the potential of Rg1 as a new therapeutic drug for AD. Our results show that the neuroprotective effect of Rg1 against Aβ25–35 insult is blocked by API-2 and PD98058, but not by SP600125 or SB203580, suggesting that signaling involving ERK1/2 and Akt, but not JNK or p38 MAPK, may be involved in the action of Rg1. It was also previously demonstrated that no significant change in p38 or JNK phosphorylation was observed after Aβ25–35 exposure (Wu et al., 2012). These results imply that the Akt and ERK1/2 signaling pathways are involved in the neuroprotective effect of Rg1 against damage induced by Aβ25–35. Additionally, western blot analysis of ERK1/2 and Akt phosphorylation showed that Rg1 post-treatment reversed the Aβ25–35-induced inactivation of ERK1/2, supporting previous studies. Wu et al. (2012) found that U0126, another specific inhibitor of MEK, inhibited Rg1-induced Erk phosphorylation. In addition, we confirmed that Rg1 post-treatment also reversed inactivation of Akt induced by Aβ25–35, and that API-2 counteracted this effect. Together, these results provide evidence that the mechanisms underlying the neuroprotective effect of Rg1 against Aβ25–35 insult involve Akt and ERK signaling.
In conclusion, the results from the present study demonstrate that Rg1 induces neurite outgrowth in cultured hippocampal neurons through Akt and ERK signaling. Moreover, Rg1 post-treatment prevents Aβ25–35-induced apoptosis via Akt and ERK signaling. Rg1 activation of Akt and ERK signaling needs to be explored further. These observations suggest that ginsenoside has potential as a treatment for progressive neurodegenerative diseases such as AD.
Acknowledgments
We thank Ryan Splittgerbe (Ph.D., University of Nebraska Medical Center, USA) for corrections of the paper.
Footnotes
Funding: This study was financially supported by the National Program on Key Basic Research Project of China (973 Program), No. 2010CB945600, 2011CB965100; the National Natural Science Foundation of China, No. 81070987, 30971531, 81371213; and a grant from the International Science & Technology Collaboration Program, No. 2011DF30010.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Slone-Murphy J, Robens J, Li CH, Song LP, Zhao M
References
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Chen F, Eckman EA, Eckman CB. Reductions in levels of the Alzheimer's amyloid beta peptide after oral administration of ginsenosides. FASEB J. 2006;20:1269–1271. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Choi RC, Zhu JT, Leung KW, Chu GK, Xie HQ, Chen VP, Zheng KY, Lau DT, Dong TT, Chow PC, Han YF, Wang ZT, Tsim KW. A flavonol glycoside, isolated from roots of panax, notoginseng, reduces amyloid-beta-induced neurotoxicity in cultured neurons: signaling transduction and drug development for Alzheimer's disease. J Alzheimers Dis. 2010;19:795–811. doi: 10.3233/JAD-2010-1293. [DOI] [PubMed] [Google Scholar]
- Gong L, Li SL, Li H, Zhang L. Ginsenoside Rg1 protects primary cultured rat hippocampal neurons from cell apoptosis induced by β-amyloid protein. Pharm Biol. 2011;49:501–507. doi: 10.3109/13880209.2010.521514. [DOI] [PubMed] [Google Scholar]
- Iverson LL, Mortishire-Smith RJ, Pollack SJ, Shearman MS. The toxicity in vitro of beta-amyloid protein. Biochem J. 1995;311:1–16. doi: 10.1042/bj3110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji ZN, Dong TT, Ye WC, Choi RC, Lo CK, Tsim KW. Ginsenoside Re attenuate beta-amyloid and serum-free induced neurotoxicity in PC12 cells. J Ethnopharmacol. 2006;107:48–52. doi: 10.1016/j.jep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135–140. doi: 10.1016/s0303-7207(97)00160-3. [DOI] [PubMed] [Google Scholar]
- Leung KW, Yung KK, Mak NK, Chan YS, Fan TP, Wong RN. Neuroprotective effects of ginsenoside-Rg1 in primary nigral neurons against rotenone toxicity. Neuropharmacology. 2007;52:827–835. doi: 10.1016/j.neuropharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Mielke K, Herdegen T. JNK and p38 stresskinases-degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Hanson JC, Quets-Nguyen AT, Bergeron M, Smith RC. A quantitative method for analysis of in vitro neurite outgrowth. J Neurosci Methods. 2007;164:350–360. doi: 10.1016/j.jneumeth.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Mook-Jung I, Hong H, Boo JH, Lee KH, Yun SH, Cheong MY, Joo I, Huh K, Jung MW. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res. 2001;63:509–515. doi: 10.1002/jnr.1045. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004a;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Ishige K, Rausch WD. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J Neural Transm. 2004b;111:37–45. doi: 10.1007/s00702-003-0063-1. [DOI] [PubMed] [Google Scholar]
- Rausch WD, Liu S, Gille G, Radad K. Neuroprotective effects of ginsenosides. Acta Neurobiol Exp (Wars) 2006;66:369–375. doi: 10.55782/ane-2006-1625. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudakewich M, Ba F, Benishin CG. Neurotrophic and neuroprotective actions of ginsenosides Rb1 and Rg1. Planta Med. 2001;67:533–537. doi: 10.1055/s-2001-16488. [DOI] [PubMed] [Google Scholar]
- Shi C, Zheng DD, Fang L, Wu F, Kwong WH, Xu J. Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim Biophys Acta. 2012;1820:453–460. doi: 10.1016/j.bbagen.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Shi YQ, Huang TW, Chen LM, Pan XD, Zhang J, Zhu YG, Chen XC. Ginsenoside Rg1 attenuates amyloid-beta content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimers Dis. 2010;19:977–989. doi: 10.3233/JAD-2010-1296. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–e317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Wang YH, Du GH. Ginsenoside Rg1 inhibits beta-secretase activity in vitro and protects against Abeta-induced cytotoxicity in PC12 cells. J Asian Nat Prod Res. 2009;11:604–612. doi: 10.1080/10286020902843152. [DOI] [PubMed] [Google Scholar]
- Wei C, Jia J, Liang P, Guan Y. Ginsenoside Rg1 attenuates beta-amyloid-induced apoptosis in mutant PS1 M146L cells. Neurosci Lett. 2008;443:145–149. doi: 10.1016/j.neulet.2008.07.089. [DOI] [PubMed] [Google Scholar]
- Wu J, Pan Z, Wang Z, Zhu W, Shen Y, Cui R, Lin J, Yu H, Wang Q, Qian J, Yu Y, Zhu D, Lou Y. Ginsenoside Rg1 protection against β-amyloid peptide-induced neuronal apoptosis via estrogen receptor á and glucocorticoid receptor-dependent anti-protein nitration pathway. Neuropharmacology. 2012;63:349–361. doi: 10.1016/j.neuropharm.2012.04.005. [DOI] [PubMed] [Google Scholar]