Abstract
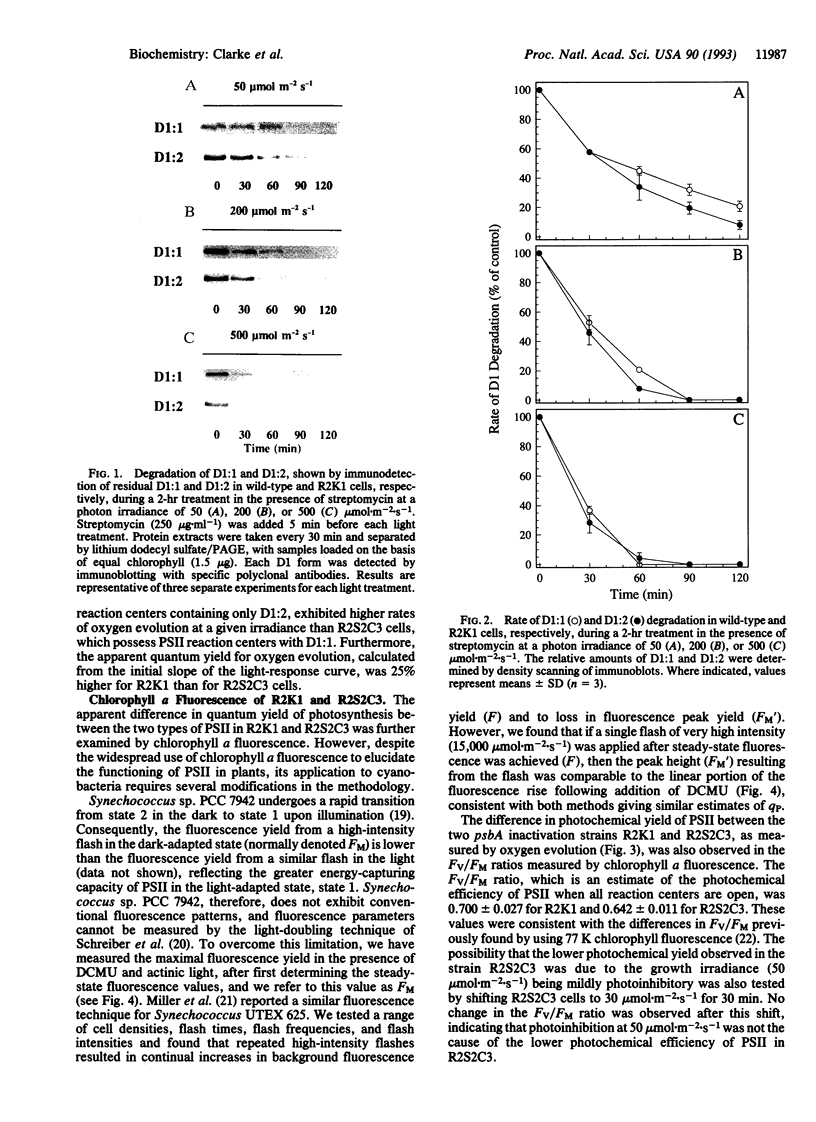
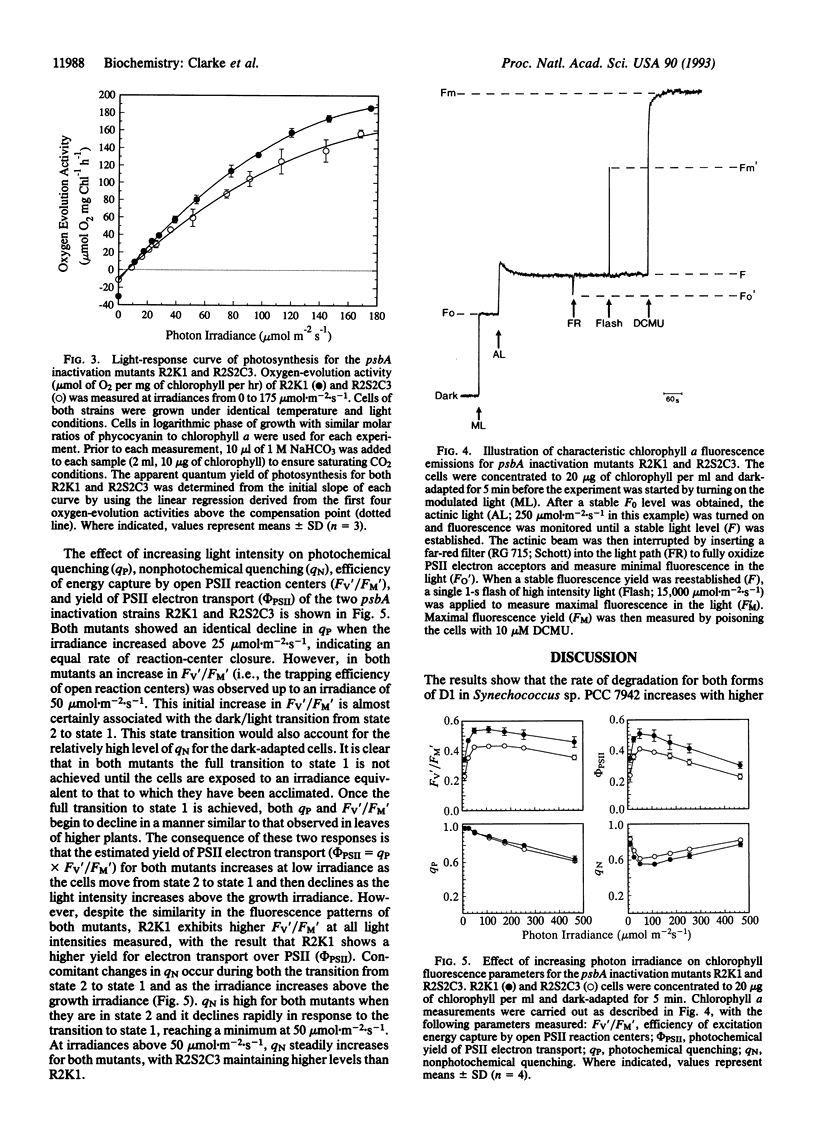
The cyanobacterium Synechococcus sp. PCC 7942 possesses a small psbA multigene family that codes for two distinct forms of the photosystem II reaction-center protein D1 (D1:1 and D1:2). We showed previously that the normally predominant D1 form (D1:1) was rapidly replaced with the alternative D1:2 when cells adapted to a photon irradiance of 50 mumol.m-2.s-1 are shifted to 500 mumol.m-2.s-1 and that this interchange was readily reversible once cells were allowed to recover under the original growth conditions. By using the psbA inactivation mutants R2S2C3 and R2K1 (which synthesize only D1:1 and D1:2, respectively), we showed that this interchange between D1 forms was essential for limiting the degree of photoinhibition as well as enabling a rapid recovery of photosynthesis. In this report, we have extended these findings by examining whether any intrinsic functional differences exist between the two D1 forms that may afford increased resistance to photoinhibition. Initial studies on the rate of D1 degradation at three photon irradiances (50, 200, and 500 mumol.m-2.s-1) showed that the rates of degradation for both D1 forms increase with increasing photon flux density but that there was no significant difference between D1:1 and D1:2. Analysis of light-response curves for oxygen evolution for the mutants R2S2C3 and R2K1 revealed that cells with photosystem II reaction centers containing D1:2 have a higher apparent quantum yield (approximately 25%) than cells possessing D1:1. Further studies using chlorophyll a fluorescence measurements confirmed that R2K1 has a higher photochemical yield than R2S2C3; that is, a more efficient conversion of excitation energy from photon absorption into photochemistry. We believe that the higher photochemical efficiency of reaction centers containing D1:2 is causally related to the preferential induction of D1:2 at high light and thus may be an integral component of the protection mechanism within Synechococcus sp. PCC 7942 against photoinhibition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. E., Janzen D. G. Non-selective afferent innervation develops in embryonic mouse spinal cord-dorsal root ganglia explants chronically exposed to GM1 ganglioside. Int J Dev Neurosci. 1989;7(1):87–92. doi: 10.1016/0736-5748(89)90047-6. [DOI] [PubMed] [Google Scholar]
- Barbato R., Frizzo A., Friso G., Rigoni F., Giacometti G. M. Photoinduced degradation of the D1 protein in isolated thylakoids and various photosystem II particles after donor-side inactivations. Detection of a C-terminal 16 kDa fragment. FEBS Lett. 1992 Jun 15;304(2-3):136–140. doi: 10.1016/0014-5793(92)80604-f. [DOI] [PubMed] [Google Scholar]
- Barber J., Andersson B. Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci. 1992 Feb;17(2):61–66. doi: 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- Bouyoub A., Vernotte C., Astier C. Functional analysis of the two homologous psbA gene copies in Synechocystis PCC 6714 and PCC 6803. Plant Mol Biol. 1993 Jan;21(2):249–258. doi: 10.1007/BF00019941. [DOI] [PubMed] [Google Scholar]
- Bustos S. A., Schaefer M. R., Golden S. S. Different and rapid responses of four cyanobacterial psbA transcripts to changes in light intensity. J Bacteriol. 1990 Apr;172(4):1998–2004. doi: 10.1128/jb.172.4.1998-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. K., Critchley C. Synthesis of early heat shock proteins in young leaves of barley and sorghum. Plant Physiol. 1990 Oct;94(2):567–576. doi: 10.1104/pp.94.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. K., Critchley C. The identification of a heat-shock protein complex in chloroplasts of barley leaves. Plant Physiol. 1992 Dec;100(4):2081–2089. doi: 10.1104/pp.100.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. K., Soitamo A., Gustafsson P., Oquist G. Rapid interchange between two distinct forms of cyanobacterial photosystem II reaction-center protein D1 in response to photoinhibition. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9973–9977. doi: 10.1073/pnas.90.21.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Rivas J., Andersson B., Barber J. Two sites of primary degradation of the D1-protein induced by acceptor or donor side photo-inhibition in photosystem II core complexes. FEBS Lett. 1992 Apr 27;301(3):246–252. doi: 10.1016/0014-5793(92)80250-k. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B. M., Gaba V., Mattoo A. K., Edelman M. Identification of a primary in vivo degradation product of the rapidly-turning-over 32 kd protein of photosystem II. EMBO J. 1987 Oct;6(10):2865–2869. doi: 10.1002/j.1460-2075.1987.tb02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson C., Debus R. J., Osiewacz H. D., Gurevitz M., McIntosh L. Construction of an Obligate Photoheterotrophic Mutant of the Cyanobacterium Synechocystis 6803 : Inactivation of the psbA Gene Family. Plant Physiol. 1987 Dec;85(4):1021–1025. doi: 10.1104/pp.85.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa Z., Oquist G., Gustafsson P. Photoinhibition and Recovery of Photosynthesis in psbA Gene-Inactivated Strains of Cyanobacterium Anacystis nidulans. Plant Physiol. 1990 May;93(1):1–6. doi: 10.1104/pp.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R. D., Schaefer M. R., Golden S. S. Transcriptional and posttranscriptional components of psbA response to high light intensity in Synechococcus sp. strain PCC 7942. J Bacteriol. 1992 Jun;174(11):3775–3781. doi: 10.1128/jb.174.11.3775-3781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol. 1989 Jul;171(7):3973–3981. doi: 10.1128/jb.171.7.3973-3981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Light availability influences the ratio of two forms of D1 in cyanobacterial thylakoids. J Biol Chem. 1989 May 5;264(13):7412–7417. [PubMed] [Google Scholar]
- Svensson B., Vass I., Cedergren E., Styring S. Structure of donor side components in photosystem II predicted by computer modelling. EMBO J. 1990 Jul;9(7):2051–2059. doi: 10.1002/j.1460-2075.1990.tb07372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




