Abstract
Purpose:
Patients with Parkinson’s disease (PD) show inefficiencies in cognitive performance including working memory functions. Since these problems impact on quality of life and overall well-being, the current study was aimed at improving patients’ situations by evaluating the computerized cognitive training tool, BrainStim.
Method:
A total of 19 healthy controls (HCs) and six patients with PD were included in the study. While all PD patients received cognitive training, the HC sample was subdivided into 12 subjects with training (HC-T) and 10 subjects without (HC-NT). Participants underwent a double baseline assessment, a post-training assessment, and a 3-month follow up on neuropsychological tests and self-report measures on fatigue and depression. Training was administered between the second baseline and postassessment. It comprised 16 supervised sessions according to a standardized training protocol over 4 weeks.
Results:
Significant improvements in verbal and visuospatial short-term and long-term memory were found in both training groups. In addition, the HC-T improved on mental speed, and verbal and visuospatial working memory. Both training groups showed stable results for all short-term visuospatial measures after 3 months. Further, the HC-T showed stable results for working memory, verbal, and visuospatial short-term and long-term memory.
Conclusions:
The efficacy of the applied computerized cognitive training tool BrainStim could be verified in patients with PD and healthy age-matched controls. The preliminary findings highlighted the suitability of a specific cognitive intervention to improve cognitive inefficiencies in patients with PD as well as in healthy older people. Further research on cognitive training in combination with PD drug therapy is needed to better understand the mutual interaction and to offer optimal therapeutic approaches to patients.
Keywords: BrainStim, cognitive rehabilitation, computerized training, Parkinson’s disease, working memory
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative illness with an age-dependent prevalence increase. It is often accompanied by psychiatric symptoms such as depression (25–50%), anxiety, phobias, and panic attacks (about 40%) [Aarsland et al. 2009]. Some 15,000 Swiss inhabitants and 6.3 million people worldwide currently suffer from PD [European Parkinson’s Disease Association, 2014]. The disease is based on a breakdown of certain nerve cells within the midbrain (substantia nigra pars compacta) that are responsible for the production of the central neurotransmitter dopamine [Dickson et al. 2009]. A lack of dopamine can cause dysfunction in physical and mental mechanisms including motor symptoms such as bradykinesia, rigidity, postural instability, and resting tremor but also cognitive loss, even in the early stages of the disease [Aarsland et al. 2009]. Patients with PD often show mild cognitive impairment in at least one cognitive domain such as episodic memory (69%), executive functions (54%), visuospatial/construction (46%), and working memory/attention (35%) [Pfeiffer et al. 2014], which is most obviously associated with a faster thinning of the gray matter [Hanganu et al. 2014]. There also seems to be a positive correlation between bradykinesia, working memory, and mental flexibility on the one hand, and postural instability, gait disturbances, and changes in visuospatial memory on the other [Moustafa et al. 2013]. Cognitive inefficiencies often remain undiagnosed and untreated [McDowell and Chesselet, 2012], though they are an indicator of poorer quality of life independent of other disease factors [Lawson et al. 2014]. Working memory in particular, including information processing speed, episodic memory, and mental flexibility, which are important processes for everyday functioning, has been given attention [Lewis et al. 2003, 2004]. The working memory model was conceptualized by Baddeley and Hitch and comprises three main components called the phonological loop, the visuospatial sketchpad, and the central executive [Baddeley and Hitch, 1974]. The phonological loop and visuospatial sketchpad are short-term storage systems (slave systems) that maintain and process verbal and visuospatial information. The central executive controls the information flow from and to both slave systems. Due to increasing awareness of cognitive deficiencies in PD, current research focuses on drug therapy to solve not only motor symptoms but also to deal with mental dysfunction [Brusa et al. 2013]. Lewis and colleagues found a positive association between L-3,4-dihydroxyphenylalanine (L-DOPA) medication and working memory measured by accuracy and response time during an intra-/extra-dimensional set-shifting task [Lewis et al. 2004]. However, findings on cognitive benefit out of dopamine replacements are mixed [McDonald et al. 2011]. Certainly, there is already evidence in favor of computerized cognitive training in healthy subjects by demonstrating improvements in working memory and changes in brain biochemistry concerning the density of cortical dopamine D1 receptors [McNab et al. 2009]. Some research on cognitive rehabilitation in patients with PD revealed promising results in terms of supporting mental activity [Cerasa et al. 2014; Petrelli et al. 2014; Zimmermann et al. 2014; Reuter et al. 2012; París et al. 2011; Sammer et al. 2006]. Thus, drug therapy combined with computerized cognitive training might achieve a more favorable benefit when compared with pharmacotherapy alone [Brusa et al. 2013]. To our knowledge, there is no computerized cognitive training tool specifically targeting working memory functions in patients with PD. Penner and colleagues developed the computerized training tool BrainStim to improve specifically the key features of working memory [Penner et al. 2006]. The effectiveness of the program has already been proven in healthy older people [Penner et al. 2007], and patients with multiple sclerosis [Hubacher, 2015a, b; Vogt et al. 2008, 2009], and schizophrenia [Hubacher et al. 2013]. Given these previous results the present study aimed at evaluating the program’s efficacy in patients with PD. We hypothesized that: (a) the PD group would show improvements in typical working memory functions (corsi-block backward and digit span backward) on postassessment; (b) on 3-month follow up after training.
Methods
Participants
Six patients with PD (mean age 71.5 years, standard deviation [SD] 4.37 years; mean disease duration 21.2 months, SD 13.9 months) participated in the study. Patients were actually treated by dopamine agonists (i.e. pramipexole and ropinirol) or L-DOPA (levodopa and carbidopa). Exclusion criteria for the PD patients were other neurological or psychiatric diseases, a change in medication within the previous month before study entry, and an age of over 80 years.
A total of 19 healthy participants with a mean age of 69.24 years (SD 4.79 years) were recruited. Inclusion criteria for healthy controls (HCs) were an age range of 20–80 years and no history of neurological or psychiatric diseases. Participants were recruited at the senior college of the University of Basel, and through advertisement. The ethics committee of the University of Basel approved the study. All participants gave written informed consent.
Study design
All participants underwent the baseline assessment twice to control for a possible learning effect. Afterwards patients with PD received 45 min training four times per week over a period of 4 weeks. The HC group was randomly assigned either to the BrainStim training group (HC-T) or the control group without training (HC-NT). The BrainStim training was supervised by a trained psychologist and took place at the neuropsychological laboratory at the Psychological Faculty of the University of Basel. Both training groups performed the BrainStim training according to a standardized training schedule. The control group HC-NT received no intervention between baseline and postassessment and were assigned to the wait-list control group. All participants underwent postassessment after a period of 6 weeks and the follow-up examination after 3 months (see Table 1 for details).
Table 1.
Study design and applied instruments.
| First baseline | Second baseline | Intervention (4 weeks) | Postassessment after 6 weeks | Follow up after 3 months | |
|---|---|---|---|---|---|
| PD HC-NT HC-T |
ADS FSMC MWT-A BRB-N N-Back Corsi blocks Digit span Stroop |
ADS FSMC BRB-N N-Back Corsi blocks Digit span Stroop |
BrainStim ______ BrainStim |
ADS FSMC BRB-N N-Back Corsi blocks Digit span Stroop |
ADS FSMC BRB-N N-Back Corsi blocks Digit span Stroop |
ADS, Allgemeine Depressions Skala (general depression scale); BRB-N, Brief Repeatable Battery of Neuropsychological Tests; FSMC, Fatigue Scale for Motor and Cognitive Functions; HC-T, healthy control with training; HC-NT, healthy control without training; MWT, Mehrfachwahl-Wortschatz-Intelligenztest A; N-Back, adaptation from TAP (Test Battery for Attention Performance); PD, Parkinson’s disease.
BrainStim training tool
The computerized program BrainStim targets working memory functions and consists of three modules: City Map, Find Pairs, and Memorize Numbers [Penner et al. 2006].
City Map trains verbal and visuospatial working memory. Participants have to memorize either a visually or verbally presented route. In the visual condition the subject is asked to retrace by mouse-click a shown route that disappears after a certain time. At each intersection green arrows indicate a possible route and participants are required to take the right decision. The number of crossings increases with ascending level of difficulty. In the verbal condition subjects receive written instructions, for example, “After the next intersection, please turn to the left”. When subjects have finished memorizing the instruction it disappears and directions need to be retraced from memory. The second module, Find Pairs, trains visual short-term and working memory as well as the updating function of the central executive. This module is comparable to the classic card-matching game where participants have to find pairs of objects printed on cards. The number of cards in a set increases with each increasing level of difficulty. It consists of four subversions: ‘faces’, ‘simple objects’, ‘geometric shapes’, and ‘abstract pictures’. In the last module, Memorize Numbers, digits are presented on a screen for a certain period of time. Subjects have to encode the numbers and recall them from memory after an arithmetic distraction task. The number of digits increases while the task becomes more difficult.
Neuropsychological assessment
All participants underwent neuropsychological assessment including self-report questionnaires for depression (Allgemeine Depressions Skala [Hautzinger and Bailer, 1993]; German version of the Center for Epidemiologic Studies Depression Scale [Radloff, 1977]) and fatigue (Fatigue Scale for Motor and Cognitive Functions [Penner et al. 2005]. Verbal intelligence was measured using the Mehrfachwahl-Wortschatz-Intelligenztest A Lehrl, 1991]. To assess working memory, the Paced Auditory Serial Addition Test (PASAT) from the Brief Repeatable Battery of Neuropsychological Tests (BRB-N) [Rao, 1990], the 2-back and 3-back tasks adapted from the Test Battery for Attention Performance (TAP) [Zimmermann and Fimm, 1992], and the Corsi block and digit span backward from the Wechsler Memory Scale-Revised (WMS-R) [Härting et al. 2000], were applied. Short-term memory was examined using the 1-back task adapted from the TAP, and the Corsi block and digit span forward from the WMS-R [Härting et al. 2000]. Verbal and visuospatial learning and delayed recall were tested with the Selective Reminding Test (SRT) from the BRB-N and the 10/36 Spatial Recall Test (SPART) from the BRB-N. For processing speed we used the Symbol Digit Modalities Test (SDMT) [Smith, 1973], and for selective attention, the Stroop Test (Victoria Stroop Color-Word version) [Regard, 1981]. Executive functions and semantic retrieval were assessed using the Word List Generation Test (WLG) from the BRB-N. For SRT, PASAT, and WLG parallel versions were applied for the different assessments to control for potential learning effects.
Statistical analysis
In total, 25 participants were enrolled in the study including 19 HCs and six patients with PD. Due to the small sample size and the not normally distributed data (Kolmogorov–Smirnov test and Levene’s test), nonparametric tests were applied and z values calculated corrected for sex, age, and education. First, the Kruskal–Wallis test (H) was used to calculate between-group comparisons for baseline assessment. Second, the Wilcoxon signed-rank test (W) with a p value of 0.05 was applied to check for within-group differences between the conditions baseline, postintervention, and 3-month follow up. Third, z-value differences and Cohen’s d for within-group effect sizes were calculated [d = (MPD – MHC)/√((SDPD2 * (NPD-1) + SDHC2 * (NHC-1))/(NPD + NHC - 2)); d = .2, small effect; d = .5, moderate effect; d = .8, large effect]. Fourth, training results were taken from the BrainStim log files. The first and last training sessions were compared within the training groups using the Wilcoxon signed-rank test.
The cut-off value of z ⩽ –1.68 for the BRB-N test battery was derived from the German validation study by Scherer and colleagues [Scherer et al. 2004]. The pathological cut-off for the Corsi blocks and digit span (WMS-R) and the Stroop test was a z value of < –1, which corresponds to 1 SD from the mean using the underlying test norms. z values for the N-Back task were calculated using mean and SD from the HC group.
Results
Between-group comparisons for baseline assessment
Table 2 shows the baseline characteristics of six patients (three women: three men), 12 HC-T (five women: seven men), and seven HC-NT (three women: four men). No significant differences were found among the groups with respect to age (mean (M) (SD): PD 71.5 (4.37); HC-T 70.04 (4.53); HC-NT 67.86 (5.72); p = 0.169), education (education: 0 = secondary school, 1 = college, 2 = university; M (SD): PD 2 (0.0); HC-T 1.33 (0.65); HC-NT 1.43 (0.54); p = 0.059), depression, and fatigue. Mean fatigue scores were all below the clinically relevant cut-off value of ⩾ 43. Depression mean scores were all below the determined cut-off value of 23 for the German version. Group comparisons using the Kruskal–Wallis test revealed significant differences for mental speed, and verbal short-term and working memory. Post-hoc analyses using the Mann–Whitney U test between the PD group and both HC groups revealed significantly worse performance for the PD group in information processing speed, short-term memory, verbal long-term memory, and working memory.
Table 2.
Baseline characteristics of Parkinson’s disease with training, and healthy controls with and without training.
| Parkinson’s disease |
Healthy control with training |
Healthy control without training |
(H) p | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Questionnaires | |||||||
| ADS | −0.28 | 0.66 | −0.93 | 0.61 | 1.50 | 0.53 | *0.053 |
| FSMC | 31.67 | 7.71 | 34.21 | 9.42 | 36.14 | 8.89 | 0.381 |
| Working memory | |||||||
| Corsi block backward | −1.15 | 0.86 | −0.10 | 0.81 | 0.01 | 0.99 | 0.073 |
| Digit span backward | 0.09 | 0.97 | −0.37 | 0.68 | −0.18 | 0.81 | 0.587 |
| 2-back ACC | 1.20ƒ | – | 0.02 | 1.01 | −0.33ª | 1.50 | 0.276 |
| 2-back RT | −0.38ƒ | – | 0.22 | 1.04 | −0.86ª | 0.42 | 0.173 |
| 3-back ACC | – | – | 0.21 | 0.86º | −0.74ª | 1.48 | 0.240 |
| 3-back RT | – | – | −0.17 | 1.08º | 0.61ª | 0.09 | 0.143 |
| PASAT | −1.47 | 0.92 | −0.54 | 0.85 | 0.44 | 0.43 | *0.005 |
| Short-term memory | |||||||
| Corsi block forward | −0.55 | 0.52 | −0.08 | 0.89 | −0.18 | 1.08 | 0.392 |
| Digit span forward | 0.06 | 0.72 | −0.41 | 0.66 | −0.28 | 0.55 | 0.309 |
| 1-back ACC | −0.69 | 1.78 | −0.03 | 0.65 | 0.14 | 0.98 | 0.560 |
| 1-back RT | −1.27 | 1.49 | −0.15 | 1.25 | 0.18 | 0.41 | 0.135 |
| SPART | −0.07 | 1.59 | 0.48 | 1.03 | 0.42 | 0.90 | 0.793 |
| SRT-LTS | −1.46 | 1.49 | 0.27 | 1.01 | −0.41 | 0.93 | *0.036 |
| SRT-CLTR | −1.09 | 1.18 | 0.23 | 0.96 | −0.23 | 0.71 | 0.070 |
| Mental speed | |||||||
| SDMT | −1.33 | 0.68 | 0.43 | 1.30 | 0.53 | 1.54 | *0.009 |
| WLG | −0.94 | 1.24 | 0.35 | 1.00 | 0.63 | 0.58 | *0.047 |
| Long-term memory | |||||||
| SRT-DR | −2.06 | 2.02 | 0.13 | 0.96 | −0.11 | 1.35 | 0.066 |
| SPART-DR | 0.36 | 1.36 | 0.79 | 0.84 | 0.21 | 0.67 | 0.250 |
| Interference/inhibition | |||||||
| Stroop | −0.41 | 0.40 | 0.13 | 1.02 | −0.34 | 0.56 | 0.651 |
All scores are z values except for FSMC; (H) p, Kruskal–Wallis test; *, significance at an alpha level of .05; ƒ = N = 1; º = N = 7; ª = N = 2. ACC, accuracy; ADS, Allgemeine Depressions Skala (general depression scale); FSMC, Fatigue Scale for Motor and Cognitive Functions; M, mean; PASAT, Paced Auditory Serial Addition Test; RT, reaction time; SD, standard deviation; SDMT, Symbol Digit Modalities Test; SPART, Spatial Recall Test; SPART-DR, Spatial Recall Test Delayed Recall; SRT-CLTR, Selective Reminding Test Consistent Long-Term Retrieval; SRT-DR, Selective Reminding Test Delayed Recall; SRT-LTS, Selective Reminding Test Long-Term Storage; WLG, Word List Generation.
Within-group and between-group comparisons for PD, HC-T, and HC-NT for baseline and postassessment
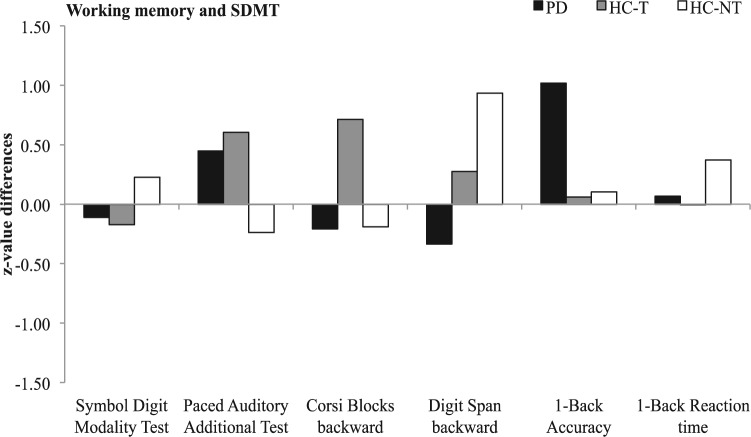
The Kruskal–Wallis test (H) revealed a significant difference in verbal and visuospatial short-term memory (Corsi blocks forward, Selective Reminding Test Consistent Long-Term Retrieval [SRT-CLTR]). Since we hypothesized that PD patients would show improved outcomes after training, further analyses using the Wilcoxon signed-rank test with a 1-tailed p value of 0.05 were applied to follow up this assumption. First, a within-group comparison for baseline and postassessment was calculated for all three groups (see Table 3). The PD group showed significant improvements for visual short-term memory (SPART) and verbal long-term memory (Selective Reminding Test Delayed Recall [SRT-DR]) comparing baseline and postassessment. Significantly improved performance within the HC-T group for baseline and postassessment was found for verbal short-term and long-term memory (digit span forward; Selective Reminding Test Long-Term Storage [SRT-LTS]; SRT-CLTR; SRT-DR), visual short-term memory (SPART), mental speed and working memory (SDMT; PASAT), and verbal and visuospatial working memory (digit span backward; Corsi block backward). The HC-NT group showed a significant increase in verbal short-term memory (SRT-LTS; SRT-CLTR; digit span forward), visuospatial short-term (Corsi block forward) and verbal working memory (digit span backward). z-value differences especially for working memory and mental speed are displayed in Figure 1.
Table 3.
z-value differences of baseline and postassessment. Within-group comparisons (Cohen’s d effect sizes and Wilcoxon signed-rank test (W)) as well as between-group comparisons using the Kruskal–Wallis (H) test are shown.
| Parkinson’s disease |
Healthy control with training |
Healthy control without training |
(H) p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z | d | (W) p | Z | d | (W) p | Z | d | (W) p | ||
| Questionnaires | ||||||||||
| ADS | −0.47 | −0.62 | 0.156 | −0.46 | −0.85 | *0.046 | 0.09 | −0.06 | 0.406 | 0.39 |
| FSMC | 0.24 | 0.29 | 0.234 | 0.05 | 0.07 | 0.423 | 0.29 | 0.16 | 0.234 | 0.57 |
| Working memory | ||||||||||
| Corsi block backward | 0.68 | 0.66 | 0.078 | 0.50 | 0.52 | *0.021 | −0.31 | −0.44 | 0.234 | 0.09 |
| Digit span backward | −0.05 | −0.04 | 0.500 | 0.38 | 0.62 | *0.049 | 0.35 | 0.35 | *0.047 | 0.59 |
| 2-back ACC | – | – | – | 0.63 | 0.75 | 0.313 | 0.33 | – | – | 0.55 |
| 2-back RT | – | – | – | −0.07 | −0.06 | 0.313 | −0.86 | – | – | 0.38 |
| 3-back ACC | – | – | – | 0.52 | 0.71 | 0.063 | 0.74 | – | – | – |
| 3-back RT | – | – | – | −0.69 | −0.75 | 0.063 | 0.61 | – | – | – |
| PASAT | 0.05 | 0.05 | 0.563 | 0.31 | 0.37 | *0.033 | −0.22 | −0.11 | 0.125 | 0.81 |
| Short-term memory | ||||||||||
| Corsi block forward | −0.15 | −0.31 | 0.344 | 0.27 | 0.26 | 0.209 | 0.89 | 0.76 | *0.008 | *0.03 |
| Digit span forward | 0.29 | 0.38 | 0.125 | 0.34 | 0.37 | *0.021 | 0.83 | 0.47 | *0.031 | 0.66 |
| 1-back ACC | 0.73 | 0.43 | 0.375 | 0.30 | 0.40 | 0.172 | −0.10 | −0.08 | 0.313 | 0.75 |
| 1-back RT | 0.54 | 0.36 | 0.313 | 0.07 | 0.06 | 0.455 | 0.18 | 0.42 | 0.148 | 0.62 |
| SPART | 0.94 | 0.57 | *0.047 | 1.11 | 0.95 | *0.001 | 0.28 | 0.11 | 0.234 | 0.12 |
| SRT-LTS | 0.71 | 0.47 | 0.109 | 1.22 | 1.46 | *<0.001 | 1.19 | 0.75 | *0.008 | 0.78 |
| SRT-CLTR | 0.40 | 0.31 | 0.078 | 1.16 | 1.20 | *<0.001 | 1.09 | 0.82 | *0.016 | *0.05 |
| Mental speed | ||||||||||
| SDMT | 0.20 | 0.27 | 0.156 | 0.32 | 0.24 | *0.053 | 0.15 | 0.20 | 0.398 | 0.71 |
| WLG | −0.22 | −0.19 | 0.313 | −0.43 | −0.43 | 0.065 | −0.36 | −0.13 | 0.234 | 0.86 |
| Long-term memory | ||||||||||
| SRT-DR | 1.39 | 0.88 | *0.047 | 0.94 | 1.30 | *0.001 | 0.46 | 0.34 | 0.313 | 0.21 |
| SPART-DR | 0.50 | 0.34 | 0.063 | 0.27 | 0.23 | 0.072 | 0.04 | –0.14 | 0.500 | 0.42 |
| Interference/inhibition | ||||||||||
| Stroop | 0.09 | 0.11 | 0.500 | −0.20 | −0.24 | 0.311 | −0.02 | 0.08 | 0.500 | 0.84 |
z = z-value differences between pre- and postassessment within groups; d = Cohen’s d effect size within groups with d = .2 small effect, d = .5 moderate effect, d = .8 large effect; (W) p, Wilcoxon signed-rank test for within-group comparisons; (H) p, Kruskal–Wallis test for between-group comparisons; *, 1-tailed significance p ⩽ 0.05. ACC, accuracy; ADS, Allgemeine Depressions Skala (general depression scale); FSMC, Fatigue Scale for Motor and Cognitive Functions; M, mean; PASAT, Paced Auditory Serial Addition Test; RT, reaction time; SD, standard deviation; SDMT, Symbol Digit Modalities Test; SPART, Spatial Recall Test; SPART-DR, Spatial Recall Test Delayed Recall; SRT-CLTR, Selective Reminding Test Consistent Long-Term Retrieval; SRT-DR, Selective Reminding Test Delayed Recall; SRT-LTS, Selective Reminding Test Long-Term Storage; WLG, Word List Generation.
Figure 1.
Differences in z values between baseline and postintervention assessment for the trained cognitive domains of working memory and speed. Negative values imply a decrease in performance; positive values represent an increase.
Training tool BrainStim
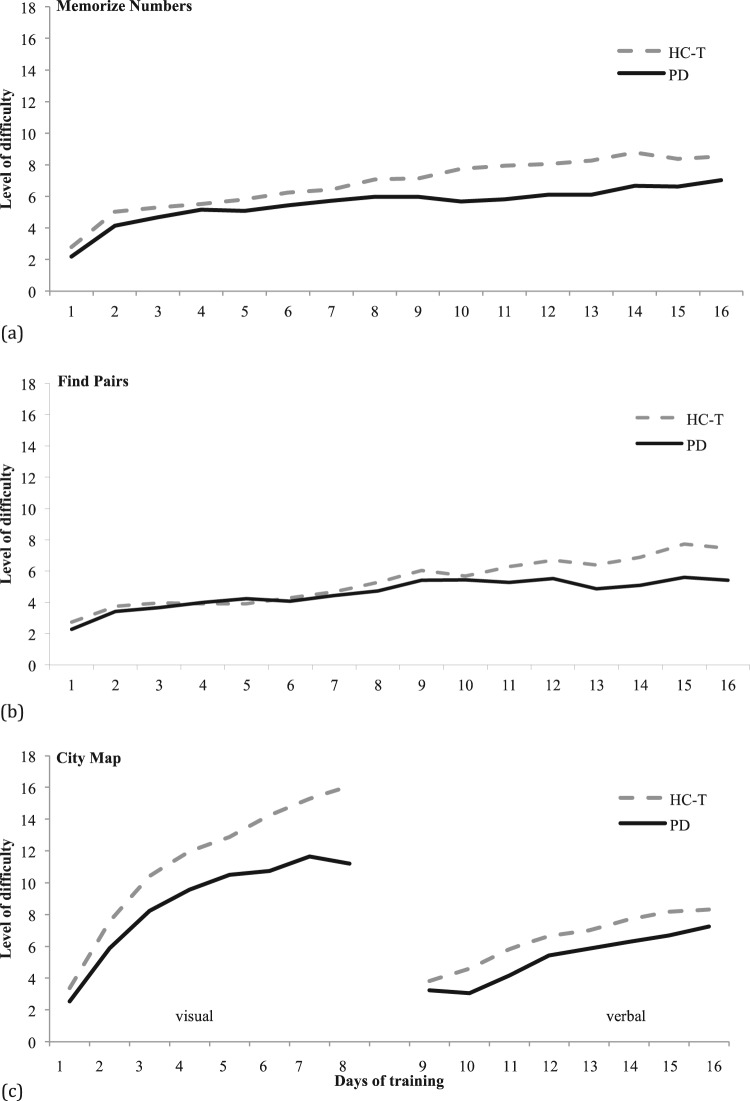
Table 4 shows group means and SDs for the first and last BrainStim training session. Both training groups (PD and HC-T) succeeded in increasing the level of difficulty over the training period of 16 sessions (Figure 2). In detail, both groups displayed significant improvements in training performance for City Map visual instructions (zHC-T = −3.059, p < 0.001; zPD = −1.992, p = 0.031) and City Map verbal instructions (zHC-T = −2.275, p = 0.010; zPD = −1.782, p = 0.047), Find Pairs (zHC-T = −3.059, p < 0.001; zPD = −1.782, p = 0.047), as well as for Memorize Numbers (zHC-T = −3.059,p < 0.001; zPD = −2.201, p = 0.016).
Table 4.
Means and standard deviations of the first and last BrainStim training session for the Parkinson’s disease and healthy control with training groups.
| Parkinson’s disease (n = 6) |
Healthy control with training (n = 12) |
|||||||
|---|---|---|---|---|---|---|---|---|
| First training |
Last training |
First training |
Last training |
|||||
| M | SD | M | SD | M | SD | M | SD | |
| City Map visual instructions | 4.33 | 0.50 | 13.92 | 2.86 | 5.37 | 0.60 | 18.23 | 2.47 |
| City Map verbal instructions | 6.58 | 3.19 | 8.8 | 2.62 | 4.45 | 0.79 | 10.47 | 1.63 |
| Find Pairs | 4.83 | 0.84 | 9.82 | 4.92 | 5.79 | 0.98 | 13.27 | 4.13 |
| Memorize Numbers | 3.22 | 0.73 | 8.09 | 1.70 | 3.78 | 0.64 | 9.53 | 3.76 |
M, mean; SD, standard deviation.
Figure 2.
Averaged achievements during training sessions for healthy controls with training and patients with Parkinson’s disease in the modules Memorize Numbers, Find Pairs, and City Map with visual and verbal instructions. The vertical axis represents the level of difficulty, the horizontal axis the training days. The level of difficulty has been calculated by the mean of correct answers during each session.
Within-group and between-group comparison for PD, HC-T, and HC-NT for baseline versus 3-month follow up
Table 5 shows significant differences between groups in short-term memory (1-back reaction time [RT]; SRT-CLTR), mental speed (SDMT), and verbal and visuospatial long-term memory (SRT-DR; Spatial Recall Test Delayed Recall [SPART-DR]). Within-group comparison revealed significantly better values for verbal and visuospatial short-term memory (SRT-LTS; SPART; Corsi block forward) in the PD group. Significant improvements in the HC-T group were found for verbal short-term and long-term memory (SRT-LTS; SRT-CLTR; SRT-DR), visuospatial short-term and long-term memory (SPART; SPART-DR), and visuospatial working memory (PASAT; Corsi block backward), whereas a significant decrease was observed for 1-back RT. The HC-NT group showed better results for verbal short-term memory (1-back RT; SRT-LTS; SRT-CLTR; digit span forward), verbal working memory (digit span backward), and verbal fluency (WLG). Cohen’s d within-group effect sizes revealed moderate to large effect sizes on visuospatial short-term memory (Corsi block forward and SPART) in the PD and HC-T group only (Table 5). z-value differences especially for working memory and mental speed are displayed in Figure 3.
Table 5.
z-value differences of baseline assessment and 3-month follow up. Shown are Cohen’s d effect sizes and Wilcoxon signed-rank test (W) for within-group comparisons and between-group comparisons using the Kruskal–Wallis (H) test.
| Parkinson’s disease |
Healthy controls with training |
Healthy controls without training |
(H) p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z | d | (W) p | Z | d | (W) p | Z | d | (W) p | ||
| Questionnaires | ||||||||||
| ADS | 0.11 | 0.12 | 0.500 | −0.07 | −0.11 | 0.281 | 0.17 | 0.27 | 0.234 | *0.02 |
| FSMC | 0.33 | 0.38 | 0.219 | −0.02 | −0.02 | 0.417 | 0.24 | 0.24 | 0.094 | 0.58 |
| Working memory | ||||||||||
| Corsi block backward | −0.21 | −0.16 | 0.500 | 0.71 | 0.63 | *0.042 | −0.19 | −0.41 | 0.344 | 0.09 |
| Digit span backward | −0.34 | −0.28 | 0.188 | 0.27 | 0.38 | 0.168 | 0.93 | 0.94 | *0.016 | 0.14 |
| 2-back ACC | – | – | – | 0.95 | 0.99 | 0.250 | – | – | – | – |
| 2-back RT | – | – | – | 0.53 | 0.54 | 0.250 | – | – | – | – |
| 3-back ACC | – | – | – | 0.87 | 1.09 | 0.500 | – | – | – | – |
| 3-back RT | – | – | – | 1.60 | 1.58 | 0.250 | – | – | – | – |
| PASAT | 0.45 | 0.45 | 0.219 | 0.60 | 0.80 | *<0.001 | −0.24 | −0.18 | 0.453 | 0.07 |
| Short-term memory | ||||||||||
| Corsi block forward | 0.85 | 1.23 | *0.031 | 0.57 | 0.64 | 0.057 | 0.51 | 0.35 | 0.125 | 0.85 |
| Digit span forward | −0.10 | −0.10 | 0.625 | 0.22 | 0.25 | 0.336 | 0.91 | 0.54 | *0.039 | 0.36 |
| 1-back ACC | 1.02 | 0.67 | 0.250 | 0.06 | 0.09 | 0.156 | 0.10 | 0.15 | 0.500 | 0.77 |
| 1-back RT | 0.07 | 0.05 | 0.406 | 0.00 | 0.00 | *0.003 | 0.37 | 0.75 | *0.039 | *0.03 |
| SPART | 0.94 | 0.65 | *0.031 | 0.84 | 0.79 | *0.001 | −0.21 | −0.47 | 0.352 | 0.07 |
| SRT-LTS | 0.62 | 0.41 | *0.016 | 0.77 | 0.76 | *0.016 | 1.33 | 0.87 | *0.008 | 0.08 |
| SRT-CLTR | 0.14 | 0.10 | 0.203 | 1.02 | 1.12 | *0.002 | 1.19 | 0.95 | *0.016 | *0.03 |
| Mental speed | ||||||||||
| SDMT | −0.11 | −0.14 | 0.313 | −0.17 | −0.13 | 0.268 | 0.23 | 0.27 | 0.133 | *0.02 |
| WLG | 0.39 | 0.25 | 0.219 | 0.21 | 0.22 | 0.188 | 0.56 | 0.97 | *0.031 | 0.07 |
| Long-term memory | ||||||||||
| SRT-DR | 0.64 | 0.35 | 0.063 | 0.62 | 0.77 | *0.012 | 0.64 | 0.54 | 0.063 | *0.03 |
| SPART-DR | 0.42 | 0.34 | 0.156 | 0.68 | 0.88 | *0.003 | −0.39 | −0.60 | 0.313 | *0.03 |
| Interference/inhibition | ||||||||||
| Stroop | −0.14 | −0.37 | 0.422 | −0.17 | −0.19 | 0.120 | −0.13 | −0.14 | 0.422 | 0.43 |
z = z-value differences between pre- and postassessment within groups; d = Cohen’s d effect size within groups with d = .2 small effect, d = .5 moderate effect, d = .8 large effect; (W) p, Wilcoxon signed-rank test for within-group comparisons; (H) p, Kruskal–Wallis test for between-group comparisons; *, 1-tailed significance p ⩽ 0.05. ACC, accuracy; ADS, Allgemeine Depressions Skala (general depression scale); FSMC, Fatigue Scale for Motor and Cognitive Functions; M, mean; PASAT, Paced Auditory Serial Addition Test; RT, reaction time; SD, standard deviation; SDMT, Symbol Digit Modalities Test; SPART, Spatial Recall Test; SPART-DR, Spatial Recall Test Delayed Recall; SRT-CLTR, Selective Reminding Test Consistent Long-Term Retrieval; SRT-DR, Selective Reminding Test Delayed Recall; SRT-LTS, Selective Reminding Test Long-Term Storage; WLG, Word List Generation.
Figure 3.
Differences in z values between baseline and follow-up assessment after 3 months for the trained cognitive domains working memory and speed. Negative values imply a decrease in performance; positive values represent an increase.
Discussion
Since patients with PD suffer from cognitive decline in domains especially important for daily living, this study aimed to evaluate the effectiveness of a computerized working memory training tool in patients with PD. Since the program BrainStim has already been applied successfully in healthy older subjects [Penner et al. 2007], and in patients with multiple sclerosis [Vogt et al. 2008], and schizophrenia [Hubacher et al. 2013], clinical application and methodological quality have been proven. Thus, we aimed to reveal comparable findings in patients with PD.
Baseline analyses showed a significantly worse performance for the PD group compared with the HCs in several tasks measuring information processing speed and verbal and visuospatial memory. These results are consistent with current research findings on cognitive inefficiencies in patients with PD [Pfeiffer et al. 2014; Hanganu et al. 2014].
Given these domain-specific problems it was intriguing to find out whether the computerized training tool BrainStim may be able to improve these inefficiencies within 16 training sessions. According to BrainStim log files the PD group was able to manage the increasing cognitive complexity in all three modules using suitable cognitive strategies reflecting participants’ learning ability. These changes were statistically significant when the first training session was compared with the last. As shown in Figure 2 the PD group had a lower performance on average achievements during training sessions in comparison with the HC-T group. This result indicates that the learning curve of patients with PD is flatter when compared with matched controls and that either more training sessions are needed to achieve similar results or that the learning capacity in general is limited due to the neurodegenerative process. In the PD group there were significant improvements in visual short-term memory on postassessment, which is consistent with other studies using BrainStim as training tool [Hubacher et al. 2013; Vogt et al. 2008]. Contrary to previous research the PD group showed significantly increased results in verbal long-term memory on postassessment and a trend on 3-month follow up with stable effect sizes [Parìs et al. 2011; Petrelli et al. 2014]. Since training tasks differed from the tests applied in the neuropsychological examination and parallel test versions were used on postassessment, these results may reflect learning of appropriate strategies rather than simple practice effects. This assumption could also be confirmed by improved visual and verbal short-term memory on the 3-month follow up showing that the learned strategies were even applied after finishing the training.
In comparison to the PD group, the HC-T group improved in almost all tests after training. These findings are in line with results from the study by Penner and colleagues on healthy older subjects [Penner et al. 2007], and confirm the therapeutic potential of specific computerized training, not only for the injured, but also for the normal aging brain.
Of interest is the specific effect of training on measures of working memory and speed. As demonstrated in Figure 1 by z-value differences, patients with PD improved or remained stable in these measures directly after training and even showed larger training effects on three tasks than HCs with training. However, when looking at z-value differences between baseline assessment and 3-month follow up it becomes obvious that performance could not be maintained over time. Since HCs also showed decreases after 3 months, cognitive training has to be regarded as a permanent treatment option, which should at best regularly accompany medication.
There are some limitations that we are aware of and that need to be addressed. The study did not include a PD group without training or a true placebo group because of recruitment difficulties. The PD sample was too small to draw general conclusions. However, since training effects were found even in this small group, it can be expected that results would become more pronounced in a larger cohort. It is important to consider the impact of drug therapy on cognitive improvement during additional cognitive training. Analyzing descriptive data revealed that two participants out of the PD group managed to solve the 2-back and 3-back tasks after training. Both participants were given pramipexole, which is known to stimulate dopamine D2 receptor activation [Domellöf et al. 2013], and therefore might have had a positive influence on working memory performance [Moustafa et al. 2013].
To sum up, the results showed that training with BrainStim impressively improved cognitive performance in patients with PD and that training effects in some cognitive domains were still visible after 3-month follow up. The trained group of HCs showed the largest benefit from training. Both training groups enjoyed the training sessions since they were designed to relate closely to everyday life in an entertaining manner. The continuous adaptation of the level of difficulty to participants’ performance seems to act both as motivation and reward for success. Two healthy participants who were learning a new language at the time even reported that they felt that they learned new vocabulary easier than before. These findings underline the relevance of such an easy to apply cognitive training, which strengthens not only cognitive functioning but also seems to support self-efficacy and emotional well-being.
Acknowledgments
Natalia Adamski and Matthias Adler contributed equally as first authors.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Natalia Adamski, Department of Cognitive Psychology and Methodology, University of Basel, Switzerland.
Matthias Adler, Department of Cognitive Psychology and Methodology, University of Basel, Switzerland.
Klaus Opwis, Department of Cognitive Psychology and Methodology, University of Basel, Switzerland.
Iris-Katharina Penner, COGITO Center for Applied Neurocognition and Neuropsychological Research and Neurology Department, University Hospital, Merowingerplatz 1, 40225 Düsseldorf, Germany.
References
- Aarsland D., Marsh L., Schrag A. (2009) Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord 24: 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A., Hitch G. (1974) Working memory. Psychol Learn Motiv 8: 47–89. [Google Scholar]
- Brusa L., Pavino V., Massimetti M., Bove R., Iani C., Stanzione P. (2013) The effect of dopamine agonists on cognitive functions in non-demented early-mild Parkinson’s disease patients. Funct Neurol 28: 13–17. [PMC free article] [PubMed] [Google Scholar]
- Cerasa A., Gioia M., Salsone M., Donzuso G., Chiriaco C., Realmuto S., et al. (2014) Neurofunctional correlates of attention rehabilitation in Parkinson’s disease: an explorative study. Neurol Sci 1–8. [DOI] [PubMed] [Google Scholar]
- Dickson D., Fujishiro H., Orr C., DelleDonne A., Josephs K., Frigerio R., et al. (2009) Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 15: 1–5. [DOI] [PubMed] [Google Scholar]
- Domellöf M., Forsgren L., Elgh E. (2013) Persistence of associations between cognitive impairment and motor dysfunction in the early phase of Parkinson’s disease. J Neurol 260: 2228–2236. [DOI] [PubMed] [Google Scholar]
- European Parkinson’s Disease Association. (2014) Prevalence of Parkinson’s Disease. Brussels: European Parkinson’s Disease Association; http://www.epda.eu.com/en/parkinsons/life-with-parkinsons/part-1/prevalence-of-parkinsons-disease (accessed 26 February 2014). [Google Scholar]
- Härting C., Markowitsch H., Neufeld H., Calabrese P., Deisinger K. (2000) Wechsler Gedächtnistest – Revidierte Fassung WMS-R. Bern: Hans Huber. [Google Scholar]
- Hanganu A., Bedetti C., Degroot C., Mejia-Constain B., Lafontaine A., Soland V., et al. (2014) Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain 137: 1120–1129. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M. (1993) Allgemeine Depressionsskala (ADS). Weinheim: Beltz PVU. [Google Scholar]
- Hubacher M., DeLuca J., Weber P., Steinlin M., Kappos L., Opwis K., et al. (2015a) Cognitive rehabilitation of working memory in juvenile multiple sclerosis – effects on cognitive functioning, functional MRI and network related connectivity. Restor Neurol Neurosci 33: 713–725. [DOI] [PubMed] [Google Scholar]
- Hubacher M., Kappos L., Weier K., Stöcklin M., Opwis K., Penner I. (2015b) Case-based fMRI analysis after cognitive rehabilitation in MS: a novel approach. Frontiers Neurol 6: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubacher M., Weiland M., Penner I., Calabrese P., Stoppe G., Stöcklin M., et al. (2013) Working memory training in patients with chronic schizophrenia: a pilot study. Psychiatry J 2013: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson R., Yarnall A., Duncan G., Khoo T., Breen D., Barker R., et al. (2014) Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Parkinsonism Relat Disord 20: 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S., Merz J., Burkhard G., Fischer S. (1991) Mehrfachwahl-Wortschatz-Intelligenztest (MWT-A). Manual. Balingen, Germany: Spitta Verlag. [Google Scholar]
- Lewis S., Dove A., Robbins T., Barker R., Owen A. (2003) Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23: 6351–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Slabosz A., Robbins T., Barker R., Owen A. (2004) Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia 43: 823–832. [DOI] [PubMed] [Google Scholar]
- MacDonald P., MacDonald A., Seergobin K., Tamjeedi R., Ganjavi H., Provost J., et al. (2011) The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson’s disease: support from functional MRI. Brain 134: 1447–1463. [DOI] [PubMed] [Google Scholar]
- McDowell K., Chesselet M. (2012) Animal models of the non-motor features of Parkinson’s disease. Neurobiol Disease 46: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F., Varrone A., Farde L., Jucaite A., Bystritsky P., Forssberg H., et al. (2009) Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science 323: 800–802. [DOI] [PubMed] [Google Scholar]
- Moustafa A. A., Bell P., Eissa A. M., Hewedi D. H. (2013) The effects of clinical motor variables and medication dosage on working memory in Parkinson’s disease. Brain and Cognition 82(2): 137–145. [DOI] [PubMed] [Google Scholar]
- París A., Saleta H., de la Cruz Crespo Maraver M., Silvestre E., Freixa M., Torrellas C., et al. (2011) Blind randomized controlled study of the efficacy of cognitive training in Parkinson’s disease. Mov Disord 26: 1251–1258. [DOI] [PubMed] [Google Scholar]
- Penner I., Kobel M., Stöcklin M., Opwis K., Calabrese P. (2007). BrainStim – hirnstimulation als Präventions- und Therapiemassnahme? Neurogeriatrie 4: 109–115. [Google Scholar]
- Penner I., Kobel M., Opwis K. (2006) BrainStim – a recently developed tool to train different aspects of working memory. In: Proceedings of the INS/GNP Conference, pp. 17–19. [Google Scholar]
- Penner I., Vogt A., Rselli C., Stöcklin M., Opwis K., Kappos L. (2005) The FSMC (Fatigue Scale for Motor and Cognitive Functions): a new patient reported outcome measure for cognitive and motor fatigue in multiple sclerosis. Mult Scler 11: 264. [Google Scholar]
- Petrelli A., Kaesberg S., Barbe M., Timmermann L., Fink G., Kessler J., et al. (2014) Effects of cognitive training in Parkinson’s disease: a randomized controlled trial. Parkinsonism Relat Disord 20: 1196–1202. [DOI] [PubMed] [Google Scholar]
- Pfeiffer H., Løkkegaard A., Zoetmulder M., Friberg L., Werdelin L. (2014) Cognitive impairment in early-stage non-demented Parkinson’s disease patients. Acta Neurol Scan 129: 307–318. [DOI] [PubMed] [Google Scholar]
- Radloff L. (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1: 385–401. [Google Scholar]
- Rao S.; Cognitive Function Group of the National Multiple Sclerosis Society. (1990) A Manual for the Brief Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. Milwaukee, WI: Medical College of Wisconsin. [Google Scholar]
- Regard M. (1981) Stroop Test – Victoria Version. Victoria, BC: Neuropsychological Laboratory, University of Victoria. [Google Scholar]
- Reuter I., Mehnert S., Oechsner M., Engelhardt M. (2012) Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson’s disease. J Aging Res ID 235765: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammer G., Reuter I., Hullmann K., Kaps M., Vaitl D. (2006) Training of executive functions in Parkinson’s disease. J Neurol Sci 248: 115–119. [DOI] [PubMed] [Google Scholar]
- Scherer P., Baum K., Bauer H., Göhler H., Miltenburger C. (2004) Normierung der Brief Repeatable Battery of Neuropsychological Tests (BRB-N) für den deutschsprachigen Raum. Nervenarzt 75: 984–990. [DOI] [PubMed] [Google Scholar]
- Smith A. (1973). Symbol Digit Modality Test. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Vogt A., Kappos L., Calabrese P., Stocklin M., Gschwind L., Opwis K., et al. (2009) Working memory training in patients with multiple sclerosis – comparison of two different training schedules. Restor Neurol Neurosci 27: 225–235. [DOI] [PubMed] [Google Scholar]
- Vogt A., Kappos L., Stöcklin M., Gschwind L., Opwis K., Penner I. (2008) BrainStim – Wirksamkeit eines neu entwickelten kognitiven Trainingsprogramms bei MS. Neurologie & Rehabilitation 14: 93–101. [Google Scholar]
- Zimmermann P., Fimm B. (1992) Testbatterie zur Aufmerksamkeitsprüfung. Würselen, Germany: Psytest. [Google Scholar]
- Zimmermann R., Gschwandtner U., Benz N., Hatz F., Schindler C., Taub E., et al. (2014) Cognitive training in Parkinson disease cognition-specific vs nonspecific computer training. Neurology 82: 1219–1226. [DOI] [PubMed] [Google Scholar]