Abstract
Niemann-Pick type C (NP-C) disease is a neurovisceral disorder caused by mutations in the NPC1 and NPC2 genes. It is characterized by lysosomal storage of a broad range of lipids as a result of abnormal intracellular lipid trafficking. Typically patients develop neurodegeneration; however, the speed of disease progression is variable. The exact functions of NPC1 and NPC2 proteins have not been determined and therefore the molecular pathophysiology of NP-C is still not clearly understood. Due to the disease’s rarity and clinical heterogeneity, delays from symptom onset to diagnosis and treatment initiation are common. Current therapeutic approaches focus on multidisciplinary symptom control and deceleration (rather than reversal) of disease progression. Thus identification of cases at early stages of disease is particularly important. Recent advances in genetic and biochemical testing have resulted in the generation of relatively non-invasive, quick and cost-effective laboratory assays that are highly sensitive and specific and have the capacity to enhance the clinicians’ ability to reach a diagnosis earlier. Miglustat is a compound recently licensed in many countries for the treatment of NP-C that has been shown to decelerate neurological regression, whereas many other promising drugs are currently being trialled in preclinical models or human studies. This review summarizes key clinical, genetic and biochemical features of NP-C, suggests a simple diagnostic investigation strategy and gives an overview of available therapeutic options as well as potential novel treatments currently under development.
Keywords: biochemical markers, diagnosis, genetics, miglustat, Niemann-Pick type C disease (NP-C), NPC1, NPC2
Introduction
Niemann-Pick disease type C (NP-C; OMIM: NP-C1 #257220, NP-C2 #607625) is a neurodegenerative disorder caused by mutations in NPC1 and NPC2 genes [Carstea et al. 1997; Naureckiene et al. 2000]. At a cellular level, it is characterized by accumulation of a broad range of lipids, including unesterified cholesterol and sphingolipids, in the lysosomes and late endosomes [Pentchev et al. 1985; Sokol et al. 1988; Vanier and Millat, 2003; Lloyd-Evans and Platt, 2010; Vanier, 2010]. The lipid accumulation and storage is associated with the multisystemic clinical manifestations of NP-C. The exact NPC1 and NPC2 protein functions are unknown [Vance and Karten, 2014; Vanier, 2015] and the mechanisms leading to abnormal storage and subsequent cellular malfunction and neurodegeneration have not, to date, been fully elucidated. A number of hypotheses have been proposed, including the suggestion that sphingosine storage in the endolysosomal compartment leads to impaired calcium homeostasis and plays an integral part in disease pathophysiology [Lloyd-Evans et al. 2008].
NP-C disease is very heterogeneous in its presentation. It has a variable age of onset and a range of (often nonspecific) visceral, neurological and psychiatric clinical features can arise at different disease stages and progress at different rates [Patterson et al. 2012]. NP-C has an estimated prevalence of 1 in 90,000–120,000 live births, although recent studies suggest this figure to be an underestimate, with many patients failing to reach a diagnosis [Vanier, 2010; Mengel et al. 2013; McKay Bounford and Gissen, 2014; Wassif et al. 2016]. One of the main reasons behind the diagnostic delay is believed to be the nonspecific clinical presentation, thus making detection and diagnosis at an early stage of disease difficult. Specialist biochemical and molecular genetic tests are required to confirm the diagnosis; however, the major diagnostic delay is due to the lack of awareness of NP-C among non-specialist clinical practitioners [Wraith et al. 2009; Patterson et al. 2013].
Timely and accurate NP-C diagnosis is crucial to enable prompt initiation of appropriate clinical management, especially in view of the presence of: (1) disease modifying drugs that can slow down disease progression [Patterson et al. 2015]; and (2) novel therapeutic approaches currently in preclinical development (or at the clinical trial stage) with promising results in animal or cell models [Kirkegaard et al. 2010; Kalmar et al. 2014; Vite et al. 2015]. In 2012, updated recommendations on the diagnosis and treatment of NP-C were published [Patterson et al. 2012]. Moreover, an increased understanding of NP-C genetics and biochemical features has recently enabled the development of laboratory tests that not only aim to aid clinicians to achieve diagnosis in a more timely fashion but are also simpler and more cost-effective than those traditionally available. However, diagnostic and management difficulties persist.
This article provides an overview of available diagnostic methods that can be utilized when NP-C is suspected, as well as their advantages and limitations, but also focuses on therapeutic strategies that can be applied once the diagnosis has been made.
Current diagnostic techniques
History taking and clinical examination
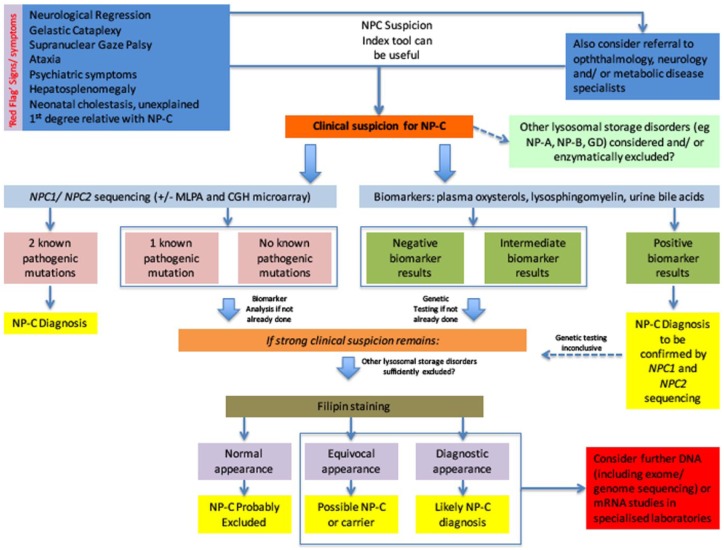
Clinical examination is the initial and most crucial step when assessing patients and considering the diagnosis of NP-C. Of paramount importance is the need for increased clinical awareness and suspicion, especially in view of the disease’s rarity and variable, nontypical presentation. In about 90% of the patients, a progressive neurodegenerative involvement is the dominant feature [Vanier, 2015]. The combined presence of visceral, neurological and/or psychiatric symptoms should prompt clinicians to include NP-C in their differential diagnosis; however, atypical presentations in NP-C patients are common. The age at onset of neurological manifestations has been linked with prognosis, with earlier onset forms progressing faster and associated with greater mortality and morbidity [Mengel et al. 2013]. In each case, clinicians should look out for ‘red flag’ signs and symptoms that point towards the disorder and which are summarized in Figure 1. These include neonatal cholestasis, hepatosplenomegaly, vertical supranuclear gaze palsy, gelastic cataplexy, neurological regression (with or without dementia), progressive ataxia, dystonia and dysphagia as well as psychiatric symptoms (psychosis or depression).
Figure 1.
Proposed diagnostic algorithm for patients with suspected Niemann-Pick type C disease.
Although clinically heterogeneous, several ‘red flag’ signs and symptoms exist, which should alert clinicians towards suspecting and investigating for the presence of the disorder. The differential diagnosis is often broad and includes other lysosomal storage disorders that also need to be considered early on. In any case, there should be a low threshold in referring suspected cases to specialist clinics. Genetic and biomarker analysis can be performed early on in the diagnostic pathway, provided these tests are available to the clinician. Positive biomarker results should prompt NPC1 and NPC2 gene testing. Identification of two known pathogenic mutations (one on each allele) via sequencing confirms the diagnosis, while studies looking for exon or whole gene deletions might also prove diagnostic in a few cases. In clinically suspicious cases of inconclusive genotype, other lysosomal storage disorders should be excluded and other tests (such as filipin staining or advanced genotypic studies) might be considered.
+/-, test might need to be considered; CGH, comparative genomic hybridization; DNA, deoxyribonucleic acid; GD, Gaucher disease; MLPA, multiplex ligation-dependent probe amplification; mRNA, messenger ribonucleic acid; NP-A, Niemann-Pick type A; NP-B, Niemann-Pick type B; NP-C, Niemann-Pick type C.
There are currently several tools available online for clinicians which can aid in identifying patients who may warrant further investigation, such as the NP-C Suspicion Index Tool [Wijburg et al. 2012] (http://www.npc-si.com). The tool generates a risk prediction score based on the presence of specific clinical manifestations and family history, and provides a strong indication of those patients who should be referred for further investigations. Its application in paediatric populations has shown limited utility in younger patients (less than 4 years of life) and is unlikely to help in diagnosing disease at an early stage when symptoms are nonspecific [Wraith et al. 2014]. Overall, there should be a low threshold for referring suspected cases to appropriate specialist neurometabolic services where available [Wraith et al. 2009; Patterson et al. 2012], not only to establish or refute the diagnosis but also to ensure optimal utilization of diagnostic testing and appropriate multidisciplinary management if the diagnosis is reached.
Laboratory testing
Laboratory-based diagnostic tests are essential for diagnosing NP-C. Below, we focus on the genetic and biochemical diagnostic approaches that can be applied when NP-C is suspected and also provide a suggested algorithm, which is summarized in Figure 1. The algorithm is modifiable according to each test’s availability, with clinicians able to prioritize some tests compared with others if more readily accessible (and/or less costly) in their practice. The appropriate use of these tests will help in prompt patient identification and treatment initiation.
Routine/nonspecific laboratory testing
Commonly performed laboratory routine tests in patients suspected with NP-C include full blood count, renal and liver function tests, plasma lipids and unconjugated bilirubin levels. These often yield unremarkable results. However, mild thrombocytopenia can be seen in patients with hypersplenism and liver function can be deranged in patients with cholestatic liver disease. Although plasma transaminases are generally normal, aspartate aminotransferase elevation and low high-density lipoprotein (HDL) cholesterol have been reported [Wraith et al. 2009].
Although the chitotriosidase assay has previously been proposed as a screening test for NP-C, this marker is, however, neither specific nor sensitive for NP-C [Ries et al. 2006]. Chitotriosidase is mildly elevated in many cases of NP-C but normal values are also possible [McKay Bounford and Gissen, 2014], whereas in other lysosomal storage disorders, such as Gaucher disease (GD) and Niemann-Pick type A (NB-A) and B (NP-B), very high levels are usually seen [Guo et al. 1995; Wajner et al. 2004]. The presence of elevated chitotriosidase would have to be paired with (hepato)splenomegaly and normal activities of acid sphingomyelinase (excluding NP-A and NP-B) and β-glucocerebrosidase (excluding GD), for NP-C to be further considered [McKay Bounford and Gissen, 2014]. In addition, 10% of the population has a pseudodeficiency mutation, which makes this test uninterpretable [Ries et al. 2006].
Genetic testing
NP-C is inherited in an autosomal recessive manner. NPC1 is located on chromosome 18q11-q12 [Carstea et al. 1997], whereas NPC2 maps to chromosome 14q24.3 [Naureckiene et al. 2000]. NPC1 gene mutations are present in 95% of cases and NPC2 mutations are present in approximately 4%; the remainder of patients are biochemically proven cases who do not have identified mutations [Millat et al. 2001a; Wraith et al. 2009; Patterson et al. 2012]. More than 350 different NPC1 and NPC2 gene mutations have been reported in patients with confirmed diagnoses [McKay Bounford and Gissen, 2014].
Although often not done as an initial test (or traditionally requested to support suspicious biochemical/ cell biology results), genetic testing is paramount in the NP-C diagnostic process. The identification of two alleles with demonstrated disease-causing mutations in NPC1 or NPC2 is currently considered definitive proof of an NP-C diagnosis. For example, genetic analysis is necessary to confirm a diagnosis of NP-C following abnormal results with disease biomarkers, or inconclusive filipin staining results [Patterson et al. 2012; Vanier and Latour, 2015]. Identification of NPC1 or NPC2 mutations allows family studies, antenatal diagnosis and carrier testing to be performed [Patterson et al. 2012].
Previously uncommon, sequencing of both NPC1 and NPC2 genes has recently become more readily available as part of the routine diagnostic process and a greater proportion of patients are now diagnosed via genetic testing as a primary diagnostic tool [Patterson et al. 2012; Bauer et al. 2013]. The most frequently currently employed method of genetic analysis for NP-C is Sanger sequencing, which utilizes polymerase chain reaction (PCR) to target the 30 coding exons, and intron–exon boundaries, of the NPC1 and NPC2 genes. Alternatively, targeted next-generation sequencing (NGS) methods may be applied. NGS has the potential to reduce costs associated with genetic testing for NP-C, particularly if the genes concerned are included on a multigene panel along with other genes considered relevant to the patient phenotype [Bruce et al. 2010]. In general, sequence changes that result in the introduction of premature stop codons (i.e. nonsense, frameshift and conserved splice-site mutations) produce truncated mRNA species that are usually targeted for breakdown (nonsense-mediated decay; NMD) rather than translation into proteins. Sequence changes that result in small protein sequence changes (missense and in-frame mutations) may also be disease-causing. Lastly, sequencing may identify intronic sequence variants that can disrupt RNA splicing and lead to NMD. However, missense and intronic variants are not necessarily disease-causing and several such polymorphisms exist in NPC1 and NPC2 [McKay Bounford and Gissen, 2014].
Despite its promise as a diagnostic tool, genetic sequencing sometimes identifies mutations clearly associated with disease on only one allele or provides negative results [Vanier, 2010; Bauer et al. 2013], even in patients where there is strong clinical suspicion and/or supportive biochemical test results pointing towards NP-C. Additionally, many families have ‘private’ sequence variants that have not been reported/published elsewhere [McKay Bounford and Gissen, 2014]. Hence, in a number of suspected patients, confirmation of a diagnosis is not possible through genetic sequencing alone.
Inconclusive genotype in the presence of convincing NP-C clinical and laboratory phenotypic features raises the possibility that mutations in other, yet unidentified, genes could cause NP-C, which would further increase the complexity of the genotype–phenotype correlation. Moreover, deep intronic mutations or mutations in regulatory/promoter regions, not regularly identified through standard genetic sequencing techniques, may also result in aberrant protein expression. Indeed, mutations in NPC1 introns have been identified in patients with NP-C and have been found to result in splicing defects [Di Leo et al. 2004]. Therefore, sequencing of only exons and exon–intron boundaries would miss such mutations. Full genomic DNA sequencing is currently possible using NGS methods but this is, in turn, also likely to identify sequence variants that are difficult to interpret [Plon et al. 2008; Richards et al. 2015]. Therefore, this strategy should be limited to patients with a suggestive clinical and/or biochemical picture where routine sequencing has not identified two known pathogenic mutations. Investigations of such cases would sometimes also require RNA and protein studies and, as such, are often outside the scope of service laboratories. Additionally, genomic rearrangements, such as exonic deletions or whole gene deletions, are also reported to rarely cause NP-C. Although microarray comparative genomic hybridization (CGH) testing can pick up large deletions, testing for this type of mutations would generally require quantitative methodologies and is most frequently performed using multiplex ligation-dependent probe amplification (MLPA) [Patterson et al. 2012; McKay Bounford and Gissen, 2014]. In cases of missense or intronic changes of unknown significance, parental/familial DNA testing, the use of in silico protein and splicing prediction tools and adherence to recently published guidelines on variant interpretation [Houdayer et al. 2008; Min et al. 2015; Richards et al. 2015] may assist in assigning their pathogenicity.
Diagnostic biomarkers
Due to several scientific advances in recent years (mainly in mass spectrometry methods), a number of novel and cost-effective techniques have now become available for studying and diagnosing NP-C. These methods are much more convenient, less invasive and easier to perform compared to traditional techniques such as filipin staining, which requires a skin biopsy and specialist expertise, is time-consuming and expensive. Currently available (and perhaps other, yet to be discovered) biochemical markers could possibly substantially reduce diagnostic delays but also prove useful in establishing disease severity and monitoring disease progression and response to therapeutic interventions. Although still largely provided through specialist clinical and research mass spectrometry laboratories, these techniques are now becoming more readily available around the world. Due to their advantages, they have the potential to be used as first-line targeted tests, before genetic analysis, when a diagnosis of NP-C is suspected.
In view of the above, we propose that genotype and biochemical marker analysis (if available) may precede or, in the advent of convincing results, substitute other NP-C specific tests (like the filipin staining) in the clinicians’ diagnostic pathway, a summary of which is provided in Figure 1. The practical implementation of the algorithm is dependent on each test’s local availability.
Plasma oxysterols
In NP-C, unesterified cholesterol accumulates in the lysosomes but increased production of reactive oxygen species (ROS) secondary to oxidative stress also occurs [Tint et al. 1998; Reddy et al. 2006; Zhang et al. 2008; Zampieri et al. 2009]. ROS leads to conversion of unesterified cholesterol into cholesterol oxidation products (mainly cholestane-3β,5α,6β-triol and 7-ketocholesterol). After having been noted to be elevated in NPC1 mice models [Zhang et al. 2008], various studies have shown that plasma oxysterol levels are high in patients with NPC1 and NPC2 mutations [Porter et al. 2010; Jiang et al. 2011; Boenzi et al. 2014; Zhang et al. 2014; Reunert et al. 2015]. There seems to be clear discrimination between NP-C patients and controls, providing a high sensitivity for this assay, and cholestane-3β,5α,6β-triol might be better than 7-ketocholesterol in discriminating between the two groups [Porter et al. 2010; Jiang et al. 2011; Boenzi et al. 2014; Klinke et al. 2015]. However, high plasma oxysterol concentrations can also be found in unaffected NPC1 heterozygotes [Porter et al. 2010; Jiang et al. 2011] and in other disorders, including NP-A and NP-B [Lin et al. 2014; Klinke et al. 2015], lysosomal acid lipase deficiency [Reunert et al. 2014], peroxisomal disorders and Smith–Lemli–Opitz syndrome [Pajares et al. 2015]. Other difficulties encountered include the assay’s limited availability to general clinical practice; at present, oxysterol testing is only successfully performed by a limited number of specialized laboratories. Sample exposure to high temperatures, haemolysis and long transportation times from collection to the laboratory could result in erroneous (falsely elevated) test results due to oxidation [Helmschrodt et al. 2014].
Plasma lysosphingolipids
Apart from cholesterol, various other types of lipids accumulate in NP-C, including sphingomyelin, multiple glycosphingolipids and sphingosine [Lloyd-Evans and Platt, 2010]. Plasma lysosphingolipids, previously proven useful as disease biomarkers in Fabry disease and GD [Aerts et al. 2008; Dekker et al. 2011], have recently also been identified as potential diagnostic markers in NP-C [Bauer et al. 2013; Welford et al. 2014; Giese et al. 2015]. Plasma lysosphingomyelin (SPC) has been reported to have very high sensitivity and specificity in distinguishing between treatment-naïve NP-C patients and control subjects [Welford et al. 2014] and seems to be elevated in NP-C patients independent of age. However, some overlap between patients and controls can be seen in very few cases. More recently, another biomarker (lysosphingomyelin-509, with a similar structure to SPC) was suggested to be superior to SPC and oxysterols at differentiating patients with NP-C from healthy controls and NPC1 carriers [Giese et al. 2015]. Plasma oxysterols are similarly elevated in NP-A, NP-B and NP-C [Lin et al. 2014], whereas lysosphingolipids (and especially lysosphingomyelin-509) might be better in differentiating these disease pathologies. Therefore, lysosphingolipids pose as potentially very useful NP-C biochemical markers, while the prospect of screening for other sphingolipidoses (such as GD and Fabry disease) with the same assay makes their use even more alluring. However, data regarding the utility of lysosphingolipids in NP-C are still scarce and mostly based on retrospective studies; hence, the assay’s exact sensitivity and specificity still needs to be established.
Urine bile acids
High urinary concentrations of 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholen-24-oic acid (SNAG-Δ5−CA) (and its glycine- and taurine- amides) in NP-C patients compared with healthy controls have recently been identified [Alvelius et al. 2001; Maekawa et al. 2013]. Other studies have demonstrated that di-docosahexaenoyl (22:6) bis(monoacylglycerol) phosphate (di-22:6-BMP), a marker of drug-induced phospholipidosis [Baronas et al. 2007; Thompson et al. 2012], also appears in high urinary concentrations in NP-C patients compared with controls [Liu et al. 2014]. These findings suggest that urinary bile acids may be another potentially powerful biomarker in the detection of NP-C. Again, potential benefits include the test’s noninvasive nature and cost-effectiveness compared to more traditional diagnostic methods. At present, though, minimal data is available regarding these markers’ sensitivity, specificity and ability to discriminate between NP-C patients, controls and carriers (as well other disorders) and further research into urinary (and plasma) bile acids is warranted.
Filipin staining (and cholesterol esterification test)
The demonstration of impaired intracellular cholesterol transport and homeostasis has traditionally been considered the ‘gold standard’ diagnostic test for NP-C [Pentchev et al. 1985; Vanier et al. 1991; Wraith et al. 2009; Vanier, 2010]. The test involves a skin biopsy, after which cultured skin fibroblasts are exposed to an low-density lipoprotein (LDL) enriched medium for 24 hours. Filipin, a fluorescent chemical compound isolated from Streptomyces filipinensis that specifically binds to unesterified cholesterol [Bornig and Geyer, 1974], is subsequently used to measure sequestration and accumulation of cholesterol in the fibroblasts using fluorescence microscopy. In normal fibroblasts, fewer than 10% of cells would be expected to stain positive for filipin. In contrast, due to cholesterol accumulation, skin fibroblasts from 80–85% of patients with NP-C demonstrate numerous strongly fluorescent (cholesterol-filled) perinuclear vesicles in more than 80% of cells. A smaller proportion of NP-C patients are found to have less obvious filipin staining and are described as having a ‘variant’ phenotype. ‘Variant’ profiles have been linked to specific mutations in NPC1 [Millat et al. 2001b; Sevin et al. 2007]. It has also been suggested that ‘variant’ filipin staining is more commonly associated with adult-onset disease or might point towards overall milder disease severity and slower progression rate [Sevin et al. 2007; Walterfang And Velakoulis, 2010]. Moreover, an abnormal filipin test can also be encountered in heterozygote carriers and in a number of other disorders (including MEGDEL syndrome, Smith–Lemli–Opitz syndrome and Tangier disease), whereas a variant phenotype, suggestive of ‘possible NP-C’, can also be seen in patients with NP-A or NP-B [Vanier, 1997, 2010; Wortmann et al. 2012; Platt et al. 2014; Sechi et al. 2014; Vanier and Latour, 2015]. All the above can complicate the diagnostic process.
The Cholesterol Esterification Test is based on the inability of NP-C cells to traffic cholesterol from the endolysosomal compartment to the endoplasmic reticulum where cholesterol is esterified. Deficient cholesterol esterification can be detected by measuring cholesteryl oleate formation after the LDL challenge, in the presence of [3H]oleic acid [Vanier et al. 1991]. Overall, the assay is labour-intensive and has reduced sensitivity compared with filipin staining; thus it is no longer performed in most diagnostic laboratories.
Currently available treatments for NP-C
In the absence of a disease cure, management approaches in NP-C focus on trying to delay the onset and progression of symptoms, but also on their early recognition and appropriate multidisciplinary management. Due to the disease’s rarity, care is often led or coordinated by large academic centres with the appropriate staff and expertise; clinicians are encouraged to refer NP-C patients to such establishments, especially when there is lack of resources to deal with the multitude of issues these patients are likely to encounter. Management strategies are similar to other progressive neurodegenerative disorders and based on close collaboration between healthcare professionals to achieve holistic care. This includes: neurological management of extrapyramidal and pyramidal disorders, seizures and sleep disturbance; appropriate psychiatric input for behavioural concerns and neuropsychiatric symptoms; pain management; management of gastrointestinal (GI) issues such as constipation, gastro-oesophageal reflux and swallowing difficulties; regular assessment of feeding and nutritional status; appropriate orthopaedic management of secondary complications (contractures, hip dislocation, kyphoscoliosis); preventative measures and prompt treatment of respiratory complications; physiotherapy, occupational therapy, speech and language therapy; input from psychology and social services when needed; and genetic counselling for family members, as appropriate.
Substrate reduction therapy
N-butyldeoxynojirimycin (miglustat) (Zavesca®, Actelion Pharmaceuticals Ltd) is a small imino-sugar that partially inhibits glucosylceramide synthase and the synthesis of all glucosylceramide-based glycosphingolipids. Miglustat was initially approved for the treatment of GD type I [Elstein et al. 2007; Abian et al. 2011; Shemesh et al. 2015]. After evidence of benefit in animal models of NP-C [Zervas et al. 2001; Stein et al. 2012], and following several clinical trials [Patterson et al. 2007, 2010; Wraith et al. 2010], miglustat has been approved for the treatment of neurological manifestations in paediatric and adult NP-C patients in the European Union and a number of other countries. However, its use for NP-C disease remains off-label in the USA. Recent studies suggested that miglustat has a beneficial effect on dysphagia, a major factor of mortality and morbidity in NP-C [Walterfang et al. 2012]. In a longitudinal study examining data from the International Registry for NP-C disease, the clinical severity composite scale (a disease-specific scale based on ambulation, manipulation, language and swallowing which is used as a marker of disease severity and neurodisability status in many centres) [Iturriaga et al. 2006; Yanjanin et al. 2010] remained stable or even improved in the majority of patients who received continuous miglustat therapy for a period of approximately 2 years [Patterson et al. 2015]. This effect was more pronounced in patients with later onset of neurological manifestations compared with others with onset during infancy or childhood.
Miglustat side effects include GI disturbances such as diarrhoea, flatulence, abdominal discomfort, nausea and vomiting. These are the most frequent adverse effects associated with miglustat therapy and are present in up to 80% of patients during the first 6 months compared with 50–60% in the ensuing period [Remenova et al. 2015]. GI disturbances have been reported as the most common reason for miglustat discontinuation [Hollak et al. 2009; Belmatoug et al. 2011]. These are caused by inhibition of intestinal disaccharidase enzymes leading to suboptimal hydrolysis of carbohydrates and osmotic diarrhoea, whereas subsequent bacterial fermentation of unabsorbed material leads to flatulence, abdominal distension and discomfort. Transient weight decrease, presumably due to carbohydrate malabsorption, is also often reported in the first few weeks of treatment. Dietary modifications such as reduced sucrose, maltose and lactose consumption have been shown to improve the drug’s GI tolerability and ameliorate changes in body weight, particularly if initiated at or before the start of therapy. Gradual dose escalation at treatment initiation may also reduce GI disturbances. Recently, a randomized double-blind, placebo-controlled study pointed towards increased miglustat GI tolerability when administered in conjunction with Saccharomyces bulardii probiotics, as the yeast produces an enzyme that breaks down sucrose [Remenova et al. 2015]. Other, less commonly reported miglustat-associated side effects include tremor and peripheral neuropathy; however, these can also feature in the disease even without miglustat administration [Hollak et al. 2009; Patterson et al. 2015].
Acetyl-DL-leucine
Acetyl-DL-leucine, a modified amino acid was administered to 12 NP-C patients aged from 13 to 32 years in a recent prospective, open-label study [Bremova et al. 2015]. A month after treatment, the Assessment and Rating of Ataxia (SARA) scale [Schmitz-Hubsch et al. 2006; Subramony, 2007], as well as dexterity in the dominant hand, showed an improvement of statistical significance. This improvement in cerebellar signs and symptoms led to reduction in the composite disease severity score used [Pineda et al. 2010], but also improvement in quality of life for patients and family members, as assessed by a questionnaire. Adverse effects in the form of transient dizziness were reported in 1/12 patients. Hence, acetyl-DL-leucine has the potential of being a well-tolerated adjunctive therapy for cerebellar signs and symptoms in NP-C.
Other therapeutic compounds with no established benefit and treatments under development
Various compounds have been tested in NP-C animal and cell models, a few having shown potential that has not translated to clinical practice.
Cholesterol-lowering agents such as cholestyramine, lovastatin and nicotinic acid, as well as a low cholesterol diet, are ineffective in altering the neurological course in NP-C disease [Patterson et al. 1993; Somers et al. 2001].
Curcumin, the active ingredient of turmeric, has been shown to elevate cytosolic calcium in vitro, normalize NPC1 disease cellular phenotypes and prolong survival of NP-C mice [Lloyd-Evans et al. 2008]. However, other studies suggested the lack of curcumin’s efficiency in preventing neurodegeneration in mice [Borbon et al. 2012].
Histone deacetylase (HDAC) inhibitors have shown promise in correcting cholesterol storage defects in human NPC1 mutant cells by increasing expression of the low activity mutant NPC1 protein [Munkacsi et al. 2011; Pipalia et al. 2011]. HDACs are part of a vast family of enzymes that have crucial roles in numerous biological processes, largely through their repressive influence on transcription; hence, HDAC inhibitors have long been used in psychiatry and various brain disorders and are being investigated as possible treatments for several neurological diseases[Kazantsev and Thompson, 2008; Haberland et al. 2009]. LBH589 (panobinostat) and vorinostat, orally available HDAC inhibitors that cross the blood–brain barrier and have been trialled for oncological disorders, seem to restore cholesterol homeostasis in cultured NPC1 mutant fibroblasts [Pipalia et al. 2011; Helquist et al. 2013]. Again, no data deriving from human studies are currently available; however a phase I/II clinical trial of vorinostat is currently in progress [ClinicalTrials.gov identifier: NCT02124083].
Arimoclomol induces expression of a group of chaperone proteins called heat shock proteins (HSPs). The heat shock response is an endogenous cytoprotective mechanism responsible for inducing the synthesis of HSPs. Under stress conditions, upregulation of certain classes of HSPs has been shown to protect cells from excessive cellular damage, thereby providing a natural cellular protection system. HSP-enabled prevention of protein misfolding, aggregation and abnormal degradation has been found to be neuroprotective in a number of neurodegenerative disease models including amyotrophic lateral sclerosis [Kalmar et al. 2014]. HSP upregulation was also seen to ameliorate intracellular lipid accumulation in NP-C cells [Kirkegaard et al. 2010]. A prospective non-therapeutic observational study in NP-C patients is currently underway and will be followed by enrolment into a phase II/III study [ClinicalTrials.gov identifier: NCT02435030].
Neurosteroids, particularly allopregnanolone, were shown in mouse models to increase lifespan, delay the onset of neurological symptoms, and result in neuronal survival and reduction of cholesterol and ganglioside accumulation [Griffin et al. 2004]. Enhanced myelination and improvement of autophagic/lysosomal function were also shown [Mellon et al. 2008; Liao et al. 2009]. Studies on human fibrobasts suggested that allopregnanolone’s effect might be due to reduction of oxidative stress [Zampieri et al. 2009]. However, no data on the effect of neurosteroids in human subjects currently exist.
2-Hydroxypropyl-beta-cyclodextrin (HPBCD) is a cyclic oligosaccharide with a hydrophilic exterior and a hydrophobic interior, enhancing the solubility of poorly water-soluble compounds (such as cholesterol) via formation of compound-cyclodextrin complexes. Through this structure, it acts as an excipient [Loftsson and Brewster, 2012], helping molecules cross membranes. Initially, allopregnanolone, complexed with hydroxypropyl-β-cyclodextrin (HPBCD), was deemed beneficial in Npc1−/− mice [Griffin et al. 2004]. However, subsequent studies showed that HPBCD alone was effective in reducing neurodegeneration, cholesterol and ganglioside storage and prolonging lifespan [Davidson et al. 2009; Liu et al. 2010]. Hence, cyclodextrins emerged as possible treatments for NP-C, as they have been shown in cell-based systems to mediate efflux of cholesterol from within the cell [Yancey et al. 1996; Atger et al. 1997]. The mechanism by which HPBCD decelerates the neurodegenerative process is unclear. HPBCD does not cross the blood–brain barrier [Pontikis et al. 2013] and is most active in slowing disease progression and clearing cholesterol from the brain if injected intraventricularly in mice [Aqul et al. 2011]. Lately, studies on a feline model [Vite et al. 2015] showed that subcutaneous HPBCD administration ameliorated hepatic disease, but doses sufficient to affect the central nervous system resulted in pulmonary toxicity. In contrast, when administered into the cisterna magna, HPBCD prevented the onset of cerebellar dysfunction in presymptomatic cats for more than 12 months and resulted in a reduction in Purkinje cell loss and near normal concentrations of cholesterol and sphingolipids. Administration of intracisternal HPBCD to NP-C cats with ongoing cerebellar dysfunction slowed disease progression, increased survival time and decreased the accumulation of brain gangliosides. An increase in hearing threshold was identified as an adverse effect. Intravenous HPBCD was trialled on two NP-C patients [Matsuo et al. 2013] and had little or no effect in controlling or delaying neurological symptoms, although it resulted in improvement of liver size and function. A 12-year old patient with NPC1 was recently given HPBCD intrathecally every 2 weeks and response was assessed after 27 injections [Maarup et al. 2015]. The NP-C severity scale score improved over 18 months, with all neurological functions remaining stable and a 1-point improvement shown in eye movements. High frequency hearing loss was observed bilaterally, investigators attributing this to the study drug rather than disease progression. More studies to further evaluate the safety and efficacy of HPBCD in NP-C patients are underway [ClinicalTrials.gov identifier: NCT01747135].
Conclusion
NP-C is a devastating neurodegenerative disorder with heterogeneous presentation. Its rarity, heterogeneity and, at least until recently, the lack of easy-to-perform, accurate and cost-effective diagnostic tests, often lead to severe delays from symptom onset to diagnosis. Diagnosing NP-C early is critical in view of substrate reduction therapies that can decelerate disease progression, but also because of promising new therapeutic approaches currently being developed. Recent advances in gene sequencing and mass spectrometry methods have resulted in tests that are highly specific, sensitive and less invasive than others previously considered to be gold standards for diagnosing NP-C. These assays promise to reduce diagnostic delays and enable instigation of treatment as soon as possible. However, these techniques are not readily available to all clinicians around the world or can only be accessed through research laboratories. Hence, raised clinical awareness, as well as low threshold for referring patients with suspected NP-C to specialized centres, is warranted, whereas consulting available diagnostic and management recommendations [Patterson et al. 2012] should also be considered. In the absence of a cure, therapeutic approaches focus on symptom control and aim to maintain neurological function as long as possible; however, eventual neurological regression and a limited lifespan are currently inevitable. While many clinical trials are currently underway, more research is needed in elucidating the disease’s underlying pathogenetic mechanisms, identifying potential novel therapeutic compounds and also discovering new biochemical markers that can enhance the monitoring of disease progression and response to therapeutic interventions.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.P. receives research funds from Actelion to study biomarkers in neurometabolic disorders including NP-C. P.G. is a scientific advisory board member of Vtesse and has received research grants from Actelion.
References
- Abian O., Alfonso P., Velazquez-Campoy A., Giraldo P., Pocovi M., Sancho J. (2011) Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase. Mol Pharm 8: 2390–2397. [DOI] [PubMed] [Google Scholar]
- Aerts J., Groener J., Kuiper S., Donker-Koopman W., Strijland A., Ottenhoff R., et al. (2008) Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A 105: 2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvelius G., Hjalmarson O., Griffiths W., Bjorkhem I., Sjovall J. (2001) Identification of unusual 7-oxygenated bile acid sulfates in a patient with Niemann-Pick disease, type C. J Lipid Res 42: 1571–1577. [PubMed] [Google Scholar]
- Aqul A., Liu B., Ramirez C., Pieper A., Estill S., Burns D., et al. (2011) Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J Neurosci 31: 9404–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atger V., De La Llera Moya M., Stoudt G., Rodrigueza W., Phillips M., Rothblat G. (1997) Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J Clin Invest 99: 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronas E., Lee J., Alden C., Hsieh F. (2007) Biomarkers to monitor drug-induced phospholipidosis. Toxicol Appl Pharmacol 218: 72–78. [DOI] [PubMed] [Google Scholar]
- Bauer P., Balding D., Klunemann H., Linden D., Ory D., Pineda M., et al. (2013) Genetic Screening for Niemann-Pick disease type C in adults with neurological and psychiatric symptoms: findings from the ZOOM study. Hum Mol Genet 22: 4349–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmatoug N., Burlina A., Giraldo P., Hendriksz C.J., Kuter D., Mengel E., et al. (2011) Gastrointestinal disturbances and their management in miglustat-treated patients. J Inherit Metab Dis 34: 991–1001. [DOI] [PubMed] [Google Scholar]
- Boenzi S., Deodato F., Taurisano R., Martinelli D., Verrigni D., Carrozzo R., et al. (2014) Anew simple and rapid LC-ESI-MS/MS method for quantification of plasma oxysterols as dimethylaminobutyrate esters. Its successful use for the diagnosis of Niemann-Pick type C disease. Clin Chim Acta 437C: 93–100. [DOI] [PubMed] [Google Scholar]
- Borbon I., Hillman Z., Duran E., Jr., Kiela P.R., Frautschy S., Erickson R. (2012) Lack of efficacy of curcumin on neurodegeneration in the mouse model of Niemann-Pick C1. Pharmacol Biochem Behav 101: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornig H., Geyer G. (1974) Staining of Cholesterol with the fluorescent antibiotic ‘filipin’. Acta Histochem 50: 110–115. [PubMed] [Google Scholar]
- Bremova T., Malinova V., Amraoui Y., Mengel E., Reinke J., Kolnikova M., et al. (2015) Acetyl-DL-leucine in Niemann-Pick type C: a case series. Neurology 85: 1368–1375. [DOI] [PubMed] [Google Scholar]
- Bruce C., Smith M., Rahman F., Liu Z., McMullan D., Ball S., et al. (2010) Design and validation of a metabolic disorder resequencing microarray (BRUM1). Hum Mutat 31: 858–865. [DOI] [PubMed] [Google Scholar]
- Carstea E., Morris J., Coleman K., Loftus S., Zhang D., Cummings C., et al. (1997) Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277: 228–231. [DOI] [PubMed] [Google Scholar]
- Davidson C., Ali N., Micsenyi M., Stephney G., Renault S., Dobrenis K., et al. (2009) Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One 4: e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker N., Van Dussen L., Hollak C., Overkleeft H., Scheij S., Ghauharali K., et al. (2011) Elevated plasma glucosylsphingosine in Gaucher disease: relation to phenotype, storage cell markers, and therapeutic response. Blood 118: e118–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leo E., Panico F., Tarugi P., Battisti C., Federico A., Calandra S. (2004) A point mutation in the lariat branch point of intron 6 of NPC1 as the cause of abnormal pre-mRNA splicing in Niemann-Pick type C disease. Hum Mutat 24: 440. [DOI] [PubMed] [Google Scholar]
- Elstein D., Dweck A., Attias D., Hadas-Halpern I., Zevin S., Altarescu G., et al. (2007) Oral maintenance clinical trial with miglustat for type I Gaucher disease: switch from or combination with intravenous enzyme replacement. Blood 110: 2296–2301. [DOI] [PubMed] [Google Scholar]
- Giese A., Mascher H., Grittner U., Eichler S., Kramp G., Lukas J., et al. (2015) A Novel, highly sensitive and specific biomarker for Niemann-Pick type C1 disease. Orphanet J Rare Dis 10: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin L., Gong W., Verot L., Mellon S. (2004) Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med 10: 704–711. [DOI] [PubMed] [Google Scholar]
- Guo Y., He W., Boer A., Wevers R., De Bruijn A., Groener J., et al. (1995) Elevated plasma chitotriosidase activity in various lysosomal storage disorders. J Inherit Metab Dis 18: 717–722. [DOI] [PubMed] [Google Scholar]
- Haberland M., Montgomery R., Olson E. (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmschrodt C., Becker S., Thiery J., Ceglarek U. (2014) Preanalytical standardization for reactive oxygen species derived oxysterol analysis in human plasma by liquid chromatography-tandem mass spectrometry. Biochem Biophys Res Commun 446: 726–730. [DOI] [PubMed] [Google Scholar]
- Helquist P., Maxfield F., Wiech N., Wiest O. (2013) Treatment of Niemann-Pick type C disease by histone deacetylase inhibitors. Neurotherapeutics 10: 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollak C., Hughes D., Van Schaik I., Schwierin B., Bembi B. (2009) Miglustat (Zavesca) in type 1 Gaucher Disease: 5-year results of a post-authorisation safety surveillance programme. Pharmacoepidemiol Drug Saf 18: 770–777. [DOI] [PubMed] [Google Scholar]
- Houdayer C., Dehainault C., Mattler C., Michaux D., Caux-Moncoutier V., Pages-Berhouet S., et al. (2008) Evaluation of in silico splice tools for decision-making in molecular diagnosis. Hum Mutat 29: 975–982. [DOI] [PubMed] [Google Scholar]
- Iturriaga C., Pineda M., Fernandez-Valero E., Vanier M., Coll M. (2006) Niemann-Pick C disease in Spain: clinical spectrum and development of a disability scale. J Neurol Sci 249: 1–6. [DOI] [PubMed] [Google Scholar]
- Jiang X., Sidhu R., Porter F., Yanjanin N., Speak A., Te Vruchte D., et al. (2011) A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J Lipid Res 52: 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar B., Lu C., Greensmith L. (2014) The role of heat shock proteins in amyotrophic lateral sclerosis: the therapeutic potential of arimoclomol. Pharmacol Ther 141: 40–54. [DOI] [PubMed] [Google Scholar]
- Kazantsev A., Thompson L. (2008) Therapeutic Application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 7: 854–868. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T., Roth A., Petersen N., Mahalka A., Olsen O., Moilanen I., et al. (2010) HSP70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 463: 549–553. [DOI] [PubMed] [Google Scholar]
- Klinke G., Rohrbach M., Giugliani R., Burda P., Baumgartner M., Tran C., et al. (2015) LC-MS/MS based assay and reference intervals in children and adolescents for oxysterols elevated in Niemann-Pick diseases. Clin Biochem 48: 596–602. [DOI] [PubMed] [Google Scholar]
- Liao G., Cheung S., Galeano J., Ji A., Qin Q., Bi X. (2009) Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in NPC1-/- mouse brain. Brain Res 1270: 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N., Zhang H., Qiu W., Ye J., Han L., Wang Y., et al. (2014) Determination of 7-ketocholesterol in plasma by LC-MS for Rapid diagnosis of acid SMase-deficient Niemann-Pick disease. J Lipid Res 55: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Ramirez C., Miller A., Repa J., Turley S., Dietschy J. (2010) Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res 51: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Tengstrand E., Chourb L., Hsieh F. (2014) Di-22:6-bis(monoacylglycerol)phosphate: a clinical biomarker of drug-induced phospholipidosis for drug development and safety assessment. Toxicol Appl Pharmacol 279: 467–476. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E., Morgan A., He X., Smith D., Elliot-Smith E., Sillence D., et al. (2008) Niemann-Pick disease type C1 Is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med 14: 1247–1255. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E., Platt F. (2010) Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic 11: 419–428. [DOI] [PubMed] [Google Scholar]
- Loftsson T., Brewster M. (2012) Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J Pharm Sci 101: 3019–3032. [DOI] [PubMed] [Google Scholar]
- Maarup T., Chen A., Porter F., Farhat N., Ory D., Sidhu R., et al. (2015) Intrathecal 2-hydroxypropyl-beta-cyclodextrin in a single patient with Niemann-Pick C1. Mol Genet Metab 116: 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M., Misawa Y., Sotoura A., Yamaguchi H., Togawa M., Ohno K., et al. (2013) LC/ESI-MS/MS analysis of urinary 3β-sulfooxy-7β-N-acetylglucosaminyl-5-cholen-24-oic acid and its amides: new biomarkers for the detection of Niemann-Pick type C disease. Steroids 78: 967–972. [DOI] [PubMed] [Google Scholar]
- Matsuo M., Togawa M., Hirabaru K., Mochinaga S., Narita A., Adachi M., et al. (2013) Effects of cyclodextrin in two patients with Niemann-Pick type C disease. Mol Genet Metab 108: 76–81. [DOI] [PubMed] [Google Scholar]
- McKay Bounford K., Gissen P. (2014) Genetic and Laboratory diagnostic approach in Niemann Pick disease type C. J Neurol 261 Suppl 2: S569–S575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S., Gong W., Schonemann M. (2008) Endogenous and Synthetic neurosteroids in treatment of Niemann-Pick type C disease. Brain Res Rev 57: 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel E., Klunemann H., Lourenco C., Hendriksz C., Sedel F., Walterfang M., et al. (2013) Niemann-Pick disease type C symptomatology: an expert-based clinical description. Orphanet J Rare Dis 8: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millat G., Chikh K., Naureckiene S., Sleat D., Fensom A., Higaki K., et al. (2001a) Niemann-Pick disease type C: spectrum of HE1 mutations and genotype/phenotype correlations in the NPC2 group. Am J Hum Genet 69: 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millat G., Marcais C., Tomasetto C., Chikh K., Fensom A., Harzer K., et al. (2001b) Niemann-Pick C1 disease: correlations between NPC1 mutations, levels of NPC1 protein, and phenotypes emphasize the functional significance of the putative sterol-sensing domain and of the cysteine-rich luminal loop. Am J Hum Genet 68: 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min F., Wang S., Zhang L. (2015) Survey of Programs used to detect alternative splicing isoforms from deep sequencing data in silico. Biomed Res Int 2015: 831352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkacsi A., Chen F., Brinkman M., Higaki K., Gutierrez G., Chaudhari J., et al. (2011) An ‘exacerbate-reverse’ strategy in yeast identifies histone deacetylase inhibition as a correction for cholesterol and sphingolipid transport defects in human Niemann-Pick type C disease. J Biol Chem 286: 23842–23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naureckiene S., Sleat D., Lackland H., Fensom A., Vanier M., Wattiaux R., et al. (2000) Identification of HE1 as the second gene of Niemann-Pick C disease. Science 290: 2298–2301. [DOI] [PubMed] [Google Scholar]
- Pajares S., Arias A., Garcia-Villoria J., Macias-Vidal J., Ros E., De Las Heras J., et al. (2015) Cholestane-3β,5α,6β-triol: high levels in Niemann-Pick type C, cerebrotendinous xanthomatosis, and lysosomal acid lipase deficiency. J Lipid Res 56: 1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Di Bisceglie A., Higgins J., Abel R., Schiffmann R., Parker C., et al. (1993) The Effect of cholesterol-lowering agents on hepatic and plasma cholesterol in Niemann-Pick disease type C. Neurology 43: 61–64. [DOI] [PubMed] [Google Scholar]
- Patterson M., Hendriksz C., Walterfang M., Sedel F., Vanier M., Wijburg F. (2012) Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol Genet Metab 106: 330–344. [DOI] [PubMed] [Google Scholar]
- Patterson M., Mengel E., Vanier M., Schwierin B., Muller A., Cornelisse P., et al. (2015) Stable or improved neurological manifestations during miglustat therapy in patients from the International Disease Registry for Niemann-Pick disease type C: an observational cohort study. Orphanet J Rare Dis 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Mengel E., Wijburg F., Muller A., Schwierin B., Drevon H., et al. (2013) Disease and patient characteristics in NP-C patients: findings from an international disease registry. Orphanet J Rare Dis 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Vecchio D., Jacklin E., Abel L., Chadha-Boreham H., Luzy C., et al. (2010) Long-term miglustat therapy in children with Niemann-Pick disease type C. J Child Neurol 25: 300–305. [DOI] [PubMed] [Google Scholar]
- Patterson M., Vecchio D., Prady H., Abel L., Wraith J. (2007) Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol 6: 765–772. [DOI] [PubMed] [Google Scholar]
- Pentchev P., Comly M., Kruth H., Vanier M., Wenger D., Patel S., et al. (1985) A defect in cholesterol esterification in Niemann-Pick disease (type C) patients. Proc Natl Acad Sci U S A 82: 8247–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M., Perez-Poyato M., O’Callaghan M., Vilaseca M., Pocovi M., Domingo R., et al. (2010) Clinical Experience with miglustat therapy in pediatric patients with Niemann-Pick disease type C: A case series. Mol Genet Metab 99: 358–366. [DOI] [PubMed] [Google Scholar]
- Pipalia N., Cosner C., Huang A., Chatterjee A., Bourbon P., Farley N., et al. (2011) Histone deacetylase inhibitor treatment dramatically reduces cholesterol accumulation in Niemann-Pick type C1 mutant human fibroblasts. Proc Natl Acad Sci U S A 108: 5620–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt F., Wassif C., Colaco A., Dardis A., Lloyd-Evans E., Bembi B., et al. (2014) Disorders of cholesterol metabolism and their unanticipated convergent mechanisms of disease. Annu Rev Genomics Hum Genet 15: 173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plon S., Eccles D., Easton D., Foulkes W., Genuardi M., Greenblatt M., et al. (2008) Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat 29: 1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontikis C., Davidson C., Walkley S., Platt F., Begley D. (2013) Cyclodextrin alleviates neuronal storage of cholesterol in Niemann-Pick C disease without evidence of detectable blood-brain barrier permeability. J Inherit Metab Dis 36: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter F., Scherrer D., Lanier M., Langmade S., Molugu V., Gale S., et al. (2010) Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J., Ganley I., Pfeffer S. (2006) Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PLoS One 1: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenova T., Morand O., Amato D., Chadha-Boreham H., Tsurutani S., Marquardt T. (2015) A double-blind, randomized, placebo-controlled trial studying the effects of Saccharomyces boulardii on the gastrointestinal tolerability, safety, and pharmacokinetics of miglustat. Orphanet J Rare Dis 10: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reunert J., Kannenberg F., Fobker M., Marquardt T. (2014) Improved diagnostics of Niemann Pick type C by oxysterol analysis. Journal of Inherited Metabolic Disease 37: 22–185. [Google Scholar]
- Reunert J., Lotz-Havla A.S., Polo G., Kannenberg F., Fobker M., Griese M., et al. (2015) Niemann-Pick type C-2 disease: identification by analysis of plasma cholestane-3β,5α,6β-triol and further insight into the clinical phenotype. JIMD Rep 23: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., et al. (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M., Schaefer E., Luhrs T., Mani L., Kuhn J., Vanier M., et al. (2006) Critical assessment of chitotriosidase analysis in the rational laboratory diagnosis of children with Gaucher disease and Niemann-Pick disease type a/B and C. J Inherit Metab Dis 29: 647–652. [DOI] [PubMed] [Google Scholar]
- Schmitz-Hubsch T., Du Montcel S., Baliko L., Berciano J., Boesch S., Depondt C., et al. (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66: 1717–1720. [DOI] [PubMed] [Google Scholar]
- Sechi A., Dardis A., Zampieri S., Rabacchi C., Zanoni P., Calandra S., et al. (2014) Effects of miglustat treatment in a patient affected by an atypical form of Tangier disease. Orphanet J Rare Dis 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevin M., Lesca G., Baumann N., Millat G., Lyon-Caen O., Vanier M., et al. (2007) The adult form of Niemann-Pick disease type C. Brain 130: 120–133. [DOI] [PubMed] [Google Scholar]
- Shemesh E., Deroma L., Bembi B., Deegan P., Hollak C., Weinreb N., et al. (2015) Enzyme replacement and substrate reduction therapy for Gaucher disease. Cochrane Database Syst Rev 3: CD010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol J., Blanchette-Mackie J., Kruth H., Dwyer N., Amende L., Butler J., et al. (1988) Type C Niemann-Pick disease. lysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J Biol Chem 263: 3411–3417. [PubMed] [Google Scholar]
- Somers K., Brown D., Fulton R., Schultheiss P., Hamar D., Smith M., et al. (2001) Effects of dietary cholesterol restriction in a feline model of Niemann-Pick type C disease. J Inherit Metab Dis 24: 427–436. [DOI] [PubMed] [Google Scholar]
- Stein V., Crooks A., Ding W., Prociuk M., O’Donnell P., Bryan C., et al. (2012) Miglustat improves Purkinje cell survival and alters microglial phenotype in feline Niemann-Pick Disease type C. J Neuropathol Exp Neurol 71: 434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramony S. (2007) SARA – a new clinical scale for the assessment and rating of ataxia. Nat Clin Pract Neurol 3: 136–137. [DOI] [PubMed] [Google Scholar]
- Thompson K., Haskins K., Rosenzweig B., Stewart S., Zhang J., Peters D., et al. (2012) Comparison of the diagnostic accuracy of di-22:6-bis(monoacylglycerol)phosphate and other urinary phospholipids for drug-induced phospholipidosis or tissue injury in the rat. Int J Toxicol 31: 14–24. [DOI] [PubMed] [Google Scholar]
- Tint G., Pentchev P., Xu G., Batta A., Shefer S., Salen G., et al. (1998) Cholesterol and oxygenated cholesterol concentrations are markedly elevated in peripheral tissue but not in brain from mice with the Niemann-Pick type C phenotype. J Inherit Metab Dis 21: 853–863. [DOI] [PubMed] [Google Scholar]
- Vance J., Karten B. (2014) Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J Lipid Res 55: 1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier M. (1997) Phenotypic and genetic heterogeneity in Niemann-Pick disease type C: current knowledge and practical implications. Wien Klin Wochenschr 109: 68–73. [PubMed] [Google Scholar]
- Vanier M. (2010) Niemann-Pick disease type C. Orphanet J Rare Dis 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier M. (2015) Complex lipid trafficking in Niemann-Pick disease type C. J Inherit Metab Dis 38: 187–199. [DOI] [PubMed] [Google Scholar]
- Vanier M., Latour P. (2015) Laboratory diagnosis of Niemann-Pick disease type C: the filipin staining test. Methods Cell Biol 126: 357–375. [DOI] [PubMed] [Google Scholar]
- Vanier M., Millat G. (2003) Niemann-Pick disease type C. Clin Genet 64: 269–281. [DOI] [PubMed] [Google Scholar]
- Vanier M., Rodriguez-Lafrasse C., Rousson R., Gazzah N., Juge M., Pentchev P.G., et al. (1991) Type C Niemann-Pick disease: spectrum of phenotypic variation in disruption of intracellular ldl-derived cholesterol processing. Biochim Biophys Acta 1096: 328–337. [DOI] [PubMed] [Google Scholar]
- Vite C., Bagel J., Swain G., Prociuk M., Sikora T., Stein V., et al. (2015) Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann-Pick type C1 disease. Sci Transl Med 7: 276ra226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajner A., Michelin K., Burin M., Pires R., Pereira M., Giugliani R., et al. (2004) Biochemical characterization of chitotriosidase enzyme: comparison between normal individuals and patients with Gaucher and with Niemann-Pick diseases. Clin Biochem 37: 893–897. [DOI] [PubMed] [Google Scholar]
- Walterfang M., Chien Y., Imrie J., Rushton D., Schubiger D., Patterson M. (2012) Dysphagia as a Risk factor for mortality in Niemann-Pick disease type C: systematic literature review and evidence from studies with miglustat. Orphanet J Rare Dis 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M., Velakoulis D. (2010) Niemann-Pick disease type C in adulthood – a psychiatric and neurological disorder. European Psychiatric Review 3: 16–20. [Google Scholar]
- Wassif C., Cross J. (2016) High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet Med 18: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford R., Garzotti M., Marques Lourenco C., Mengel E., Marquardt T., Reunert J., et al. (2014) Plasma lysosphingomyelin demonstrates great potential as a diagnostic biomarker for Niemann-Pick disease type C in a retrospective study. PLoS One 9: e114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijburg F., Sedel F., Pineda M., Hendriksz C., Fahey M., Walterfang M., et al. (2012) Development of a suspicion index to aid diagnosis of Niemann-Pick disease type C. Neurology 78: 1560–1567. [DOI] [PubMed] [Google Scholar]
- Wortmann S., Vaz F., Gardeitchik T., Vissers L., Renkema G., Schuurs-Hoeijmakers J., et al. (2012) Mutations in the phospholipid remodeling gene SERAC1 impair mitochondrial function and intracellular cholesterol trafficking and cause dystonia and deafness. Nat Genet 44: 797–802. [DOI] [PubMed] [Google Scholar]
- Wraith J., Baumgartner M., Bembi B., Covanis A., Levade T., Mengel E., et al. (2009) Recommendations on the diagnosis and management of Niemann-Pick Disease type C. Mol Genet Metab 98: 152–165. [DOI] [PubMed] [Google Scholar]
- Wraith J., Sedel F., Pineda M., Wijburg F., Hendriksz C., Fahey M., et al. (2014) Niemann-Pick type C Suspicion Index Tool: analyses by age and association of manifestations. J Inherit Metab Dis 37: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith J., Vecchio D., Jacklin E., Abel L., Chadha-Boreham H., Luzy C., et al. (2010) Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metab 99: 351–357. [DOI] [PubMed] [Google Scholar]
- Yancey P., Rodrigueza W., Kilsdonk E., Stoudt G., Johnson W., Phillips M., et al. (1996) Cellular Cholesterol Efflux Mediated by Cyclodextrins. Demonstration of Kinetic Pools and Mechanism of Efflux. J Biol Chem 271: 16026–16034. [DOI] [PubMed] [Google Scholar]
- Yanjanin N., Velez J., Gropman A., King K., Bianconi S., Conley S., et al. (2010) Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet 153b: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri S., Mellon S., Butters T., Nevyjel M., Covey D., Bembi B., et al. (2009) Oxidative stress in NPC1 deficient cells: protective effect of allopregnanolone. J Cell Mol Med 13: 3786–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas M., Somers K., Thrall M., Walkley S. (2001) Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol 11: 1283–1287. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang Y., Lin N., Yang R., Qiu W., Han L., et al. (2014) Diagnosis of Niemann-Pick disease type C with 7-ketocholesterol screening followed by NPC1/NPC2 gene mutation confirmation in Chinese patients. Orphanet J Rare Dis 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Coleman T., Langmade S., Scherrer D., Lane L., Lanier M., et al. (2008) Niemann-Pick C1 protects against atherosclerosis in mice via regulation of macrophage intracellular cholesterol trafficking.J Clin Invest 118: 2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]