The Hippo signaling pathway is a central regulator of organ size in diverse animals from insects to mammals.1,2 Genetic perturbation of this pathway in mouse models results in massively enlarged organs accompanied by tumor formation.3-5 Given its essential role in normal growth control in animal development, one would predict that the Hippo pathway is a target of gene mutations in cancer. To date, the evidence supporting this hypothesis has been limited. In Journal of Clinical Oncology, Chen et al6 have begun to fill this knowledge gap by identifying a missense mutation in YAP (also known as YAP1 or YAP65), a key component of the Hippo pathway, as a germline risk allele for lung adenocarcinoma.
The Hippo signaling pathway was initially discovered as a growth-inhibitory mechanism in the fruit fly Drosophila melanogaster, a classic model organism for developmental biologists.7,8 In Drosophila, this pathway comprises several tumor suppressor proteins, including two kinases, Hippo (Hpo) and Warts (Wts), that signal through a core kinase cascade to converge on the phosphorylation and inactivation of an oncogene called Yorkie (Yki; Fig 1). Yki functions as a transcriptional coactivator for a DNA-binding transcription factor called Scalloped to facilitate the transcription of growth-promoting genes such as cell cycle regulators and antiapoptotic proteins.9-11 Hippo-mediated phosphorylation of Yki inactivates the growth-promoting activity of Yki by excluding the phosphorylated Yki from the nucleus.3
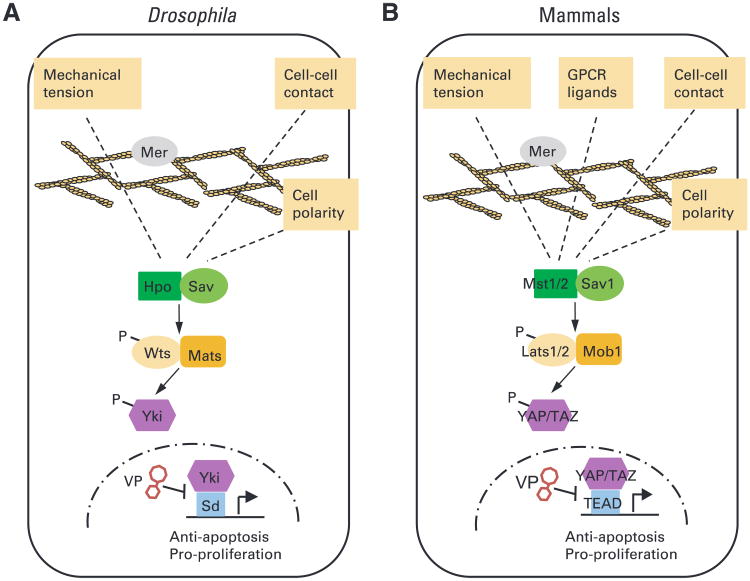
Fig 1.
The diagrams of the Hippo signaling pathway in (A) Drosophila melanogaster and (B) mammals highlight the core kinase cascade and upstream regulatory signals. For simplicity, most of the regulatory proteins upstream of the kinase cascade are not included in the diagram, except for Merlin (Mer), a membrane- and cytoskeleton-interacting protein that plays a conserved role in regulating Hippo signaling in both Drosophila and mammals. The illustration includes verteporfin (VP), a small-molecule inhibitor of Yki and YAP that disrupts the Yki-Sd (Drosophila) or YAP-TEAD (mammals) complex.8a In Drosophila, the core kinase cascade involves a kinase complex between Hippo (Hpo) and its partner Salvador (Sav), which phosphorylates and activates another kinase complex containing Warts (Wts) and its partner Mob as tumor suppressor (Mats). The activated Wts-Mats complex, in turn, phosphorylates and inactivates Yki. Only unphosphorylated Yki can enter the nucleus, where it partners with Scalloped (Sd) to activate the transcription of progrowth target genes. Loss of Hpo, Sav, Wts, or Mats results in constitutive nuclear localization of Yki, elevated expression of progrowth target genes, and tissue overgrowth. In mammals, the core kinase cascade comprises Mst1/2 (Hpo homologs), Sav1 (Sav homolog), Lats1/2 (Wts homolog), and Mob1 A/B (Mats homolog), which converge on the phosphorylation of YAP/TAZ (Yki homolog). Only unphosphorylated YAP/TAZ can enter the nucleus and partner with TEAD1/2/3/4 (Sd homolog) to activate the transcription of progrowth genes. Small-molecule inhibitors of YAP such as VP may be useful for the treatment of the subpopulation of patients with lung cancer who carry the R331W allele. GPCR, G protein-coupled receptor; P, phosphorylation.
The Hippo pathway is conserved in mammals wherein counterpart tumor suppressors function through a similar kinase cascade to inactivate YAP and a related protein called TAZ, which are the two mammalian counterparts of Yki3,12 (Fig 1). Recent studies suggest that the Hippo pathway is regulated by many biologic inputs such as cell polarity, adhesion and mechanical forces, and secreted ligands.13,14 Although the exact mechanisms by which these biologic inputs are modulated spatially and temporally to precisely terminate organ growth at appropriate size during development remain to be determined, it is known that developmental regulation of Hippo signaling in both Drosophila and mammals requires an upstream regulator called Merlin, a “4.1, ezrin, radixin, moesin” domain-containing adaptor protein localized to the cell cortex15-17 (Fig 1).
In both Drosophila and mice, inactivation of Hippo pathway tumor suppressors, or activation of the oncogene Yki/YAP, leads to tremendous tissue hyperplasia characterized by excessive cell proliferation and diminished apoptosis, two hallmarks of cancer. Indeed, in several mouse tissues, these genetic manipulations also result in tumorigenesis.3-5 In contrast to the spectacular phenotypes in animal studies, mutations in Mstl/2 and Latsl/2, the human counterparts of Hpo and Wts, respectively, are extremely rare in human cancers. Instead, these genes were reported to be silenced by hypermethylation in certain cancers.18-20 The only tumor suppressor related to the Hippo pathway that has been consistently linked to human cancer is the upstream regulator Merlin. Merlin, also called NF2, was discovered two decades ago as a tumor suppressor gene whose mutations cause neurofibromatosis 2, an inherited autosomal dominant disorder characterized by the development of schwannomas and meningiomas affecting the nervous system.21,22 Somatic mutations of NF2 are also frequently found in mesotheliomas.23 It is not immediately clear why mutations of the core components of the Hippo pathway have not been more frequently detected in human cancers. This could simply be a matter of statistical improbability. Unlike Drosophila, humans encode two homologues of Hpo (Mstl and Mst2) and Wts (Latsl and Lats2). Thus a human cell has to encounter four instead of two hits at the relevant genetic loci to abolish Hpo or Wts activity. In contrast, NF2 is the sole Merlin homolog in humans.
Although genetic redundancy may in principle account for the dearth of mutations in tumor suppressor genes of the Hippo pathway, gain-of-function mutations in the oncogenes of the pathway should not be subjected to the same constraints. Supporting this view, the YAP gene locus on human chromosome 11q22 is amplified in various tumors such as lung, pancreas, oral, esophagus, liver, and ovarian carcinomas.24-29 However, the frequency of YAP amplification in these tumors is relatively low (5% to 10%). To complicate matters further, the YAP gene locus was also reported to undergo frequent loss of heterozygosity in breast cancer.30 Indeed, although the prevailing view holds that YAP functions as a growth-promoting oncogene, YAP has also been proposed to function as a tumor suppressor gene in some contexts.30,31
Against this backdrop, the identification by Chen et al6 of an R331W missense mutation in YAP as a germline risk allele for lung adenocarcinoma is notable for several reasons. First and foremost, this information can be immensely valuable for early detection and disease prevention of lung adenocarcinoma. As beautifully illustrated by the authors, even though the R331W mutation is a rare allele, the high penetrance of mutant carriers to have lung adenocarcinoma and related lung lesions warrants the use of low-dose computed tomography scans as a preventive measure to this high-risk subpopulation.6 This practice allowed the authors to diagnose a stage I adenocarcinoma in one carrier who would otherwise become aware of the disease only at a much later stage. In addition, it provides unbiased clinical evidence that further implicate the Hippo signaling pathway as a cancer-relevant pathway. Finally, the dominant nature of the R331W mutation in increasing lung cancer risk and its gain-of-function activity in cellular assays provides further evidence supporting YAP as a bona fide oncogene and further validates the widespread interest of developing small-molecule inhibitors of YAP. Indeed, recent studies have demonstrated the proof of principle that YAP inhibitors can be successfully developed by identifying small molecules that disrupt the physical interaction between YAP and its transcription factor partner.8a Thus, YAP may be a promising and pharmacologically viable target for lung cancer prevention and treatment.
Like many good studies, the work of Chen et al6 raises interesting questions that warrant further investigation. Although the authors showed that the R331W missense mutation increases the colony formation ability and invasion potential of a lung cancer cell line in culture, the precise mechanism by which the R331W mutation confers predisposition to lung cancer remains unknown. Does the mutation increase the transcriptional activity, nuclear localization, or protein abundance of YAP? It is noteworthy that two patients who had lung cancer with the R331W allele in the Chen et al6 study also had breast cancer. A more systematic survey of the R331W carriers will be required to better appreciate the tissue-specific effect, or the lack thereof, of this allele in cancer predisposition. If the R331W allele predisposes patients to only lung adenocarcinoma but not other cancers, it will be extremely interesting to investigate how this mutation has such a selective effect on lung cancer development It was shown recently that YAP plays a critical role in the self-renewal of airway stem cells.32,33 Perhaps a better understanding of how the Hippo-YAP pathway is uniquely regulated in the lung progenitor cells may provide some insights into this question. We have now come full circle, in as much as developmental biology has informed cancer biology, our understanding of cancer genome landscapes presents a rich opportunity for deeper exploration of basic developmental processes.
Acknowledgments
Supported in part by Grants No. EY015708 and DK098424 from the National Institutes of Health and NF130090 and PR130920 from the Department of Defense.
Footnotes
Author's Disclosures of Potential Conflicts of Interest:Disclosures provided by the authors are available with this article at www.jco.org.
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Duojia Pan: No relationship to disclose
References
- 1.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haider G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HY, Yu SL, Ho BC, et al. R331W missense mutation of oncogene YAP1 is a germline risk allele for lung adenocarcinoma with medical actionability. J Clin Oncol. 2015;33:2303–2310. doi: 10.1200/JCO.2014.59.3590. [DOI] [PubMed] [Google Scholar]
- 7.Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 8a.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S, Liu Y, Zheng Y, et al. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Koontz LM, Liu-Chittenden Y, Yin F, et al. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Ren F, Zhang Q, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 15.Hamaratoglu F, Willecke M, Kango-Singh M, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin F, Yu J, Zheng Y, et al. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seidel C, Schagdarsurengin U, Blümke K, et al. Frequent hypermethylation of MST1 and MST2 in soft tissue sarcoma. Mol Carcinog. 2007;46:865–871. doi: 10.1002/mc.20317. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Li X, Hu J, et al. Promoter hypermethylation-mediated down-regulation of LATS1 and LATS2 in human astrocytoma. Neurosci Res. 2006;56:450–458. doi: 10.1016/j.neures.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi Y, Miyoshi Y, Takahata C, et al. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin Cancer Res. 2005;11:1380–1385. doi: 10.1158/1078-0432.CCR-04-1773. [DOI] [PubMed] [Google Scholar]
- 21.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neurofibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 22.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi AB, Mitsunaga SI, Cheng JQ, et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A. 1995;92:10854–10858. doi: 10.1073/pnas.92.24.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Z, Zhu WG, Morrison CD, et al. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis clAP1 and clAP2 as candidate oncogenes. Hum Mol Genet. 2003;12:791–801. doi: 10.1093/hmg/ddg083. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-L A, Northcott PA, Dalton J, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snijders AM, Schmidt BL, Fridlyand J, et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005;24:4232–4242. doi: 10.1038/sj.onc.1208601. [DOI] [PubMed] [Google Scholar]
- 27.Bashyam MD, Bair R, Kim YH, et al. Array-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancer. Neoplasia. 2005;7:556–562. doi: 10.1593/neo.04586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imoto I, Yang ZQ, Pimkhaokham A, et al. Identification of clAPI as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Res. 2001;61:6629–6634. [PubMed] [Google Scholar]
- 29.Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- 31.Barry ER, Morikawa T, Butler BL, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahoney JE, Mori M, Szymaniak AD, et al. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao R, Fallon TR, Saladi SV, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30:151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]