Abstract
Purpose
Metastatic pancreatic adenocarcinoma is considered a uniformly fatal disease with a median survival of 1 year with modern chemotherapy. While a subset of patients achieve prolonged survival, few of the factors that define this group of patients are known.
Methods
For the determination of overall survival (OS), 549 patients with histologically confirmed metastatic pancreatic adenocarcinoma were evaluated. Emphasis was placed on treatment history and family history of breast, ovarian, and pancreatic cancers. To ensure a uniform metastatic population, patients treated with prior locoregional therapies (i.e., surgery or radiotherapy) were excluded as were patients with a prior history of stage I–III disease.
Results
Patients with family history or pedigree history of cancer had superior OS. This was especially true in patients with three or more relatives with either breast, ovarian, or pancreatic cancers [hazard ratio (HR) 0.49, 95 % confidence interval (CI) 0.30–0.80, p = 0.003]. First-line platinum chemotherapy was associated with a poor survival (hazard ratio for death 1.74, 95 % CI 1.12–2.71, p = 0.01) for patients without a family history of these cancers but not for those without such a history (p = 0.31). In fact, as the number of relatives with these cancers increased, the OS survival improved for individuals receiving first-line platinum therapy (HR 0.76, 95 % CI 0.65–0.89, p = 0.0004), which was not the case for those receiving other therapies (p = 0.98).
Conclusions
Treatment with platinum chemotherapy in patients with a family history of breast, ovarian, or pancreatic cancers was associated with a longer survival, whereas platinum use in patients without such a family history of cancer was associated with poor survival. These findings suggest that family history may serve as a predictive marker for platinum use in patients with metastatic pancreatic adenocarcinoma.
Keywords: Pancreatic adenocarcinoma, Family history, BRCA, Chemotherapy, Survival
Introduction
Adenocarcinoma of the pancreas is a devastating disease with 45,000 cases expected in the USA in 2013. Greater than 80 % of patients have metastatic disease at time of diagnosis and have a median survival of 6 months. Until recently, the standard of care for metastatic disease had been the nucleoside analogue, gemcitabine [1]. However, this convention has been challenged by recent studies showing significant survival advantage with FOLFIRINOX (5-FU, irinotecan, and oxaliplatin) [2] and other combination chemotherapies [3–5].
Few individual patients enjoy a long-term survival benefit from chemotherapy. The clinical or molecular determinants that identify such patients are not known. However, several reports have suggested that cancers from patients with germ-line mutations in DNA repair pathways are highly sensitive to DNA-damaging agents [6–10]. The best described models show selective tumor killing with agents that generate DNA interstrand crosslinks (ICL) in tumors with loss of function mutations in the BRCA2/Fanconi Anemia pathway [11, 12]. Defects in this pathway have been well-described in familial breast, ovarian, and pancreatic cancer syndromes [13–21]. Clinical trials evaluating this approach, using ICL-inducing agents such as cisplatin or mitomycin C, have been challenging since patients with both germ-line mutations in BRCA2 and pancreatic cancer are rare.
To better define the subgroup of patients with long-term survival, we reviewed cases of metastatic pancreatic adenocarcinoma from our two institutions with an emphasis on a family history of tumors (breast, ovarian, and pancreatic) that might suggest defects in DNA repair susceptible to DNA-damaging agents such as platinum drugs and better define the ‘BRCAness’ subpopulation. We hypothesized that patients with this family history might have such defects (whether characterized or not) and would have preferential benefit from platinum-based therapy.
Methods
Study design
Patient records with a diagnosis of American Joint Committee on Cancer (AJCC) stage IV pancreatic cancer were identified from the local cancer registry at Johns Hopkins-affiliated hospitals (JHU) from 1995 to 2009 and from the M.D. Anderson Cancer Center (MDACC) tumor registries (2005–2009) and were reviewed for confirmation of clinical stage and treatment at initial presentation according to an IRB-approved protocol. The registry database contained variables reported to the local cancer registry. All patients treated with upfront modalities other than chemotherapy, such as radiation or surgery, were excluded by chart review. Patients with initial consultation records indicating non-metastatic or locally advanced disease at treatment were also excluded. Platinum chemotherapy was defined as cisplatin, carboplatin, or oxaliplatin. All pathology was reviewed centrally at Johns Hopkins or the M.D. Anderson Cancer Center. Information on grade was not routinely recorded since tissue diagnosis was made only by fine-needle aspiration sampling for some tumors.
Statistical analysis
The primary outcome variable was overall survival (OS), which was calculated as the time from pathologic diagnosis to date of death from the tumor registry database, chart review, or social security death index. Patients without confirmed deaths were censored at date of last contact. The number of family members diagnosed with pancreatic, breast, and ovarian cancers was recorded for each patient through first to third degree relatives (immediate family, grandparents, and first- and second-degree cousins). We compared demographic and clinical features between patient cohorts with Chi-square tests (or Fisher’s exact tests) for categorical variables and t tests (or Wilcoxon rank-sum tests) for continuous variables, as appropriate. Kaplan–Meier techniques were used to estimate the survivor function, percent surviving at 1 year, and the median time to death with 95 % confidence intervals. Differences between groups were assessed using the log-rank test. Cox proportional hazards models were used to estimate hazard ratios with 95 % confidence intervals as well as to compare groups in multivariate models, i.e., after adjusting for age, race, liver metastases, and cohort.
Results
To obtain a uniform population of patients with metastatic adenocarcinoma, charts from 1425 patients were initially screened at JHU and MDACC. Eight-hundred and seventy-six patients were excluded for histology other than adenocarcinoma, inappropriate staging of locally advanced tumors, initial treatment with cytoreductive surgery or chemoradiation, patients not treated with initial chemotherapy, or patients without family history data available.
A total of 549 individuals from Johns Hopkins and M.D. Anderson with metastatic pancreatic adenocarcinoma met the eligibility criteria (Table 1). Of the cases reviewed, 78 % had at least one family member diagnosed with cancer, while those with a family history of pancreatic, ovarian, or breast cancer represented 36 % of the cohort. A family history of pancreatic cancer specifically was seen in 15 % of the cohort. The clinical characteristics were in general well balanced between those individuals at both institutions. Exceptions were noted in race, the presence of lung and peritoneal metastases, and the year of diagnosis. In addition, platinum chemotherapy was more commonly utilized at M.D. Anderson (79 vs. 34 %). These differences may be explained by the increased use of platinum in recent years (p < 0.0001) and for individuals without liver metastases (p = 0.05) both of which were more common at M.D. Anderson. At Johns Hopkins, cisplatin was the most commonly used platinum (60 %) followed by oxaliplatin (37 %) and carboplatin (3 %). Similarly, at M.D. Anderson, patients were most commonly treated with cisplatin (60 %) followed by oxaliplatin (28 %) and carboplatin (2 %).
Table 1.
Comparison of patients in the Johns Hopkins and MD Anderson cohorts
| Overall (N = 549) | Johns Hopkins (N = 243) | MD Anderson (N = 306) | p value | |
|---|---|---|---|---|
| Age (years) | ||||
| Median (range) | 62 (30–89) | 62 (30–89) | 62 (34–87) | 0.30 |
| Gender | ||||
| Female | 235 (43 %) | 113 (47 %) | 122 (40 %) | 0.14 |
| Male | 314 (57 %) | 130 (53 %) | 184 (60 %) | |
| Race | ||||
| White | 452 (82 %) | 209 (86 %) | 243 (79 %) | 0.004 |
| Black | 57 (10 %) | 26 (11 %) | 31 (10 %) | |
| Other | 40 (7 %) | 8 (3 %) | 32 (10 %) | |
| Year of diagnosis | ||||
| 1995–1999 | 13 (2 %) | 13 (5 %) | 0 (0 %) | <0.0001 |
| 2000–2005 | 74 (13 %) | 65 (27 %) | 9 (3 %) | |
| 2005–2010 | 462 (84 %) | 165 (68 %) | 297 (97 %) | |
| Liver metastases | ||||
| No | 122 (22 %) | 45 (19 %) | 77 (25 %) | 0.064 |
| Yes | 427 (78 %) | 198 (81 %) | 229 (75 %) | |
| Lung metastases | ||||
| No | 434 (79 %) | 202 (83 %) | 232 (76 %) | 0.045 |
| Yes | 115 (21 %) | 41 (17 %) | 74 (24 %) | |
| Peritoneal metastases | ||||
| No | 437 (80 %) | 205 (84 %) | 232 (76 %) | 0.014 |
| Yes | 112 (20 %) | 38 (16 %) | 74 (24 %) | |
| Family history of cancera | ||||
| None | 122 (22 %) | 61 (25 %) | 61 (20 %) | 0.45 |
| Pancreatic | 57 (10 %) | 21 (9 %) | 36 (12 %) | |
| Breast or ovarian | 118 (21 %) | 48 (20 %) | 70 (23 %) | |
| Pancreatic and breast or ovarian | 25 (5 %) | 12 (5 %) | 13 (4 %) | |
| Other | 227 (41 %) | 101 (42 %) | 126 (41 %) | |
| Personal history of breast, ovarian, or pancreatic cancer | ||||
| No | 533 (97 %) | 237 (98 %) | 296 (97 %) | 0.62 |
| Yes | 16 (3 %) | 6 (2 %) | 10 (3 %) | |
| Platinum therapy | ||||
| None | 224 (41 %) | 160 (66 %) | 64 (21 %) | <0.0001 |
| First line | 258 (47 %) | 62 (26 %) | 196 (64 %) | |
| Second line | 67 (12 %) | 21 (9 %) | 46 (15 %) | |
Family history of cancer excludes personal history of cancer
Of the 243 patients treated at JHU, 160 (66 %) never received platinum therapy (Table 1), 62 (26 %) received it in the first line of therapy, and 21 (9 %) received second line platinum. At MDA, 64 (21 %) of 306 patients never received platinum, while 196 (64 %) and 46 (15 %) received it in the first and second lines of treatment, respectively. Of the 549 patients enrolled in the study, 488 (89 %) had died at the time our data were assembled and analyzed.
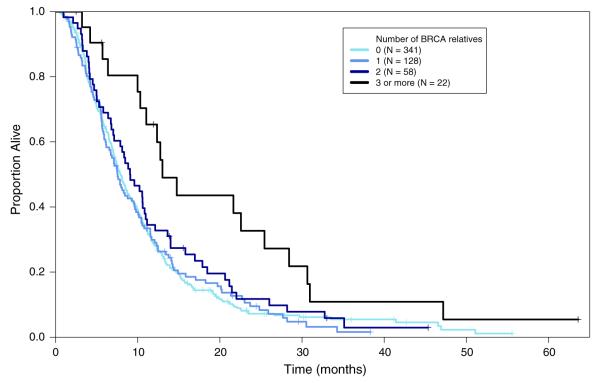
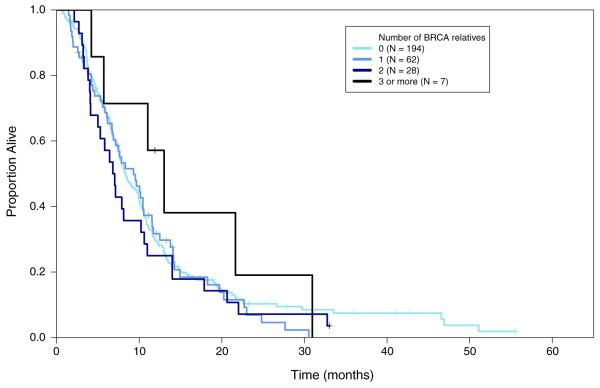
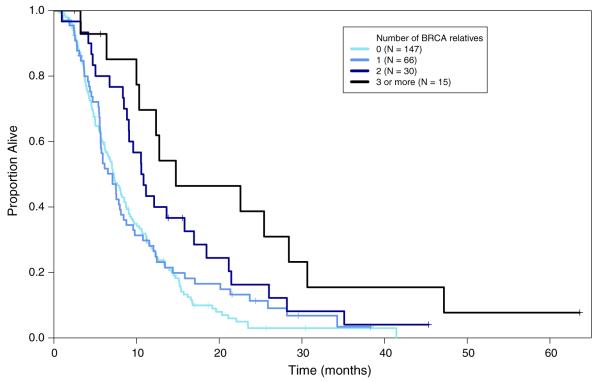
Overall the median survival (mOS) in the 549 individuals evaluated was 8.1 months (95 % CI 7.5–9.0) with 31 % being alive at 1 year. Univariate analysis of several potential prognostic variables (Table 2) revealed that the risk of death increased with African-American race (p = 0.008) and in patients with liver metastases (p = 0.003). Prolonged survival was observed in individuals with a family history of breast or ovarian cancers (mOS 8.5 months, HR 0.76, p = 0.042) and was most pronounced (mOS 14.8 months; HR 0.43; p = 0.0003) in patients with a family history of pancreatic cancer and breast or ovarian cancer. Survival was also strongly associated with the number of relatives with a BRCA-related malignancy (test of trend p = 0.009). Kaplan–Meier curves demonstrating survival for all patients (Fig. 1), patients without first-line platinum (Fig. 2), and patients who did receive first-line platinum (Fig. 3) are included.
Table 2.
Summary of risk factors associated with overall survival in the combined Johns Hopkins and MD Anderson cohorts
| Number at risk | Number of events | Median overall survival in months (95 % CI) |
Hazard ratio (95 % CI) | p values | p values test of trend | |
|---|---|---|---|---|---|---|
| Overall | 549 | 488 | 8.1 (7.5–9.0) | |||
| Age (years) | ||||||
| <50 | 70 | 59 | 9.7 (7.5–11.7) | 1.00 | 0.080 | |
| 50–59 | 147 | 132 | 8.5 (7.4–10.1) | 1.00 (0.73–1.37) | 0.99 | |
| 60–69 | 200 | 175 | 8.2 (7.0–9.9) | 1.08 (0.08–1.45) | 0.62 | |
| 70 or greater | 132 | 122 | 7.5 (6.1–8.6) | 1.25 (0.91–1.72) | 0.15 | |
| Gender | ||||||
| Female | 235 | 207 | 8.1 (7.4–9.8) | 1.00 | ||
| Male | 314 | 281 | 8.1 (7.1–9.1) | 1.04 (0.86–1.25) | 0.70 | |
| Race | ||||||
| White | 452 | 402 | 8.3 (7.6–9.4) | 1.00 | ||
| Black | 57 | 53 | 6.1 (5.0–10.0) | 1.48 (1.10–1.98) | 0.008 | |
| Other | 40 | 33 | 8.3 (6.4–11.2) | 1.01 (0.70–1.45) | 0.95 | |
| Year of diagnosis | ||||||
| 1995–1999 | 13 | 13 | 10.6 (8.5–Inf) | 1.00 | ||
| 2000–2005 | 74 | 74 | 8.0 (7.2–10.7) | 0.94 (0.52–1.71) | 0.84 | |
| 2005–2010 | 462 | 401 | 8.1 (7.3–9.1) | 0.99 (0.56–1.72) | 0.96 | |
| Liver metastases | ||||||
| No | 122 | 105 | 10.6 (9.0–12.6) | 1.00 | ||
| Yes | 427 | 383 | 7.6 (7.1–8.5) | 1.38 (1.11–1.72) | 0.003 | |
| Lung metastases | ||||||
| No | 434 | 387 | 8.1 (7.4–9.0) | 1.00 | ||
| Yes | 115 | 101 | 8.57 (7–10.61) | 0.97 (0.77–1.21) | 0.78 | |
| Peritoneal metastases | ||||||
| No | 437 | 389 | 8.1 (7.5–9.2) | 1.00 | ||
| Yes | 112 | 99 | 7.6 (6.4–10.6) | 1.00 (0.79–1.25) | 0.97 | |
| Family history of cancer | ||||||
| None | 122 | 109 | 7.5 (5.6–8.9) | 1.00 | ||
| Pancreatic | 57 | 53 | 7.1 (5.5–10.1) | 0.86 (0.61–1.20) | 0.37 | |
| Breast or ovarian | 118 | 102 | 8.5 (7.1–10.5) | 0.76 (0.57–1.00) | 0.042 | |
| Pancreatic and breast or ovarian |
25 | 22 | 14.8 (10.5–28.4) | 0.43 (0.26–0.68) | 0.0003 | |
| Other | 227 | 202 | 8.4 (7.3–9.9) | 0.75 (0.59–0.95) | 0.015 | |
| Personal history of breast, ovarian, or pancreatic cancer | ||||||
| No | 533 | 473 | 8.1 (7.4–9.0) | 1.00 | ||
| Yes | 16 | 15 | 10.6 (5.4–20.3) | 0.98 (0.58–1.64) | 0.93 | |
| Pedigree history of malignancya | ||||||
| No | 119 | 106 | 7.5 (5.6–8.90) | 1.00 | ||
| Other cancer | 222 | 197 | 8.4 (7.3–9.9) | 0.76 (0.59–0.97) | 0.022 | |
| BRCA-related | 208 | 185 | 8.8 (7.5–10.4) | 0.72 (0.56–0.92) | 0.008 | |
| Number of relatives with a breast, ovarian, or pancreatic | ||||||
| 0 | 341 | 303 | 7.9 (7.2–8.8) | 1.00 | 0.009 | |
| 1 | 128 | 113 | 7.6 (6.5–9.6) | 1.01 (0.81–1.26) | 0.92 | |
| 2 | 58 | 54 | 9.1 (7.1–11.2) | 0.85 (0.63–1.14) | 0.26 | |
| 3 or more | 22 | 18 | 13.0 (11.0–30.7) | 0.49 (0.30–0.80) | 0.004 | |
| Platinum therapy: First line | ||||||
| No | 291 | 259 | 8.3 (7.5–10.0) | 1.00 | ||
| Yes | 258 | 229 | 8.0 (7.1–9.1) | 1.04 (0.87–1.25) | 0.65 | |
After adjusting for age, African-American race, liver metastases, and cohort, only BRCA-related malignancies were significantly associated with overall survival (p = 0.488)
Fig. 1.
First-line platinum status: overall
Fig. 2.
First-line platinum status: none
Fig. 3.
First-line platinum status: present
The effect of first-line platinum treatment on survival was assessed across the different types of family history. Surprisingly, individuals without any family history of breast, ovarian, or pancreatic cancer (Table 3) fared substantially worse when treated with platinum chemotherapy as a first-line therapy (7.3 vs. 8.4 months; HR 1.42; p = 0.005). A significant decrease in survival was also noted when first-line platinum therapy was used in patients without a pedigree history (pedigree being comprised of both family history and personal history of cancer) of breast, ovarian, or pancreatic cancer (HR 1.39, p = 0.008). In both cases, the results were significantly different than those for individuals without such histories (p = 0.01 and p = 0.04, respectively, for interaction between family history and platinum variables) for whom there was no significant association between type of therapy and overall survival. The same pattern was observed for general family history, although the difference in the effect of first-line platinum therapy was not significant (test of interaction p = 0.10).
Table 3.
Interaction between family history of cancer and first-line platinum therapy
| Number at risk | Number of deaths |
Median overall survival (95 % CI) |
Adjusted hazard ratioa for stratified models (95 % CI) |
Adjusted stratified p valuea |
Adjusted inter- action p valuea |
|
|---|---|---|---|---|---|---|
| No family history of breast, ovarian, or pancreatic cancer | ||||||
| No first-line platinum | 198 | 176 | 8.4 (7.5–10.1) | 1 | 0.005 | 0.017 |
| First-line platinum | 151 | 135 | 7.3 (6.1–8.6) | 1.42 (1.11–1.80) | ||
| Family history of breast, ovarian, or pancreatic cancer | ||||||
| No first-line platinum | 93 | 83 | 7.9 (6.8–10.4) | 1 | 0.314 | |
| First-line platinum | 107 | 94 | 9.1 (7.5–11.5) | 0.85 (0.61–1.18) | ||
| No pedigree history of breast, ovarian, or pancreatic cancer | ||||||
| No first-line platinum | 194 | 172 | 8.3 (7.5–10.0) | 1 | 0.008 | 0.047 |
| First-line platinum | 147 | 131 | 7.3 (6.1–8.8) | 1.39 (1.08–1.77) | ||
| Pedigree history of breast, ovarian, or pancreatic cancer | ||||||
| No first-line platinum | 97 | 87 | 8.1 (6.8–10.5) | 1 | 0.474 | |
| First-line platinum | 111 | 98 | 8.8 (7.5–10.8) | 0.89 (0.64–1.23) | ||
| No family history of cancer | ||||||
| No first-line platinum | 71 | 63 | 8.2 (7.4–10.7) | 1 | 0.013 | 0.101 |
| First-line platinum | 51 | 46 | 5.0 (4.2–8.1) | 1.74 (1.12–2.71) | ||
| Family history of cancer | ||||||
| No first-line platinum | 220 | 196 | 8.3 (7.3–10.1) | 1 | 0.384 | |
| First-line platinum | 207 | 183 | 8.5 (7.4–10.3) | 1.10 (0.88–1.37) | ||
Models are adjusted for age, African-American race, liver metastases, and cohort (JHH vs. MDA)
To determine whether the density of relatives with breast, ovarian, or pancreatic malignancies was related to platinum sensitivity, we examined the association between the number of such malignancies in the pedigree and survival (Table 4). There was a significant difference in the effects of the number of relatives with such cancers for those receiving first-line platinum therapy as compared to those who received other therapies (test of interaction p = 0.017). The use of first-line platinum chemotherapy was associated with superior survival in patients with two relatives (HR 0.63, p = 0.032) and three relatives with these cancers (HR 0.36, p = 0.002), but no such trend was observed in patients that did not receive first-line platinum. When comparing individuals with no history of platinum therapy versus those with platinum therapy at any point (i.e., first or second line), we observed a benefit for patients with three or more relatives harboring such cancers (mOS = 21.7 months, HR 0.41, 95 % CI 0.22–0.76, p = 0.004). However, the contrast with those not receiving platinum therapy was not as strong. We also note a trend toward improved survival in patients with three or more relatives who did not receive platinum, and the comparison of the two groups was no longer statistically significant (test of interaction p = 0.19).
Table 4.
Interaction between the strength of family history of breast, ovarian, or pancreatic cancers and first-line or any platinum therapy
| Number at risk |
Number of deaths |
Median overall survival (95 % CI) |
Adjusted hazard ratioa for stratified models (95 % CI) |
Adjusted p valuea |
Adjusted interaction p valuea |
|
|---|---|---|---|---|---|---|
| No first-line platinum therapy | ||||||
| Number of relatives with breast, ovarian, or pancreatic cancer | ||||||
| 0 | 194 | 172 | 8.3 (7.5–10.0) | 1 | 0.017 | |
| 1 | 62 | 54 | 9.3 (6.8–11.6) | 1.04 (0.76–1.42) | 0.78b | |
| 2 | 28 | 27 | 6.8 (4.9–11.0) | 1.21 (0.80–1.82) | 0.36 | |
| 3 or more | 7 | 6 | 13.01 (5.7–Inf) | 0.63 (0.27–1.45) | 0.28 | |
| First-line platinum therapy | ||||||
| Number of relatives with breast, ovarian, or pancreatic cancer | ||||||
| 0 | 147 | 131 | 7.3 (6.1–8.8) | 1 | ||
| 1 | 66 | 59 | 7.1 (5.6–8.5) | 0.89 (0.65–1.23) | 0.47c | |
| 2 | 30 | 27 | 10.6 (9.0–18.5) | 0.63 (0.41–0.97) | 0.032 | |
| 3 or more | 15 | 12 | 14.8 (10.3–Inf) | 0.36 (0.18–0.68) | 0.002 | |
| No platinum therapy (any line) | ||||||
| Number of relatives with breast, ovarian, or pancreatic cancer | ||||||
| 0 | 151 | 140 | 7.5 (6.4–8.8) | 1 | 0.19 | |
| 1 | 45 | 40 | 6.9 (5.5–9.6) | 1.13 (0.78–1.63) | 0.50d | |
| 2 | 22 | 21 | 5.3 (4.0–11.0) | 1.12 (0.70–1.78) | 0.64 | |
| 3 or more | 6 | 5 | 12.0 (5.7–Inf) | 0.65 (0.26–1.60) | 0.35 | |
| Platinum therapy (any line) | ||||||
| Number of relatives with breast, ovarian, or pancreatic cancer | ||||||
| 0 | 190 | 163 | 8.3 (7.3–10.1) | 1 | ||
| 1 | 83 | 73 | 8.0 (6.5–11.5) | 0.97 (0.73–1.30) | 0.87e | |
| 2 | 36 | 33 | 10.6 (9.0–17.0) | 0.82 (0.56–1.20) | 0.30 | |
| 3 or more | 16 | 13 | 21.7 (12.3–47.2) | 0.41 (0.22–0.76) | 0.004 | |
Models are adjusted for age, African-American race, liver metastases, and cohort (JHH vs. MDA)
Test for trend among individuals not receiving first-line platinum therapy: HR 0.99, 95 % CI 0.85–1.17, p = 0.98
Test for trend among individuals receiving first-line platinum therapy: HR 0.76, 95 % CI 0.65–0.89, p = 0.0004
Test for trend among individuals not receiving any-line platinum therapy: HR 0.99, 95 % CI 0.83–1.18, p = 0.94
Test for trend among individuals receiving any-line platinum therapy: HR 0.83, 95 % CI 0.72–0.96, p = 0.0076
Discussion
These observations provide strong evidence that patients with adenocarcinoma of the pancreas, who have a strong family history breast, ovarian, and pancreatic malignancies, may have a better overall prognosis than those patients without such histories. Additionally, we have found evidence that patients with a strong family history may also be more sensitive to platinum-based chemotherapy. Equally important is the observation that platinum chemotherapy may be detrimental to patients without such a family history of cancer. The data are quite provocative as more than 20 % of patients in our cohort had a positive family history of breast, ovarian, or pancreatic cancers. This study, therefore, identifies a sizable population of patients that may derive substantial benefit from platinum-containing regimens and another that should avoid them.
The molecular mechanisms for this platinum sensitivity are not identified by this study, and we do not have sufficient tissue to retrospectively analyze the genetic makeup of these tumors. However, we note that mutations in the BRCA2/PALB2/Fanconi anemia pathway have been reported in approximately 10–12 % of familial pancreatic cancers. These molecular defects are also common to familial cases of breast and ovarian cancers. In each of these malignancies, a disruption of the pathway interferes with repair of DNA double-strand breaks through homologous recombination. Preclinical models have shown that cisplatin preferentially induces death of cells deficient in homologous recombination repair by generating intrastrand crosslinks in DNA. This has been validated in breast and pancreatic cancer cells lines with biallelic genetic defects in BRCA2 [22].
Multiple studies have shown clinical benefit with the use of platinum agents in BRCA mutant cancers. Case reports suggest that pancreatic cancer patients harboring BRCA2 mutations benefit from treatment with platinum agents. In one retrospective review, patients with BRCA mutant advanced pancreatic cancers lived longer if treated with platinum agents (22 vs. 9 months) [23]. Other case reports have similarly demonstrated patients with unusually long survival on treatment with these agents [9, 24, 25]. In other tumor types such as ovarian cancer, platinum sensitivity and improved survival accompany BRCA1/2 pathway mutations [25–27]. Likewise, patients with BRCA-associated breast cancer have high pathologic CR rates than non-BRCA when treated with platinum agents [28,29]. We hypothesize that the platinum-sensitive phenotype described in this report genotypically corresponds to either known or yet to be identified defects in the BRCA2/PALB2/Fanconi anemia pathway. A next logical step would be to further genotype the tumors of patients with strong family histories looking for undiagnosed BRCA, PALB2, or Fanconi gene mutations. Currently, no CLIA-certified assay for functional homologous recombination exists, but such an assay would also be helpful to identify defects in the BRCA2/PALB2/Fanconi pathway. One example of such an assay has been described by Mukhopadhyay et al. [30].
How will these data affect future clinical care and clinical trial design for patients with pancreatic cancer? Certainly, the benefit of other DNA-damaging agents in patients with a family history of cancer should be explored. For example, one patient in our study who did not receive platinum therapy, but who had a dense pedigree (3 + relatives) for pancreatic, breast, and ovarian cancers, survived for >2 years when treated with irinotecan, a topoisomerase inhibitor (Table 3). Other compelling therapies include radiation therapy and inhibitors of PARP [poly(ADP-ribose) polymerase], the latter which appears to be selectively active in BRCA mutations carriers [7].
In our analysis, we did not review objective responses to therapy; however, previous studies using platinum-containing regimens have reported a number of major responses, which would have clear implications not only for metastatic patients, but also in the neoadjuvant setting for resectable or borderline resectable patients. Of note, no individual prospective study has shown a significant survival benefit to platinum-containing regimens in pancreatic cancer. It is possible that the benefit that might have been obtained in patients with BRCA history might have been diluted by an absence of benefit in patients without such history. Subgroup analysis by family history was not performed in those studies [31, 32].
One limitation of our analysis is that we did not include patients treated with the FOLFIRINOX regimen, a recently published oxaliplatin-containing regimen that has demonstrated improved survival over single agent gemcitabine in pancreatic cancer patients treated for metastatic disease, as this regimen had not come into use during the time frame of our review [2]. Likewise, we have not been able to include patients treated with the gemcitabine and nab-paclitaxel regimen, also recently described [3]. Both have demonstrated increased efficacy in the metastatic setting as compared to gemcitabine alone. Clinicians currently have no biologic marker to suggest which of the two regimens might be more effective for any given patient. It may therefore be fruitful to repeat our analysis, focusing on patients treated with these regimens, in order to discern whether family history remains relevant for prognosis or prediction with these newer treatments.
An interesting question is whether our broad definition of family history introduced many sporadic cases of breast, ovarian, and pancreatic cancers into our analysis, which potentially weakened the described survival benefit. For instance, we observed little difference between patients with no or one family member affected by BRCA tumors. A more stringent and prospective evaluation of our observations will likely result in fewer patients meeting the criteria for a familial cancer syndrome, but would likely improve the statistical significance and size of the observed survival benefit.
Future comparisons will more rigorously compare biological markers of DNA repair deficits with family history and overall survival. However, for the immediate moment, we believe that family history might serve as an inexpensive and easily obtained biomarker for sensitivity to platinum agents among patients with metastatic adenocarcinoma of the pancreas.
Acknowledgments
This work is supported by The Swim Across America Laboratory at Johns Hopkins, The Banyan Gate Foundation, The Lustgarten Foundation for Pancreatic Cancer Research and Gastrointestinal SPORE grant P50CA062924
Footnotes
Conflict of interest Dr. Diaz is a founder and shareholder of Pap-gene Inc. and Personal Genome Diagnostics and licensed technology to these and other entities. These relationships are managed by the Johns Hopkins conflict of interest committee. These items disclosed by Dr. Diaz are not related to contents of this article. Dr. Klein notes that under a licensing agreement between Myriad Genetics Inc. and the Johns Hopkins University, Drs. Klein is entitled to a share of royalty received by the University on sales of products. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies. Other co-authors declare no conflict of interest with the contents of this article.
References
- 1.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.De Jesus-Acosta A, Oliver GR, Blackford A, Kinsman K, Flores EI, Wilfong LS, Zheng L, Donehower RC, Cosgrove D, Laheru D, Le DT, Chung K, Diaz LA., Jr A multicenter analysis of GTX chemotherapy in patients with locally advanced and metastatic pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2012;69(2):415–424. doi: 10.1007/s00280-011-1704-y. doi:10.1007/s00280-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriege M, Seynaeve C, Meijers-Heijboer H, et al. Sensitivity to first-line chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:3764–3771. doi: 10.1200/JCO.2008.19.9067. [DOI] [PubMed] [Google Scholar]
- 7.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. New Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi T, Tischkowitz M, Ameziane N, et al. Disruption of the Fanconi anemia–BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9:568–574. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 9.James E, Waldron-Lynch MG, Saif MW. Prolonged survival in a patient with BRCA2 associated metastatic pancreatic cancer after exposure to camptothecin: a case report and review of literature. Anticancer Drugs. 2009;20:634–638. doi: 10.1097/CAD.0b013e32832b511e. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani P, Kurtin S, Dragovich T. Response to a third-line mitomycin C (MMC)-based chemotherapy in a patient with metastatic pancreatic adenocarcinoma carrying germline BRCA2 mutation. JOP. 2008;9:305–308. [PubMed] [Google Scholar]
- 11.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 12.van der Heijden MS, Brody JR, Dezentje DA, et al. In vivo therapeutic responses contingent on Fanconi anemia/BRCA2 status of the tumor. Clin Cancer Res. 2005;11:7508–7515. doi: 10.1158/1078-0432.CCR-05-1048. [DOI] [PubMed] [Google Scholar]
- 13.Tischkowitz MD, Sabbaghian N, Hamel N, et al. Analysis of the gene coding for the BRCA2-interacting protein PALB2 in familial and sporadic pancreatic cancer. Gastroenterology. 2009;137:1183–1186. doi: 10.1053/j.gastro.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Sukhni W, Rothenmund H, Borgida AE, et al. Germline BRCA1 mutations predispose to pancreatic adenocarcinoma. Hum Genet. 2008;124:271–278. doi: 10.1007/s00439-008-0554-0. [DOI] [PubMed] [Google Scholar]
- 15.Rahman N, Seal S, Thompson D, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39:165–167. doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couch FJ, Johnson MR, Rabe K, et al. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res. 2005;65:383–386. [PubMed] [Google Scholar]
- 17.van der Heijden MS, Yeo CJ, Hruban RH, Kern SE. Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res. 2003;63:2585–2588. [PubMed] [Google Scholar]
- 18.Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomark Prev. 2007;16:342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 19.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 20.Breast Cancer Linkage C Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 21.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–5364. [PubMed] [Google Scholar]
- 22.Howlett NG, Taniguchi T, Olson S, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- 23.Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132–1138. doi: 10.1038/bjc.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonnenblick A, Kadouri L, Appelbaum L, et al. Complete remission, in BRCA2 mutation carrier with metastatic pancreatic adenocarcinoma, treated with cisplatin based therapy. Cancer Biol Ther. 2011;12:165–168. doi: 10.4161/cbt.12.3.16292. [DOI] [PubMed] [Google Scholar]
- 25.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 26.Bolton KL, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–379. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 29.Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147:401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay A, Elattar A, Cerbinskaite A, et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin Cancer Res. 2010;16:2344–2351. doi: 10.1158/1078-0432.CCR-09-2758. [DOI] [PubMed] [Google Scholar]
- 31.Heinemann V, Wilke H, Mergenthaler HG, et al. Gemcitabine and cisplatin in the treatment of advanced or metastatic pancreatic cancer. Ann Oncol. 2000;11:1399–1403. doi: 10.1023/a:1026595525977. [DOI] [PubMed] [Google Scholar]
- 32.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]