Abstract
Human papillomavirus (HPV) is responsible for increasing incidence of oropharyngeal cancer (OPC). At present, there are no biomarkers in the surveillance algorithm for HPV-positive oropharyngeal cancer (HPV-OPC). HPV16 E6 antibody precedes OPC diagnosis. If HPV16 E6 indeed precedes primary diagnosis, it is similarly expected to precede disease recurrence and may have a potential role as a biomarker for surveillance of HPV-OPC. To determine whether HPV antibody titers have a potential role as early markers of disease recurrence or prognosis a retrospective pilot study was designed to determine whether HPV16 early antibody titers E6, E7, E1 and E2 decrease after treatment of HPV16-positive OPC. Trends in pre-treatment, early- (≤6 months after treatment) and late- post treatment (>6 months after treatment) HPV16 antibody titers were examined. There were 43, 34 and 52 subjects with serum samples available for pre-treatment, early- and late- post treatment intervals. Mean pre-treatment antibody levels were higher than post-treatment antibody levels. Average antibody levels decreased significantly over time for E6 (p-trend=0.001) and E7 (p-trend<0.001). 6 disease recurrences were observed during the follow-up period (median 4.4 years). In univariate analysis, a log unit increase in pre-treatment E6 titer was significantly associated with increased risk of disease recurrence (HR 5.42, 95%CI 1.1–25.7, p=0.03). Therefore, levels of antibodies to HPV16 early oncoproteins decline after therapy. Higher E6 titers at diagnosis are associated with significant increases in risk of recurrence. These data support the prospective evaluation of HPV16 antibodies as markers of surveillance and for risk stratification at diagnosis.
Introduction
The incidence of oropharyngeal squamous cell carcinomas (OPCs) is rapidly increasing in the United States (U.S.), as well as other countries around the world (1, 2). Human papillomavirus (HPV), a sexually transmitted infection, is the recognized etiologic agent for this growing majority of OPCs (3, 4). In the U.S. HPV is the demonstrated oncogenic agent responsible for these incidence trends (4), and currently accounts for approximately 80% of OPCs diagnosed (5, 6). The presence of HPV in oropharyngeal tumors confers improved overall- and progression-free survival, relative to HPV-negative tumors (5, 6). Despite improved prognosis, up to 27% of HPV-positive patients still experience recurrence of disease, the majority of which occurs in the first two years after treatment (7–9).
Historically, even 1-year survival of patients with recurrent OPC was dismal (5–30%) [10]. However, recent data suggest that at the time of disease recurrence, HPV-positive tumor status and surgical salvage are independently associated with improved overall survival (8). Two-year overall survival is 25% greater for recurrent HPV-positive patients who undergo surgical salvage as compared to those who do not (7, 8). Therefore, if recurrent HPV-positive OPC is detected at an early stage when surgical salvage is possible, patients may have a significant improvement in overall survival, although whether improved lead time afforded by any potential biomarker would change the outcome is unknown.
At present, National Comprehensive Cancer Network (NCCN) guidelines for surveillance recommend history and physical examination at routine intervals with anatomic and metabolic imaging as clinically indicated (11). In contrast to malignancies of other anatomic sites for which biomarkers are integral to recurrent disease surveillance (e.g. prostate surface antigen titer), there are no analogous or validated biomarkers for HPV-positive OPC (HPV-OPC). Therefore, we were interested in identifying a candidate biomarker for disease status in HPV-OPC.
The presence of antibodies to HPV16 early oncoprotein E6 is strongly associated with diagnosis of OPC (OR 58.4 95%CI 24.2–138.3) [12, 13] and precedes diagnosis of OPC by ten years (14). HPV16-specific E1, E2, and E7 antibodies are similarly associated with incident HPV-OPC years before diagnosis of malignancy (14).
Data from cervical cancer literature, the paradigm of HPV-related malignancy, demonstrates a significant reduction in titer of antibodies after treatment of disease and antibody status is a significant predictor of prognosis (15, 16). Although similar reductions in E6 and E7 titers have been observed in head and neck cancer, the clinical relevance is limited by heterogeneity of HPV tumor status, histology types and anatomic sites (17, 18).
To explore whether HPV16 antibodies to E6, E1, E2 and E7 have potential as biomarkers of disease status for patients with HPV-OPC, we hypothesized that titers will decrease after treatment with curative intent.
Materials and Methods
This was a retrospective study designed to determine whether HPV16 antibody titers change after treatment. Participants with HPV-positive OPC and two or greater serology specimens available were eligible. Serology samples had been collected from patients enrolled in the Molecular Surveillance Protocol, an IRB-approved study at Johns Hopkins. Clinical characteristics of interest including age, gender, race, alcohol and smoking history, primary site of diagnosis, staging, primary treatment modality, date of last clinical visit, presence and date of first recurrence were abstracted from the electronic medical record. HPV16 tumor status was obtained from previously reported data which included quantitative PCR for HPV16 genomic DNA (≥0.1 copy per genome) and high risk HPV in situ hybridization (19). A subset analysis was restricted to participants with clinically available HPV16-positive in situ hybridization tumor status.
Serologic methods
Antibodies to HPV 16 E1, E2, E6 and E7 were measured by ELISA using the glutathione S-transferase (GST) capture method (20) with some modifications. The following reagents were generously provided by Michael Pawlita (German Cancer Research Center, Heiderlberg, Germany): cleared lystae from E. coli over expressing GST-HPV 16 E1, GST-HPV 16 E2, GST-HPV 16 E6, GST-HPV 16 E7 and GST alone. Briefly, 96-well polystyrene flat bottom MaxiSorp plates (Nunc, Naperville, IL) were coated overnight at 4°C with 200 ng/well of glutathione-casein in phosphate buffered saline, pH 7.2 (PBS), and blocked for 1 hour at 37°C with 0.5% (wt/vol) polyvinyl alcohol, MW 30,000–70,000 (Sigma-Aldrich, St Louis MO) in Blocker™ Casein in PBS (casein PVA buffer; Thermo scientific, Rockland IL). The blocked plates were incubated for 1 h at room temperature with GST-HPV antigen lysate diluted in casein PVA buffer to 0.25 μg/μl total lysate protein. Control wells were coated with GST alone at the same protein concentration. Before addition of serum samples and following each incubation step, the plates were washed 4 times with PBS containing 0.05% (vol/vol) Tween 20 (Sigma-Aldrich) in an automatic plate washer (Skanwasher 300, Skatron, Lier, Norway). Serum samples diluted 1:100 in casein PVA buffer containing GST alone lysate (0.50 mg/ml) were left to react for 1 h at 37°C. Samples were tested in duplicate on the same day but in different microtiter plates. Antigen-bound immunoglobulin was detected with peroxidase-conjugated goat antibodies against human IgG, gamma chain specific, (Southern Biotec, Birmingham, AL) diluted 1:4000 in casein PVA buffer containing 0.8% (wt/vol) polyvinylpyrrolidone, MW 360,000 (Sigma-Aldrich) and 0.05% (vol/vol) Tween 20. After 30 min at 37°C, color development was initiated by the addition of 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) hydrogen peroxide solution (Kirkegaard and Perry, Gaithersburg, MD). The reaction was stopped after 20 min by addition of 1% dodecyl sulfate and optical density (OD) is measured at 405 nm, with a reference wavelength of 490 nm, in an automated microtiter plate reader (Molecular Devices, Menlo Park, CA). The mean OD in wells with GST alone was subtracted from the mean OD in wells with the GST-HPV protein to give an antigen specific OD value.
Antibody titers to BK polyomavirus (BKPyV) were used as a control. Antibody to BKPyV capsids were measured by virus-like particle (VLP) ELISA as previously described (21). The rationale for performing this assay was to understand whether the observed changes in antibody levels were specific to HPV or applicable to antibody levels in general, and the choice of BKPyV ELISA was based on the knowledge that a majority of individuals are expected to be seropositive and thus changes in antibody level could be measured.
Statistical Analysis
The primary outcome of interest was change of OD values over time from pre-treatment to post-treatment. The overall average trend in the OD values over time was visualized using locally weighted regression (lowess) curves. Time of serum sample collection was considered as a categorical variable with respect to diagnosis and treatment. Serology samples collected from date of diagnosis and up to initiation of therapy were considered “pre-treatment”. If repeated measurements were available on the same day for an individual, an average value was used for analysis. Samples obtained up to 6 months after therapy and after 6 months were categorized as “early post-treatment” and “late post-treatment”, respectively. If a subject had greater than one sample available during a post-treatment period (“early” and “late”), an average value of the available measurements was used. Descriptive statistics of the serology data based upon the average values by treatment periods were calculated. A linear mixed-effects model was used to estimate the means of log transformed OD values across treatment periods, as well as mean differences between treatment periods (e.g. pre- and post-treatment periods), where correlated measurements within same subject were accounted for by assuming an exchangeable correlation structure.
Timing of clinical recurrence was used to determine prognosis group. The analysis was restricted to subjects who were followed for at least 2 years and none of the early dropouts were due to death. Recurrence within two years of therapy was considered poor prognosis, while no evidence of recurrence during this time period or recurrence long after 2 years post-treatment was categorized as good prognosis.
Recurrence-free survival (RFS) was defined as the date of end treatment until date of first documented disease recurrence. Patients who did not recur but died were censored at the time of death. Patients who remained alive without recurrence were censored at the time of last contact. For analyses of RFS, mean OD values were log-transformed. Association of baseline serology with RFS was evaluated using Cox proportional hazards model by univariate and multivariate analyses adjusting for alcohol consumption, smoking history, and TNM stage. All tests were considered statistically significant at P<0.05. All analyses were performed with use of SAS 9.2 (SAS Institute, Cary, NC) and R version 3.1.0 (available at http://www.r-project.org/).
Results
The overall study population consisted of 60 HPV16-positive OPC patients. The characteristics of the study population are summarized in Table 1. The majority of individuals were male (n=53, 88.3%), white (n=59, 98.3%) with advanced overall stage (n=45, 75.0%). Pre-treatment serology was available for 43 participants (71.7%).
Table 1.
Clinical Characteristics of Study Population (n=60)
| Characteristics | No. of Patients | % |
|---|---|---|
| Age at diagnosis (years) | ||
| Median (range) | 56 (29 – 84) | |
|
| ||
| Gender | ||
| Male | 53 | 88.3 |
| Female | 7 | 11.7 |
|
| ||
| Race | ||
| White | 59 | 98.3 |
| Black | 1 | 1.7 |
|
| ||
| Alcohol consumption | ||
| <14 drinks/week | 45 | 75.0 |
| ≥14 drinks/week | 12 | 20.0 |
| Unknown | 3 | 5.0 |
|
| ||
| Smoking history | ||
| Never | 25 | 41.7 |
| Ever | 32 | 53.3 |
| Unknown | 3 | 5.0 |
|
| ||
| Primary site at diagnosis | ||
| OP | 58 | 96.7 |
| UP | 1 | 1.7 |
| Unknown | 1 | 1.7 |
|
| ||
| Tumor stage | ||
| I | 30 | 50.0 |
| II | 21 | 35.0 |
| III | 4 | 6.7 |
| IV | 2 | 3.3 |
| Unknown | 3 | 5.0 |
| Nodal stage | ||
| N0–N1 | 9 | 15.0 |
| N2a | 13 | 21.7 |
| N2b | 30 | 50.0 |
| N3+ | 6 | 10.0 |
| Unknown | 2 | 3.3 |
| Overall stage | ||
| Stage 0–III | 12 | 20.0 |
| Stage IV | 45 | 75.0 |
| Unknown | 3 | 5.0 |
| Primary treatment | ||
| RT +/− chemotherapy | 23 | 38.3 |
| Surgery +/− RT +/− chemotherapy | 34 | 56.7 |
| Unknown | 3 | 5.0 |
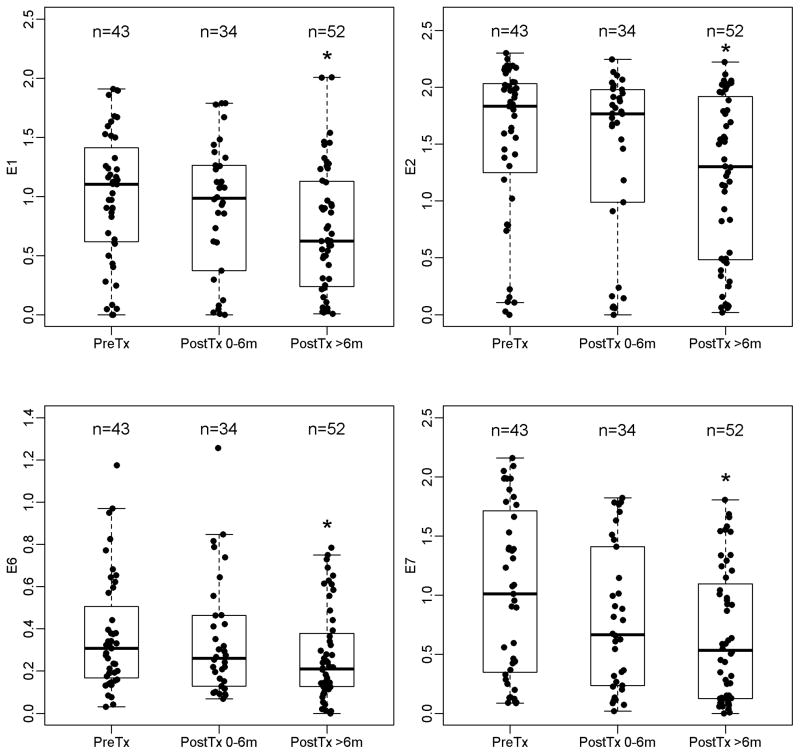
Antibody levels over time were evaluated for the study population overall. There were 43, 34 and 52 subjects with serum samples available for pre-treatment, early- and late- post-treatment intervals. Average antibody levels were higher pre-treatment than post-treatment for E6, E7, E1, and E2 (Figure 1, Supplementary Table 1). For E6, the average pre-treatment antibody level was 0.31 (range 0.03, 1.2) and declined after treatment. In the early post-treatment interval (up to six months after treatment), mean E6 level was 0.26 (range 0.07, 1.3) and in the late post-treatment interval (six months or greater after treatment) was 0.21 (range 0–0.78). Similar patterns of declining average antibody levels per treatment interval were observed for E7, E1 and E2 over time, although the mean levels for E6 were consistently lower.
Figure 1. Antibody levels by treatment period.
The length of the box is the inter-quartile range and represents the middle 50% of antibody levels. The horizontal line inside the box depicts the median. The lower and upper hinges of the box represent the 25th and 75th percentiles, respectively. The vertical dashed lines extend from the box to the upper and lower 1.5 inter-quartile values from the upper and lower hinges. The filled circles represent the actual antibody levels. Significant change from pre-treatment (P<0.05) is indicated by asterisk (*).
The trajectory of mean antibody levels over time was further modeled. Within-participant correlation of antibody levels over time was accounted for (Table 2). This analysis was restricted to 43 individuals with available pre-treatment serum samples. Compared to pre-treatment levels, the average antibody level declined in the early post-treatment interval for E6, E2, and E1, although not statistically significantly (p>0.05). For E7, average early post-treatment antibody levels were significantly lower than pre-treatment levels (p=0.03). However, when average late- post-treatment antibody levels were compared with pre-treatment levels, there were significant decreases for E6, E7 and E1 (p<0.02 for all). For E2 the decrease remained non-significant (p=0.09). Overall, the average antibody levels decreased significantly over time for E6 (p-trend=0.001) and E7 (p-trend<0.001). In a subset analysis restricted to subjects with ISH16-positive tumors, E6 and E7 declined significantly in the early post-treatment period (Table 3; E6 p=0.001; E7 p<0.001; p-trends<0.001).
Table 2.
Changes in antibody levels in patients with HPV-positive oropharyngeal cancer over time1
| Overall study population | Subset: ISH HPV16-positive subjects | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Serology | N | Mean ± SE2 | P-value3 | P-trend3 | N | Mean ± SE2 | P-value3 | P-trend3 |
|
| ||||||||
| E1 | 0.066 | 0.168 | ||||||
| Pre-Treatment | 43 | −0.508 ± 0.223 | - | 31 | −0.323 ± 0.228 | - | ||
| Early Post-Treatment | 29 | −0.559 ± 0.235 | 0.730 | 21 | −0.326 ± 0.242 | 0.985 | ||
| Late post-treatment | 37 | −0.816 ± 0.227 | 0.024 | 27 | −0.584 ± 0.232 | 0.082 | ||
|
| ||||||||
| E2 | 0.166 | 0.019 | ||||||
| Pre-Treatment | 43 | 0.032 ± 0.191 | - | 31 | 0.296 ± 0.166 | - | ||
| Early Post-Treatment | 29 | 0.047 ± 0.200 | 0.896 | 21 | 0.199 ± 0.170 | 0.212 | ||
| Late post-treatment | 37 | −0.148 ± 0.194 | 0.092 | 27 | 0.092 ± 0.167 | 0.005 | ||
|
| ||||||||
| E6 | 0.001 | <0.0001 | ||||||
| Pre-Treatment | 43 | −1.272 ± 0.148 | - | 31 | −1.175 ± 0.143 | - | ||
| Early Post-Treatment | 29 | −1.484 ± 0.163 | 0.114 | 21 | −1.471 ± 0.149 | 0.001 | ||
| Late post-treatment | 37 | −1.736 ± 0.153 | 0.0003 | 27 | −1.594 ± 0.145 | <0.0001 | ||
|
| ||||||||
| E7 | <0.0001 | <0.0001 | ||||||
| Pre-Treatment | 43 | −0.381 ± 0.187 | - | 31 | −0.265 ± 0.188 | - | ||
| Early Post-Treatment | 29 | −0.692 ± 0.201 | 0.031 | 21 | −0.530 ± 0.194 | 0.006 | ||
| Late post-treatment | 37 | −1.049 ± 0.192 | <0.0001 | 27 | −0.822 ± 0.190 | <0.0001 | ||
This analysis was restricted to subjects with available pre-treatment serology measurements
Estimated log-transformed mean. SE denotes standard error.
Comparison relative to pre-treatment measurements based on linear mixed effects models taking into account the within-subject correlation
Table 3.
Association of pre-treatment antibody levels with recurrence-free survival in patients with HPV-positive oropharyngeal cancer
| Serology parameter | Univariate analysis1 (n=43) | Multivariable analysis2 (n=41) | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Log E1 | 1.01 (0.60 – 1.69) | 0.982 | 0.97 (0.59 – 1.59) | 0.912 |
| Log E2 | 1.00 (0.56 – 1.79) | 0.999 | 0.92 (0.48 – 1.73) | 0.785 |
| Log E6 | 5.42 (1.14 – 25.7) | 0.033 | 7.06 (1.15 – 43.2) | 0.035 |
| Log E7 | 0.71 (0.32 – 1.58) | 0.403 | 0.46 (0.16 – 1.32) | 0.149 |
Cox proportional hazards model;
In multivariable analysis, alcohol consumption, smoking history, and TNM stage at diagnosis were adjusted for.
When considering average antibody levels by prognosis group, the majority were in the good prognosis group at pre-treatment (33 of 37) and early post-treatment (23 of 28). At pre-treatment, E6 antibody level was lower for the good prognosis as compared to the poor prognosis group (0.32 vs 0.61, p=0.048) and early post-treatment (0.25 vs. 0.64, p=0.045) intervals. Similar trends were observed for E7, E1 and E2, although they were not statistically significant.
Given the declines in antibody levels over time with respect to therapy, the prognostic significance of pre-treatment serology levels was explored (Table 3). There were only 6 disease recurrences observed in this study population with a median follow-up time of 4.4 years (range 0.08 – 11.9). In univariate analysis higher pretreatment E6 level (per log unit) was significantly associated with increased risk of disease recurrence (HR 5.42, 95%CI 1.1–25.7, p=0.03). After adjustment for clinically relevant factors, the robust association between increasing level of E6 antibody at pre-treatment and risk of disease recurrence remained significant (HR 7.1, 95%CI 1.2–43.2, p=0.04). Pretreatment levels of E7, E1, and E2 were not of prognostic significance (p>0.05 for all). In the subset analysis restricted to 31 subjects with ISH16-positive tumors and 5 events of recurrence, a similar magnitude of the association with increased risk of recurrence for E6 was observed, albeit not statistically significant (HR 5.5, 95%CI 0.66–46.7).
Additionally the prognostic implication of E6 antibody in the first three months after therapy was explored. In univariate analysis, each log unit increase in level of E6 antibody level was associated with a seven-fold increased risk of recurrence, although non-significant (HR=6.9, 95% CI = 0.50–95.9). To determine whether the observed declines in antibody titers were HPV-specific or systemic immune-related, BKV titers were evaluated over time. In contrast to declines in HPV16 early oncoproteins, BKV titers remained stable over time (p-value=0.30).
Discussion
Levels of antibodies against HPV16-specific oncoproteins declined after therapy in a study population of HPV-positive OPC patients. Notably, higher levels of E6 antibody at diagnosis were associated with significantly increased risk of recurrence. These observations provide critical initial data to support further investigation of serum antibodies of HPV as biomarkers of prognosis and disease status.
The growing interest in therapeutic de-intensification of HPV-OPC has highlighted the need to identify the subsets of HPV-positive patients who are at decreased risk of recurrence and therefore appropriate candidates for de-intensification (22). Present prognostic risk stratification for HPV-positive patients is limited to lifetime tobacco exposure and nodal disease (6) in the RTOG 0129 model and age, TNM stage and lifetime tobacco exposure in the Princess Margaret proposed prognostic groups (23). Other than p16 tumor status there are currently no other prognostic biomarkers available to refine de-intensification eligibility. In this study, the magnitude of the baseline antibody level is strongly associated with recurrence. If this finding is validated in a rigorous prospective study, then HPV serology may offer a novel prognostic biomarker to further stratify the risk categorization currently used for clinical trials.
To consider HPV antibody levels as a candidate biomarker for surveillance of HPV-OPC after treatment, an important first question is whether antibody levels change after treatment. In this data, significant declines are observed for HPV antibodies E6, E7 and E1 after treatment. This study builds upon a previous report of significant declines in mean antibody levels after treatment in a patient population including oral cavity, an anatomic site not relevant to HPV (17). By contrast, to evaluate the question of whether HPV antibody levels decline after therapy, this study was restricted to OPCs with HPV16-positive tumor status. Indeed other head and neck cancers which are not HPV-related are not expected to be HPV seropositive (24), and therefore any associations observed may be driven by the majority seronegative and low levels of antibodies. A similar approach of restricting to HPV-positive OPCs (determined by PCR) was recently used to demonstrate that the presence of HPV E6 serum antibodies at diagnosis was significantly associated with improved overall and progression-free survival (25). In contrast to the findings of the current study, the median antibody levels of individuals who recurred or did not recur were statistically similar and appeared to be (non-significantly) lower for patients who recurred. Of note, consistent with our findings, a decline in median antibody levels is apparent graphically when comparing pre-treatment and post-treatment levels (25).
Analogous trends have been observed in cervical cancer, however, a substantial proportion of women with cervical cancer have no immunologic response to HPV-specific antibodies and the broad distribution of HPV types responsible for cervical cancer precludes from examination of antibody response to one antibody type (26). By contrast, the majority of HPV-positive OPC patients are seropositive to HPV; in a case-control study in which HPV16 DNA was present in 72 tumors, 64 (88.9%) of these cases were seropositive to HPV 16 E6 or E7 (12). As a biomarker, the increased prevalence of this marker at diagnosis in OPC as compared to cervical cancer is appealing. Additionally, the vast majority of HPV-OPCs are HPV16-related (27). Recent data suggest that while approximately 97% of HPV-OPC are seropositive, presence of antibodies to specific antigens (E1, E2, E4, E5, E6 and E7) is highly variable (2–66%) and is affected by age (28). Realistically, HPV serology is only useful in patients with an antibody response and therefore it can be expected that for some E7 will be a better marker of disease status than E6, and vice versa.
Clinical surveillance for disease recurrence is currently similar for HPV-positive and HPV-negative OPC patients. However, whether surveillance strategies should differ for HPV-OPC changes is an area of controversy (9, 29). While most HPV-OPCs are “cured” of their disease, up to 30% of patients still experience recurrence. To date, there is no method to identify the patients who will unfortunately experience disease recurrence after therapy. Indeed, HPV-positive patients at the time of recurrence retain the phenotype of HPV-positive patients; those who recur are not the HPV-positive patients with characteristics more similar to HPV-negative patients (7–9). Therefore, serology at the time to diagnosis may be a biomarker to identify patients who need more or less intense follow-up. This could influence imaging recommendations and clinical examinations in the current surveillance paradigm.
The possibility that the decreases in HPV16 antibodies were non-specific and applicable to any antibody was explored. Others have shown that L1 serology, a measure of cumulative lifetime exposure to HPV, does not change with therapy (17). In this study, BK virus levels were used as controls and were stable over time (p=0.30). Therefore, the level of HPV16 antibody titers appears to be specific to the disease status of HPV-OPC and may be an index of the tumor or antigen “load” (13). This extends prior provocative data in a European case-control which showed the presence of E6 antibodies a decade or more prior to OPC diagnosis at the time of early carcinogenesis and antigen presentation (14).
There are several limitations of this study that warrant discussion. Most importantly, this is a retrospective pilot study performed to determine whether this hypothesis should be considered prospectively. Available samples were collected under a prospective collection protocol, however the timing and number of available samples is variable and dictated by clinical follow-up, availability of patients and prior use of serum. HPV16 ISH, which is the clinical gold standard for HPV16 detection, was only clinically available for a limited subset of subjects. However, HPV high-risk ISH in combination with quantitative PCR for HPV16 oncoproteins was available for all the cases. HPV16 is responsible for >95% of HPV-OPC, and estimates were similar in the subset analysis, therefore it is unlikely that cases included were attributed to other high-risk HPV infections. It is important to note that not all HPV-positive OPC patients are E6 or E7 seropositive. Therefore future prospective analyses should determine baseline antibody status to HPV16 and other HPV high-risk types. It was not feasible in this study to examine trends among individuals seropositive at pre-treatment. Additionally for the few HPV-OPC tumors due to other high-risk HPV types, there may be cross-reactivity. With regard to the association of pre-treatment HPV16 E6 titer and recurrence, there were limited recurrences which may explain the wide confidence intervals of the risk estimates. In addition, the calculation of risk at completion of therapy was not possible given the lack of samples consistently collected at end of treatment.
These data support further study of HPV16 serology as a candidate biomarker of prognosis at the time of diagnosis and surveillance after treatment.
Supplementary Material
Acknowledgments
Grant support: Oral Cancer Foundation, NIDCR P50DE019032-13; C.Fakhry
Footnotes
Conflict of interest disclosures: None
References
- 1.Gillison M, Chaturvedi A, Anderson W, Fakhry C. The epidemiology of HPV-positive head and neck squamous cell carcinoma. Journal Clinical Oncology. 2015;33:3235–42. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fakhry C, Andersen KK, Eisele DW, Gillison ML. Oropharyngeal cancer survivorship in Denmark, 1977–2012. Oral oncology. 2015;51:982–984. doi: 10.1016/j.oraloncology.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. Journal of Clinical Oncology. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. Journal of the National Cancer Institute. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T, Qualliotine JR, Ha PK, Califano JA, Kim Y, Saunders JR, et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer. 2015;121:1977–84. doi: 10.1002/cncr.29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar A, Garden AS, et al. Human Papillomavirus and Overall Survival After Progression of Oropharyngeal Squamous Cell Carcinoma. Journal of clinical oncology. 2014;32:3365–73. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar AK, Garden AS, et al. Reply to B. O’Sullivan et al. Journal of clinical oncology. 2015;33:1708–9. doi: 10.1200/JCO.2014.60.3555. [DOI] [PubMed] [Google Scholar]
- 10.Kostrzewa JP, Lancaster WP, Iseli TA, Desmond RA, Carroll WR, Rosenthal EL. Outcomes of salvage surgery with free flap reconstruction for recurrent oral and oropharyngeal cancer. The Laryngoscope. 2010;120:267–272. doi: 10.1002/lary.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfister DG, Ang KK, Brizel DM, Burtness BA, Busse PM, Caudell JJ, et al. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network. 2013;11:917–923. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- 12.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. The New England journal of medicine. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KS, Dahlstrom KR, Cheng JN, Alam R, Li G, Wei Q, et al. HPV16 antibodies as risk factors for oropharyngeal cancer and their association with tumor HPV and smoking status. Oral oncology. 2015;51:662–667. doi: 10.1016/j.oraloncology.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. Journal of clinical oncology. 2013;31:2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamsikova E, Ludvikova V, Tachezy R, Kovarik J, Brouskova L, Vonka V. Longitudinal follow-up of antibody response to selected antigens of human papillomaviruses and herpesviruses in patients with invasive cervical carcinoma. International journal of cancer Journal international du cancer. 2000;86:351–355. doi: 10.1002/(sici)1097-0215(20000501)86:3<351::aid-ijc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Di Lonardo A, Marcante ML, Poggiali F, Venuti A. HPV 16 E7 antibody levels in cervical cancer patients: before and after treatment. Journal of medical virology. 1998;54:192–195. [PubMed] [Google Scholar]
- 17.Koslabova E, Hamsikova E, Salakova M, Klozar J, Foltynova E, Salkova E, et al. Markers of HPV infection and survival in patients with head and neck tumors. International journal of cancer Journal international du cancer. 2013;133:1832–1839. doi: 10.1002/ijc.28194. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstein LM, Smith EM, Pawlita M, Haugen TH, Hamsikova E, Turek LP. Human papillomavirus serologic follow-up response and relationship to survival in head and neck cancer: a case-comparison study. Infectious agents and cancer. 2011;6:9. doi: 10.1186/1750-9378-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn SM, Chan JY, Zhang Z, Wang H, Khan Z, Bishop JA, et al. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA otolaryngology-- head & neck surgery. 2014;140:846–854. doi: 10.1001/jamaoto.2014.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehr P, Zumbach K, Pawlita M. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. Journal of immunological methods. 2001;253:153–162. doi: 10.1016/s0022-1759(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 21.Randhawa PS, Gupta G, Vats A, Shapiro R, Viscidi RP. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clinical and vaccine immunology. 2006;13:1057–1063. doi: 10.1128/CVI.00114-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adelstein DJ, Ridge JA, Gillison ML, Chaturvedi AK, D’Souza G, Gravitt PE, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, D. C Head & neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 23.Huang SH, Xu W, Waldron J, Siu L, Shen X, Tong L, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. Journal of clinical oncology. 2015;33:836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 24.Anantharaman D, Gheit T, Waterboer T, Abedi-Ardekani B, Carreira C, McKay-Chopin S, et al. Human papillomavirus infections and upper aero-digestive tract cancers: the ARCAGE study. Journal of the National Cancer Institute. 2013;105:536–545. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 25.Dahlstrom KR, Anderson KS, Cheng JN, Chowell D, Li G, Posner M, et al. HPV Serum Antibodies as Predictors of Survival and Disease Progression in Patients with HPV-Positive Squamous Cell Carcinoma of the Oropharynx. Clinical cancer research. 2015;21:2861–2869. doi: 10.1158/1078-0432.CCR-14-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Combes JD, Pawlita M, Waterboer T, Hammouda D, Rajkumar T, Vanhems P, et al. Antibodies against high-risk human papillomavirus proteins as markers for invasive cervical cancer. International journal of cancer Journal international du cancer. 2014;135:2453–2461. doi: 10.1002/ijc.28888. [DOI] [PubMed] [Google Scholar]
- 27.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer epidemiology, biomarkers & prevention. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 28.Anderson KS, Gerber JE, D’Souza G, Pai SI, Cheng JN, Alam R, et al. Biologic predictors of serologic responses to HPV in oropharyngeal cancer: The HOTSPOT study. Oral oncology. 2015;51:751–758. doi: 10.1016/j.oraloncology.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Sullivan B, Adelstein DL, Huang SH, Koyfman SA, Thorstad W, Hope AJ, et al. First Site of Failure Analysis Incompletely Addresses Issues of Late and Unexpected Metastases in p16-Positive Oropharyngeal Cancer. Journal of clinical oncology. 2015;33:1707–8. doi: 10.1200/JCO.2014.58.2700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.