Summary
Data from homecare electronic health records were used to explore the association of patient characteristics with re-hospitalizations of patients with heart failure (HF) during a 60-day period of telemonitoring following hospital discharge. Data from 403 Medicare patients with HF who had used telehealth from 2008 to 2010 were analysed. There were 112 all-cause (29%) and 73 cardiac-related (19%) re-hospitalizations within 60 days of being admitted to the homecare agency. In adjusted analyses, the patients’ prescribed number of medications and type of prescribed cardiac medications were significantly (P<0.05) associated with increased risk of re-hospitalization. After stratifying the sample by illness severity, age and gender, other significant (P<0.05) predictors associated with increased risk of all-cause and cardiac re-hospitalization were psychiatric co-morbidity, co-morbidities related to pulmonary and obesity within gender, beta blocker prescription in females and primary HF diagnosis in the oldest age stratum. The study’s findings may assist homecare agencies seeking to allocate resources without compromising patient care.
Introduction
More than one million hospitalizations each year in the US are due to heart failure (HF) and 25% of these hospitalized patients are readmitted within 30 days of discharge.[1,2] Telehealth may be useful in HF and some studies have reported a lower rate of hospitalization[3–6] in HF patients using telehealth. However, other studies have not found an association between telehealth and reduced hospitalizations.[7–10] Studies with negative associations between telehealth and HF outcomes had more severe HF patients in the telehealth intervention group as compared to the control group.[9,11,12]
In a recent systematic review of risk prediction models for hospital readmission rates Kansagara et al. suggested that the best choice of a model may depend on the setting, the population in which it is being used, and variables associated with the patient’s overall health and function, illness severity and social determinants of health.[13] Few studies have been conducted to assess the influence of patient characteristics on hospitalizations for patients with HF using telehealth. We have investigated the associations between the characteristics of patients with HF and re-hospitalization during a 60-day period of telemonitoring after discharge from hospital.
Methods
We conducted a retrospective chart review of electronic patient records (EPRs) from a homecare agency in New England. The agency had used electronic documentation for nursing services and telehealth for over 10 years. The patients were those admitted to the homecare agency with HF as a diagnosis who had used telehealth from 2008 to 2010. Patients with co-diagnoses of Alzheimer’s disease, wounds requiring extensive wound care, trauma, fractures and general surgery were excluded. The study was approved by the appropriate ethics committee.
EHR data sources
The Medicare dataset Outcome and Assessment Information Set (OASIS) contained the demographic and clinical characteristics of patients including age, race, gender, location (rural/urban); psychosocial status characteristics of cognitive functioning, anxiety, depression and living situation (presence or absence of caregivers); disease characteristics of ambulation, dyspnea, and number and types of co-morbidities. The homecare agency’s EHR stored the OASIS data. OASIS was also used to collect data on outcome variables of all-cause and/or cardiac re-hospitalizations for the patient. Electronic documentation of nursing visit notes, telehealth logs and scanned intake forms were used to collect data on English language ability, status (new/chronic) and type (primary/secondary) of HF diagnosis, co-morbidities and medications. The variables for each subject were collected from the time that the telehealth service began to 60 days of telemonitoring, or less if the subject was discharged earlier from the agency. We used the Elixhauser Comorbidity Index to define groups of co-morbidities.[14]
Data analysis
Log-rank tests and Cox proportional hazards model estimation was used to analyse time to re-hospitalization and time to cardiac re-hospitalization, crudely and after adjusting for covariates. Bivariate analysis was used to eliminate covariates with crude associations at a significance level of P>0.25.[15] Time to first re-hospitalization was measured from the date of the homecare agency’s telehealth service admission to the date of first re-hospitalization. All study participants were followed for 60 days; accordingly, patients without readmissions to hospital at 60 days represented censored observations. Re-hospitalizations for reasons that were non-cardiac in nature were also treated as censored observations.
Our hypothesis was that male gender, older age and greater severity of illness would modify the associations of telehealth with re-hospitalization. Therefore, we repeated our analyses, stratifying on the following key variables: gender (male/female), age (<75 years, 75-84 years, >84 years) and illness severity (high/low). The high group in the illness severity stratum was defined as subjects above the 75th percentile for number of medications, co-morbidities and days in hospital. Subjects in the high illness severity stratum included subjects with severe dyspnoea (patients scoring 3 or 4 for the OASIS item on dyspnoea) or subjects with number of medications (>16), co-morbidities (>6) or days in hospital (>17) higher than the 75th percentile.
Results
Five hundred and ninety subjects used telehealth at the homecare agency from January 2008 to May 2010. After eliminating subjects without a diagnosis of HF, 444 remained. Of these, 403 satisfied the inclusion/exclusion criteria (Figure 1).
Figure 1. Sample Selection Process.
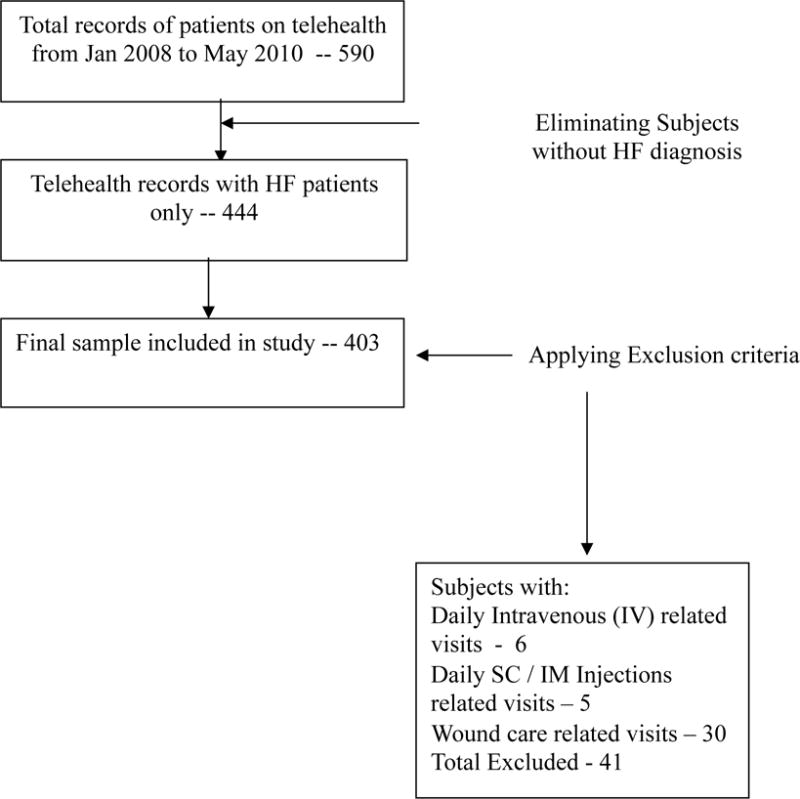
Reprinted by permission from “Association of co-morbidities with homecare nursing utilization and withdrawal from telehealth by patients with heart failure (HF)” by Radhakrishnan, K., et al. (Accepted) Journal of Cardiovascular Nursing
There were no missing data for all-cause re-hospitalizations. The cardiac-cause re-hospitalization outcome variable had 10 (3%) cases with missing data, but replacing missing data values was not necessary. Only the status of HF diagnosis (new or chronic) independent variable had missing data in 43 (11%) cases. Missing value analysis showed that the missing values occurred at random, and cases with missing data were excluded from specific analysis.[15]
Sample
The majority of the patients were Caucasian (94%), over 75 years in age (>70%), female (55%), urban (89%), lived with caregivers (69%), had chronic HF (81%) or experienced some dyspnoea (84%), see Table 1 [Production - Table 1 is for the online archive]. The average number of co-morbidities experienced by the subjects was 5.2. Blood pressure disorders (69%), vascular disorders (67%) and cardiac arrhythmia (53%) were the most common co-morbidities. The average number of medications prescribed was 13.6. Diuretics were the most common cardiac type medication prescribed (85%). About half of the patients (51%) were prescribed an Angiotensin Converting Enzyme Inhibitor/Angiotensin II Receptor Blocker (ACEI/ARB) type of cardiac medication.
Table 1.
Sample characteristics (n=403)
| Demographics | All n (%) | High illness | Low illness | Males | Females | Age<75 years | Age 75-84 years | Age>84 years |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Total | 403 (100) | 219 (54) | 184 (46) | 181 (45) | 222 (55) | 78 (19) | 110 (27) | 215 (53) |
|
| ||||||||
| Age | ||||||||
| <60 | 10 (3) | 7 (3) | 3 (2) | 6 (3) | 4 (2) | 10 (13) | – | – |
| 60–64 | 11 (3) | 7 (3) | 4 (2) | 6 (3) | 5 (2) | 11 (14) | – | – |
| 65–74 | 57 (14) | 35 (17) | 22 (11) | 30 (17) | 27 (12) | 57 (73) | – | – |
| 75–84 | 110 (27) | 62 (30) | 48 (25) | 56 (31) | 54 (24) | – | 110 (100) | – |
| 85–94 | 185 (46) | 83 (40) | 102 (53) | 74 (419) | 111 (50) | – | – | 185 (86) |
| >94 | 30 (8) | 15 (7) | 15 (8) | 9 (5) | 21 (10) | – | – | 30 (14) |
| Race | ||||||||
| American Indian/Alaska Native | 2 (0.5) | 1 (0.5) | 1 (0.5) | 1 (0.6) | 1 (0.5) | 1 (1) | 1 (0.9) | 0 |
| Asian | 4 (1) | 3 (1) | 1 (0.5) | 3 (2) | 1 (0.5) | 0 | 2 (2) | 2 (0.9) |
| African American | 9 (2) | 4 (2) | 5 (3) | 4 (2) | 5 (2) | 6 (8) | 1 (0.9) | 2 (0.9) |
| Hispanic or Latino | 5 (1) | 4 (2) | 1 (0.5) | 3 (2) | 2 (0.9) | 4 (5) | 1 (0.9) | 0 |
| Native Hawaiian/Pacific Islander | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| White | 382 (95) | 197 (94) | 185 (95) | 169 (93) | 213 (96) | 66 (85) | 105 (96) | 211 (98) |
| Unknown | 1 (0.2) | 0 | 1 (0.5) | 1 (0.6) | 0 | 0 | 0 | 0 |
| Gender | ||||||||
| Males | 181 (45) | 99 (47) | 82 (42) | 181 (100) | – | 42 (54) | 56 (519) | 83 (39) |
| Females | 222 (55) | 110 (53) | 112 (58) | – | 222 (100) | 36 (46) | 54 (49) | 132 (61) |
| Location | ||||||||
| Rural | 42 (10) | 17 (8) | 25 (13) | 24 (13) | 18 (8) | 8 (10) | 14 (13) | 20 (9) |
| Urban | 361 (90) | 192 (92) | 169 (87) | 157 (87) | 204 (92) | 70 (90) | 96 (87) | 195 (91) |
| Non-English ability | 10 (3) | 7 (3) | 3 (2) | 6 (3) | 4 (2) | 0 | 3 (3) | 7 (3) |
|
| ||||||||
| Psychosocial status | n (%) | |||||||
|
| ||||||||
| Cognitive functioning | ||||||||
| 0 - Alert | 224 (55.6) | 112 (53.6) | 112 (57.7) | 105 (58.0) | 119 (53.6) | 52 (66.7) | 70 (63.6) | 102 (47.4) |
| 1 - Requires prompting during stress | 139 (34.5) | 75 (35.9) | 64 (33.0) | 62 (34.3) | 77 (34.7) | 22 (28.2) | 32 (29.1) | 85 (39.5) |
| 2 - Requires direction for specific situation | 32 (7.9) | 16 (7.7) | 16 (8.2) | 12 (6.6) | 20 (9.0) | 4 (5.1) | 6 (5.5) | 22 (10.2) |
| 3 - Requires considerable assistance | 6 (1.5) | 4 (1.9) | 2 (1.0) | 3 (0.6) | 5 (2.3) | 0 | 1 (0.9) | 5 (2.3) |
| 4 - Totally dependant | 2 (0.5) | 2 (1.0) | 0 | 1 (0.6) | 1 (0.5) | 0 | 1 (0.9) | 1 (0.5) |
| Anxiety | ||||||||
| 0 - No anxiety | 234 (58.1) | 101 (48.3) | 133 (68.6) | 113 (62.4) | 121 (54.5) | 41 (52.6) | 57 (51.8) | 136 (63.3) |
| 1 - Less often than daily | 91 (22.6) | 44 (21.1) | 47 (24.2) | 34 (18.8) | 57 (25.7) | 16 (20.5) | 26 (23.6) | 49 (22.8) |
| 2 - Daily, not constantly | 73 (18.1) | 59 (28.2) | 14 (7.2) | 32 (17.7) | 41 (18.5) | 18 (23.1) | 26 (23.6) | 29 (13.5) |
| 3 - All the time | 5 (1.2) | 5 (2.4) | 0 | 2 (1.1) | 3 (1.4) | 3 (3.8) | 1 (0.9) | 1 (0.5) |
| Depression | 70 (17.4) | 47 (22.5) | 23 (11.9) | 33 (18.2) | 37 (16.7) | 24 (30.8) | 17 (15.5) | 29 (13.5) |
| Living situation | ||||||||
| Living alone | 122 (30.3) | 59 (28.2) | 63 (32.5) | 43 (23.8) | 79 (35.6) | 21 (26.9) | 32 (29.1) | 69 (32.1) |
| Living with others | 281 (69.7) | 150 (71.8) | 131 (67.5) | 138(76.2) | 143(64.4) | 57 (73.1) | 78 (70.9) | 146(67.9) |
|
| ||||||||
| HF disease characteristics | n (%)/Mean (SD) Range |
|||||||
|
| ||||||||
| Type of HF diagnosis | ||||||||
| Primary | 190 (47.9) | 102 (46.6) | 88 (47.8) | 81 (44.8) | 109 (49.1) | 24 (30.8) | 47 (42.7) | 119 (55.3) |
| Secondary | 213 (52.1) | 117 (53.4) | 96 (52.2) | 100 (55.2) | 113 (50.9) | 54 (69.2) | 63 (57.3) | 96 (44.7) |
| Status of HF diagnosis | ||||||||
| Newly diagnosed | 33 (8.2) | 18 (8.6) | 15 (7.7) | 15 (8.3) | 18 (8.1) | 6 (7.7) | 10 (9.1) | 17 (7.9) |
| Chronic HF | 327 (81.1) | 169 (80.9) | 158 (81.4) | 147 (81.2) | 180 (81.1) | 62 (79.5) | 86 (78.2) | 179 (83.3) |
| Unknown | 43 (10.7) | 22 (10.5) | 21 (10.8) | 19 (10.5) | 24 (10.8) | 10 (12.8) | 14 (12.7) | 19 (8.8) |
| Ambulation (at admission) | ||||||||
| 0 – Independently walk | 31 (7.7) | 14 (6.7) | 17 (8.8) | 21 (11.6) | 10 (4.5) | 8 (10.3) | 14 (12.7) | 9 (4.2) |
| 1- Use of a device to walk | 283 (70.2) | 137 (65.6) | 146 (75.3) | 128 (70.7) | 155 (69.8) | 58 (74.4) | 82 (74.5) | 143 (66.5) |
| 2 – Supervised walking only | 79 (19.6) | 49 (23.4) | 30 (15.5) | 30 (16.6) | 49 (22.1) | 10 (12.8) | 12 (10.9) | 57 (26.5) |
| 3 – Chairfast, independent using wheelchair | 5 (1.2) | 5 (2.4) | 1 (0.5) | 2 (1.1) | 3 (1.4) | 1 (1.3) | 2 (1.8) | 2 (0.9) |
| 4 – Chairfast, unable to wheel self | 5 (1.2) | 4 (1.9) | 0 | 0 | 5 (2.3) | 1 (1.3) | 0 | 4 (1.9) |
| 5 – Bedbound | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnea (at admission) | ||||||||
| 0 - Never | 24 (6.0) | 9 (4.3) | 15 (7.7) | 7 (3.9) | 17 (7.7) | 3 (3.8) | 4 (3.6) | 17 (7.9) |
| 1 – Climbing stairs | 133 (33.0) | 43 (20.6) | 90 (46.4) | 68 (37.6) | 65 (29.3) | 28 (35.9) | 33 (30.0) | 72 (33.5) |
| 2 – With dressing, bathing | 152 (37.7) | 63 (30.1) | 89 (45.9) | 59 (32.6) | 93 (41.9) | 26 (33.3) | 47 (42.7) | 79 (36.7) |
| 3 – With eating, conversation | 76 (18.9) | 76 (36.4) | 0 | 38 (21.0) | 38 (17.1) | 19 (24.4) | 22 (20.0) | 35 (16.3) |
| 4 – At rest | 18 (4.5) | 18 (8.6) | 0 | 9 (5.0) | 9 (4.1) | 2 (2.6) | 4 (3.6) | 12 (5.6) |
| No of co-morbidities | 5.19 (1.9) | 6.06 (2.1) | 4.25 (1.9) 4 | 5.4 (1.9) | 5.01 (1.9) | 5.55 (2.2) | 5. 27 (1.9) | 5. 01 (1.8) |
| 10 (1 – 11) | 10 (1-11) | (2 – 6) | 10 (1 –11) | 9 (2 – 11) | 9 (2 – 11) | 9 (2 – 11) | 10 (1 – 11) | |
| Type of co-morbidity | ||||||||
| a) Blood pressure | 277 (68.7) | 143 (68.4) | 134 (69.1) | 123 (68.0) | 154 (69.4) | 51 (65.4) | 77 (70.0) | 149 (69.3) |
| b) Vascular | 269 (66.8) | 157 (75.1) | 116 (59.8) | 141 (77.9) | 132 (59.5) | 54 (69.2) | 76 (69.1) | 143 (66.5) |
| c) Cardiac Arrhythmia | 213 (52.9) | 109 (52.2) | 100 (51.5) | 92 (50.8) | 121 (54.5) | 35 (44.9) | 57 (51.8) | 121(56.3) |
| d) Pulmonary | 185 (45.9) | 126 (60.3) | 59 (30.4) | 83 (45.9) | 102 (45.9) | 47 (60.3) | 57 (51.8) | 81 (37.7) |
| e) Diabetes mellitus | 144 (35.7) | 88 (42.1) | 56 (28.9) | 74 (40.9) | 70 (31.5) | 43 (55.1) | 50 (45.5) | 51 (23.7) |
| f) Musculoskeletal | 140 (34.7) | 89 (42.6) | 51 (26.3) | 62 (34.3) | 78 (35.1) | 22 (28.2) | 40 (36.4) | 78 (36.3) |
| g) Renal | 97 (24.1) | 66 (31.6) | 31 (16.0) | 52 (28.7) | 45 (20.3) | 17 (21.8) | 26 (23.6) | 54 (25.1) |
| h) Thyroid | 86 (21.3) | 58 (27.8) | 28 (14.4) | 26 (14.4) | 60 (27.0) | 14 (17.9) | 17 (15.5) | 55 (25.6) |
| i) Gastro-intestinal | 82 (20.4) | 56 (26.8) | 26 (13.4) | 37 (20.4) | 45 (20.3) | 13 (16.7) | 22 (20.0) | 47 (21.9) |
| j) Anaemia | 72 (17.9) | 37 (17.7) | 35(18.0) | 25 (13.8) | 47 (21.2) | 11 (14.1) | 16 (14.5) | 45 (20.9) |
| k) Depression | 55 (13.6) | 36 (17.2) | 19 (9.8) | 23 (12.7) | 32 (14.4) | 15 (19.2) | 14 (12.7) | 26 (12.1) |
| l) Benign prostrate hyperplasia(BPH) | 48 (11.9) | 31 (14.8) | 17 (8.8) | 48 (26.5) | 0 | 6 (7.7) | 15 (13.6) | 27 (12.6) |
| m) Neurological | 42 (10.4) | 24 (11.5) | 18 (9.3) | 19 (10.5) | 23 (10.4) | 7 (9.0) | 8 (7.3) | 27 (12.6) |
| n) Dementia | 33 (8.2) | 19 (9.1) | 14 (7.2) | 19 (10.5) | 14 (6.3) | 2 (2.6) | 9 (8.2) | 22 (10.2) |
| o) Anxiety | 32 (7.9) | 25 (12.0) | 7 (3.6) | 8 (4.4) | 23 (10.4) | 9 (11.5) | 5 (4.5) | 17 (7.9) |
| p) Valvular | 31 (7.7) | 12 (5.7) | 19 (9.8) | 14 (7.73) | 13 (5.8) | 11 (14.1) | 4 (3.6) | 13 (6.0) |
| q) Cancer | 23 (5.7) | 12 (5.7) | 11 (5.7) | 9 (5.0) | 14 (6.3) | 6 (7.7) | 10 (9.1) | 7 (3.3) |
| r) Post acute cardiac Event (e.g: MI) | 21 (5.2) | 12 (5.7) | 9 (4.6) | 10 (5.5) | 11 (5.0) | 5 (6.4) | 4 (3.6) | 12 (5.6) |
| s) Obesity | 17 (4.2) | 14 (6.7) | 3 (1.5) | 5 (2.8) | 12 (5.4) | 11 (14.1) | 3 (2.7) | 3 (1.4) |
| t) Skin disorders | 15 (3.7) | 9 (4.3) | 6 (3.1) | 5 (2.8) | 10 (4.5) | 3 (3.8) | 4 (3.6) | 8 (3.7) |
| u) Nutrition disorders/metabolic | 9 (2.2) | 5 (2.4) | 4 (2.1) | 2 (1.1) | 7 (3.2) | 2 (2.6) | 3 (2.7) | 4 (1.9) |
| v)Infection | 4 (1) | 2 (1.0) | 2 (1.0) | 1 (0.6) | 3 (1.4) | 2 (2.6) | 1 (0.9) | 1 (0.5) |
|
| ||||||||
| Decision support | n (%)/Mean (SD) Range |
|||||||
|
| ||||||||
| Type of cardiac medications | ||||||||
| a) ACEI/ARB | 204 (50.6) | 96 (45.9) | 108 (55.7) | 89 (49.2) | 115 (51.8) | 44 (56.4) | 53 (48.2) | 107 (49.8) |
| b) Beta-blockers | 319 (79.2) | 162 (77.5) | 157 (80.9) | 146 (80.7) | 173 (77.9) | 60 (76.9) | 88 (80.0) | 171 (79.5) |
| c) Diuretics | 344 (85.4) | 180 (86.1) | 164 (84.5) | 154 (85.1) | 190 (85.6) | 66 (84.6) | 95 (86.4) | 183 (85.1) |
| Total number of medications | 13.6 (4.8) | 15.81(4.9) | 11.21(3.3) | 13.9 (4.8) | 13.4 (4.7) | 15.8 (4.9) | 13.8 (5.2) | 12.7 (4.2) |
| 27 (4 – 31) | 26 (5 – 31) | 13 (4 – 17) | 22 (5 – 27) | 27 (4 – 31) | 19 (7 – 26) | 27 (4 – 31) | 22 (5 – 27) | |
Demographic characteristics were similar for the illness, age and gender strata, except in the over 85 years old age category, where females (61%) outnumbered males (39%). Among psychosocial characteristics, compared to the less illness severity stratum, the high illness severity stratum had four times the number of subjects with severe anxiety and twice the number of subjects with depression. More females than males lived alone (36% versus 24%). Vascular disorders were more common in males (77%) than in females (59%). All other variables were generally similar across strata (Table 1).
Of the sample, 112 (29%) experienced at least one all-cause re-hospitalization and 73 (19%) experienced at least one cardiac related re-hospitalization. The remaining cases were censored either because they did not experience re-hospitalization at the end of 60 days of being on telehealth (39), they were discharged from the homecare agency (215) or they withdrew from telehealth services (37). The reasons for discharge from the homecare agency discharge included patient or caregiver being able to demonstrate competence with health management or if homecare services were no longer needed. Patients were also discharged if they were able to travel and were no longer homebound and/or no longer eligible for homecare service.
Cox regression analysis
All-cause re-hospitalizations
The number of medications less than 10 (adjusted P=0.016) and prescription of ACE/ARB medications (adjusted P=0.004) were significantly associated with lower risk of re-hospitalization. Selected patient characteristics were also associated with a higher risk of re-hospitalization (adjusted P<0.05 in all instances): psychiatric disorders; anaemia, thyroid disorders and dyspnoea for males; renal and pulmonary co-morbidity for females; obesity and pulmonary co-morbidity for those under 75 years of age; and presence of anxiety for those aged over 84 years (Table 2).
Table 2.
Variables associated with all-cause hospitalization at P<0.1 in each stratum
| Stratum | Variables associated with all-cause hospitalization | Hazard ratio* (95% CI) | P-value |
|---|---|---|---|
| All (n=403) |
Low number of medications (<10) Medium number of medications (10-16) High number of medications (>16) Renal co-morbidity Prescription of ACEI/ARB |
0.46 (0.24 to 0.86) Reference variable 1.06 (.7 to 1.61) 1.49 (0.99 to 2.26) 0.57 (.39 to .84) |
0.016 – 0.799 0.057 0.004 |
| High illness severity (n=219) | Psychiatric co-morbidity | 0.51 (0.25 to 1.06) | 0.071 |
| Low illness severity (n=184) | Psychiatric co-morbidity Prescription of ACEI/ARB |
3.21 (1.3 to 7.9) 0.31 (0.14 to 0.67) |
0.012 0.003 |
| Males (n=181) |
Severe dyspnoea Anaemia co-morbidity Thyroid co-morbidity Living alone Neurological co-morbidity Prescription of ACEI/ARB |
1.9 (1.07 to 3.4) 2.57 (1.31 to 5.06) 2.39 (1.17 to 4.87) 0.34 (.15 to 0.79) 0.27 (0.06 to 1.15) 0.47 (0.26 to 0.87) |
0.03 0.006 0.016 0.012 0.076 0.015 |
| Females (n=222) |
Prescription of beta-blocker Renal system co-morbidity Pulmonary co-morbidity |
2.12 (1.0 to 3.06) 1.9 (1.08 to 3.37) 1.82 (1.08 to 3.06 |
0.051 0.027 0.025 |
| <75 years age (n=78) |
Prescription of beta-blocker Pulmonary co-morbidity Obesity |
4.14 (0.94 to 18.21) 3.43 (1.12 to 10.5) 2.69 (1.0 to 7.19) |
0.06 0.031 0.048 |
| 75–84 years age (n=110) | – | Model not significant | |
| >84 years age (n=215) |
No anxiety Low number of medications (<10) Medium number of medications (10-16) High number of medications (>16) Arrhythmia co-morbidity Primary HF diagnosis |
0.55 (0.3 to 1.01) 0.4 (0.18 to 0.91) Reference variable 1.46 (0.78 to 2.75) 0.6 (0.36 to 1.0) 1.68 (0.98 to 2.87) |
0.054 0.028 – 0.236 0.051 0.06 |
Increase in odds ratio associated with 1 unit increase in predictor
Cardiac-related re-hospitalizations
Patients with fewer than 10 medications (adjusted P=0.009) and prescription of ACE/ARB (adjusted P=0.027) were also significantly associated with a lower risk of cardiac related re-hospitalization. Selected patient characteristics were also associated with a higher risk of cardiac related re-hospitalization (adjusted P<0.05 in all instances): psychiatric disorders; diabetes within the high illness severity stratum; obesity for those under 75 years age; and a primary HF diagnosis for those over 84 years (Table 3).
Table 3.
Variables associated with cardiac related hospitalization at P<0.1 in each stratum
| Stratum | Variables associated with cardiac related hospitalization | Hazard ratio* (95% CI) | P-value |
|---|---|---|---|
| All (n=403) | Low number of medications (<10) Medium number of medications (10-16) High number of medications (>16) Primary HF diagnosis Prescription of ACEI/ARB |
0.32 (0.14 to 0.76) Reference variable 1.1 (0.66 to 1.84) 1.48 (0.93 to 2.36) 0.59 (0.37 to 0.94) |
0.009 – 0.718 0.096 0.027 |
| High illness severity (n=219) |
Psychiatric co-morbidity Prescription of diuretic Diabetes co-morbidity |
0.26 (0.1 to 0.82) 0.48 (0.26 to 0.89) 0.51 (0.29 to 0.89) |
0.022 0.02 0.018 |
| Low illness severity (n=184) |
Prescription of ACEI/ARB Psychiatric co-morbidity |
0.42 (0.15 to 1.16) 4.27 (1.36 to 13.41) |
0.095 0.013 |
| Males (n=181) | Prescription of ACEI/ARB | 0.62 (0.39 to 0.98) | 0.043 |
| Females (n=222) | Low number of medications (<10) Medium number of medications (10-16) High number of medications (>16) New HF diagnosis |
0.26 (0.06 to 1.12) Reference variable 1.58 (0.78 to 3.2) 2.2 (0.91 to 5.3) |
0.071 – 0.209 0.08 |
| <75 years age (n=78) |
Obesity | 4.29 (1.25 to 14.67) | 0.02 |
| 75–84 years age (n= 110) | None | Model not significant | |
| >84 years age (n=215) |
Low number of medications (<10) Medium number of medications (10-16) High number of medications (>16) Primary HF diagnosis |
0.36 (0.1 to 1.0) Reference variable 1.77 (0.85 to 3.67) 2.4 (1.12 to 4.8) |
0.056 – 0.126 0.013 |
Increase in odds ratio associated with 1 unit increase in predictor
Discussion
The gender distribution in the present study was almost identical to that of the national Medicare population, where females comprised 55% of the total Medicare beneficiaries, and the proportion of females was more than double in the oldest age category compared to males. The proportion of subjects living alone was also similar to that in the national Medicare population, which adds to the generalizability of the findings. Due to inclusion of drugs such as vitamins or health drinks, the average number of prescribed medications per patient was higher in the present study compared to the number of medications reported in patients with HF in other studies.[16–18] Anxiety and depression were more common in the high illness severity subjects than in low illness severity subjects in the present study. This is not surprising, as patients with HF who are severely ill tend to be anxious about their disease status and depressed about their quality of life.[19] The proportion of all-cause hospitalizations (29%) and cardiac related hospitalizations (19%) for HF patients using telehealth was similar to the proportion of all-cause hospitalizations (24-36%) [8,20–23] and cardiac-cause hospitalizations (12–32%) [6,8,21] in the telehealth intervention group in other studies.
A high number of medications (>16) was associated with both all-cause and cardiac-related re-hospitalizations. This is consistent with other studies, where adverse effects of poly-pharmacy on functional and health status of elderly individuals with heart failure have been reported.[8,18] Elderly patients in particular find it difficult to manage a high number of medications which can lead to health complications that result in re-hospitalizations, despite daily monitoring by telehealth. The use of multiple medications often leads to inappropriate drug use, under-prescription, low adherence, adverse drug interactions and side effects, as well as increased costs for elderly patients.[18,24] Homecare agencies could provide additional nursing resources and utilize daily monitoring by telehealth to closely assess and titrate heart failure medication therapy of elderly HF patients.
Non-prescription of ACEI/ARB was also associated with both all-cause and cardiac-related re-hospitalizations in the present study. Our findings support the literature endorsing the positive association of ACEI/ARB with reduced hospitalizations in patients with HF.[25] In the present study, ACEI/ARB were prescribed for only 50% of HF patients, compared to the 80% prescription rate for beta-blockers and diuretics. Allergic reactions or renal dysfunction could have contributed to non-prescription of ACEI/ARB. However, primary care physician (PCP) unfamiliarity with the guidelines on HF treatment may also have contributed to the low prescription rate of ACEI/ARB. Evidence supporting the association of ACEI/ARB with reduced hospitalization may persuade homecare agencies to advocate prescription of guideline-recommended HF medications for their patients.
Systematic reviews have found beneficial effects of beta-blockers in avoiding cardiac hospitalizations for patients with HF.[25] However, use of beta-blockers is suspected to be contra-indicated for patients with pulmonary disorders such as chronic obstructive pulmonary disorder (COPD).[26] The presence of pulmonary co-morbidity increased the odds of association of beta blockers with all-cause re-hospitalizations in the present study, although this relationship was observed only in female HF patients and HF patients younger than 75 years of age. Beta-blockers were not associated with cardiac re-hospitalizations. Daily telehealth monitoring could be used for gradual titration of beta-blockers, as has been recommended for patients with HF and pulmonary disorders.[26]
In previous studies, there have been increased re-hospitalizations and ER visits[27–29] for patients with HF and depression. Similarly, in our study, HF patients with psychiatric co-morbidity and using telehealth in the low illness severity stratum had higher odds of all-cause and cardiac related re-hospitalizations. Depression has been attributed to loss of interest in HF self-management[19] which might have resulted in higher odds of being hospitalized.
Surprisingly, in the high illness severity stratum, HF patients with psychiatric co-morbidity and using telehealth had lower odds of all-cause and cardiac related re-hospitalizations. Caring for HF patients with high illness severity is quite complex, and they tend to have poor outcomes irrespective of the presence of a psychiatric co-morbidity. Being diagnosed with a psychiatric co-morbidity and being prescribed anti-psychotics might have provided a protective effect which could explain the negative association with re-hospitalizations in the high illness severity stratum.
Medications for HF such as angiotensin-converting enzyme inhibitors (ACEI) or beta-blockers are normally under-prescribed for patients with HF and renal insufficiency due to suspected contra-indication in renal failure.[30] In our study, only 43% of subjects with renal co-morbidities were prescribed ACEI/ARB for HF, as recommended in the AHA guidelines. Presence of renal co-morbidity also complicates HF self-management. HF patients on dialysis were considered inappropriate for telehealth by homecare nurses because frequent weight fluctuations can trigger alarms inappropriately.[31] HF patients with renal co-morbidities have been found to have higher mortality[32] and high rates of hospitalization.[33] The lack of protocols for care delivery of heart patients with renal co-morbidities in the homecare setting could explain the positive association of renal co-morbidity with all-cause hospitalizations.
Patients admitted with HF as a primary diagnosis had higher likelihood of cardiac-related re-hospitalizations than patients admitted with HF as a secondary diagnosis, in spite of using telehealth. Primary HF diagnosis was also found to be a predictor of all-cause and cardiac related re-hospitalizations in the oldest age stratum (>85 years). Patients with primary HF have recently experienced HF exacerbation and need to cope with the complex care regimen and the new daily telehealth routine. Complex HF care management and changes to lifestyle routine in addition to coping with morbidity associated with HF might have been especially difficult for HF patients in the oldest-old age group possibly resulting in higher rate of re-hospitalizations.
Study limitations
The present study had several limitations. The patient variables were restricted to data available from the homecare EHR. HF severity measures such as ejection fraction, NYHA class or presence of implanted cardiac devices were not available. Telehealth vital sign values of blood pressure, oxygen saturation, heart rate and bodyweight that could affect outcomes were not collected for this study. Other relevant heart failure medications including aldosterone antagonists, hydralazines or nitrates were not collected for this study. A total of 94% of the sample population was Caucasian.
Conclusion
The findings of the present study contribute to our understanding of healthcare utilization by HF patients using telehealth. They could also inform practice guidelines for the delivery of homecare to HF patients within the context of their characteristics, especially co-morbidities. For example, homecare agencies and their nursing staff can anticipate the possibility of increased hospitalizations for HF patients on telehealth with high number of medications, renal or psychiatric related co-morbidities and could plan to allot resources accordingly. Such knowledge should assist home health agencies in developing tailored care-plans to attain the best patient outcomes and help them to allocate resources without compromising patient care.
Acknowledgments
The study received partial financial support through the Beta Zeta chapter of the Sigma Theta Tau association.
This manuscript was prepared during training grant T32NR009356 at University of Pennsylvania NewCourtland Center for Transitions and Aging, the training program in Individualized Care for At Risk Older Adults.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dansky KH, Vasey J, Bowles K. Impact of telehealth on clinical outcomes in patients with heart failure. Clin Nurs Res. 2008;17:182–99. doi: 10.1177/1054773808320837. [DOI] [PubMed] [Google Scholar]
- 4.Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14:300–5. doi: 10.1258/jtt.2008.071213. [DOI] [PubMed] [Google Scholar]
- 5.Benatar D, Bondmass M, Ghitelman J, Avitall B. Outcomes of chronic heart failure. Arch Intern Med. 2003;163:347–52. doi: 10.1001/archinte.163.3.347. [DOI] [PubMed] [Google Scholar]
- 6.Gambetta M, Dunn P, Nelson D, Herron B, Arena R. Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort. Prog Cardiovasc Nurs. 2007;22:196–200. doi: 10.1111/j.0889-7204.2007.06483.x. [DOI] [PubMed] [Google Scholar]
- 7.Pekmezaris R, Mitzner I, Pecinka KR, et al. The impact of remote patient monitoring (telehealth) upon Medicare beneficiaries with heart failure. Telemed J E Health. 2012;18:101–8. doi: 10.1089/tmj.2011.0095. [DOI] [PubMed] [Google Scholar]
- 8.Dar O, Riley J, Chapman C, et al. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Fail. 2009;11:319–25. doi: 10.1093/eurjhf/hfn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakefield BJ, Holman JE, Ray A, et al. Outcomes of a home telehealth intervention for patients with heart failure. J Telemed Telecare. 2009;15:46–50. doi: 10.1258/jtt.2008.080701. [DOI] [PubMed] [Google Scholar]
- 10.Bowles KH, Hanlon AL, Glick HA, et al. Clinical effectiveness, access to, and satisfaction with care using a telehomecare substitution intervention: a randomized controlled trial. Int J Telemed Appl. 2011;2011:540138. doi: 10.1155/2011/540138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowles KH, Holland DE, Horowitz DA. A comparison of in-person home care, home care with telephone contact and home care with telemonitoring for disease management. J Telemed Telecare. 2009;15:344–50. doi: 10.1258/jtt.2009.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortara A, Pinna GD, Johnson P, et al. Home telemonitoring in heart failure patients: the HHH study (Home or Hospital in Heart Failure) Eur J Heart Fail. 2009;11:312–8. doi: 10.1093/eurjhf/hfp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–98. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Tabachnick BG, Fidell LS. Using Multivariate Statistics. Fourth. Needham Heights, MA: Allyn & Bacon; 2001. [Google Scholar]
- 16.Dunlay SM, Eveleth JM, Shah ND, McNallan SM, Roger VL. Medication adherence among community-dwelling patients with heart failure. Mayo Clin Proc. 2011;86:273–81. doi: 10.4065/mcp.2010.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52:675–84. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 18.Volpe M, Chin D, Paneni F. The challenge of polypharmacy in cardiovascular medicine. Fundam Clin Pharmacol. 2010;24:9–17. doi: 10.1111/j.1472-8206.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- 19.Riegel B, Lee CS, Dickson VV. Medscape. Self care in patients with chronic heart failure. Nat Rev Cardiol. 2011;8:644–54. doi: 10.1038/nrcardio.2011.95. [DOI] [PubMed] [Google Scholar]
- 20.Giordano A, Scalvini S, Zanelli E, et al. Multicenter randomised trial on home-based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int J Cardiol. 2009;131:192–9. doi: 10.1016/j.ijcard.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Hoover Ph D CA. Home telemonitoring and heart failure outcomes. The University of Wisconsin - Milwaukee; 2008. [Google Scholar]
- 22.Morguet AJ, Kühnelt P, Kallel A, Jaster M, Schultheiss HP. Impact of telemedical care and monitoring on morbidity in mild to moderate chronic heart failure. Cardiology. 2008;111:134–9. doi: 10.1159/000119701. [DOI] [PubMed] [Google Scholar]
- 23.Scalvini S, Capomolla S, Zanelli E, et al. BenignPaletta L, Glisenti F, Giordano A. Effect of home-based telecardiology on chronic heart failure: costs and outcomes. J Telemed Telecare. 2005;11(Suppl 1):16–8. doi: 10.1258/1357633054461688. [DOI] [PubMed] [Google Scholar]
- 24.Leibundgut G, Pfisterer M, Brunner-La Rocca HP. Drug treatment of chronic heart failure in the elderly. Drugs Aging. 2007;24:991–1006. doi: 10.2165/00002512-200724120-00003. [DOI] [PubMed] [Google Scholar]
- 25.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins NM, Petrie MC, Macdonald MR, et al. Heart failure and chronic obstructive pulmonary disease the quandary of Beta-blockers and Beta-agonists. J Am Coll Cardiol. 2011;57:2127–38. doi: 10.1016/j.jacc.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Fulop G, Strain JJ, Stettin G. Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44:367–73. doi: 10.1176/appi.psy.44.5.367. [DOI] [PubMed] [Google Scholar]
- 28.Himelhoch S, Weller WE, Wu AW, Anderson GF, Cooper LA. Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries. Med Care. 2004;42:512–21. doi: 10.1097/01.mlr.0000127998.89246.ef. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan M, Simon G, Spertus J, Russo J. Depression-related costs in heart failure care. Arch Intern Med. 2002;162:1860–6. doi: 10.1001/archinte.162.16.1860. [DOI] [PubMed] [Google Scholar]
- 30.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–9. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 31.Radhakrishnan K, Jacelon C, Roche J. Perceptions on the Use of Telehealth by Homecare Nurses and Patients With Heart Failure: A Mixed Method Study. Home Health Care Management Practice. 2012 doi: 10.1177/1084822311428335. Published online first: January 13, 2012. [DOI] [Google Scholar]
- 32.Fonarow GC, Adams KF, Jr, Abraham, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 33.Ahluwalia SC, Gross CP, Chaudhry SI, et al. Impact of comorbidity on mortality among older persons with advanced heart failure. J Gen Intern Med. 2012;27:513–9. doi: 10.1007/s11606-011-1930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


