Abstract
The diverse roles of chemokines in normal immune function and many human diseases have motivated numerous investigations into the structure and function of this family of proteins. Recombinant chemokines are often used to study how chemokines coordinate the trafficking of immune cells in various biological contexts. A reliable source of biologically active protein is vital for any in vitro or in vivo functional analysis. In this chapter, we describe a general method for the production of recombinant chemokines and robust techniques for efficient refolding that ensure consistently high biological activity. Considerations for initiating development of protocols consistent with Current Good Manufacturing Practices (cGMPs) to produce biologically active chemokines suitable for use in clinical trials are also discussed.
1. INTRODUCTION
Chemokine is shorthand for chemoattractant cytokine, a family of proteins that directs the trafficking of immune cells during normal immune function and participates in many disease states (Baggiolini, 2001). For example, the chemokines CCL19 and CCL21 recruit antigen-presenting dendritic cells and naïve T-cells that express the chemokine receptor CCR7 to the lymph nodes thereby priming the immune responses (Forster, Davalos-Misslitz, & Rot, 2008). However, CCR7 and its ligands, CCL19 and CCL21, also play roles in the dissemination, migration and metastasis of cancer cells that express CCR7 (Legler, Uetz-von Allmen, & Hauser, 2014). Other chemokines play similar roles in normal immune function and human pathologies including cardiovascular disease, arthritis, asthma, cancer, HIV-AIDS, and many others. Consequently, the ~50 members of the chemokine family and their G protein-coupled receptors are intensely studied as targets for drug development (Kufareva, Salanga, & Handel, 2015; O'Hayre, Salanga, Handel, & Hamel, 2010).
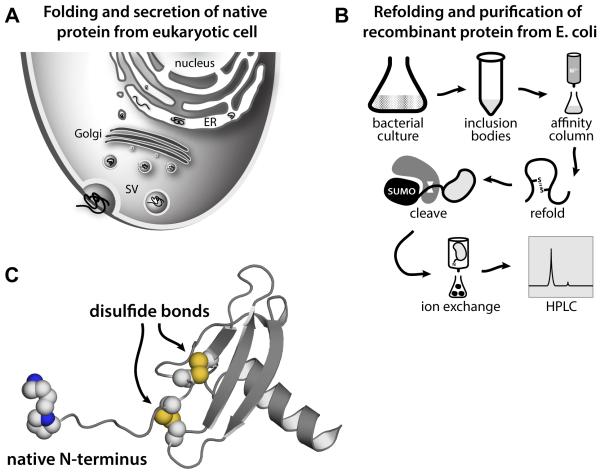
A supply of bioactive chemokine protein is essential to the study of chemokine function and to be fully active the protein must be properly folded. Figure 1 illustrates the differences in natural eukaryotic and recombinant bacterial production of chemokines, each of which is optimized to yield a natively-folded, bioactive protein.
Figure 1.
Recombinant chemokine production reproduces the natively folded bioactive protein secreted from eukaryotic cells. A) Folding, transport, and secretion of chemokines in eukaryotic cells. B) Schematic diagram of expression, purification and refolding of recombinant chemokines from E. coli. C) The 3D structure of human CXCL12 illustrates the conserved chemokine fold with structurally important disulfide bonds and functionally important native N-terminus.
The chemokine fold (Figure 1C) consists of a flexible N-terminus, an N-loop, occasionally a 310 helix, an antiparallel three-stranded β-sheet, and a C-terminal α-helix. Within the antiparallel three stranded β-sheet, the β1-strand is connected to β2-strand by the 30s loop and β2-strand is linked to β3-strand by the 40s loop. Chemokines generally contain four cysteines that form two conserved disulfide bonds that stabilize a chemokine’s tertiary structure. Two of these cysteines are located between the N-terminus and the N-loop while the others are located in the 30’s loop and the β3-strand. The first cysteine in a chemokine sequence pairs with the third cysteine in the chemokine’s 30s loop and the second cysteine in a chemokine sequence pairs with the fourth cysteine in the β3-strand. A few chemokines contain one fewer or one additional disulfide bond. The metamorphic chemokine XCL1 has only two cysteines that form one disulfide bond, which permits unfolding and interconversion between two unrelated folded structures, the canonical chemokine domain or an all β-sheet structure (Tyler, Murray, Peterson, & Volkman, 2011). Other chemokines, like CCL21 or CCL28, contain six cysteines corresponding to a novel third disulfide in addition to the two conserved disulfides (Love, Sandberg, Ziarek, Gerarden et al., 2012; Thomas, Buelow, Nevins, Jones et al., 2015).
Production of functional chemokines can be inherently challenging due to the chemokine fold. For example, chemokines, whether produced by chemical synthesis or heterologous expression in E. coli, are initially unfolded, unoxidized and nonfunctional (Clark-Lewis, 2000; Edgerton, Gerlach, Boesen, & Allet, 2000; Lu, Burns, McDevitt, Graham et al., 2009; Proudfoot & Borlat, 2000; Veldkamp, Peterson, Hayes, Mattmiller et al., 2007). In order to become functional, synthetic or recombinant chemokines must be refolded, a process that requires cysteine oxidation to achieve the correct pattern of conserved disulfide bonds (Clark-Lewis, 2000; Edgerton et al., 2000; Lu et al., 2009; Proudfoot et al., 2000; Veldkamp et al., 2007). Approaches for refolding chemokines have included: dialysis, infinite dilution, and on-column techniques (Clark-Lewis, 2000; Edgerton et al., 2000; Lu et al., 2009; Proudfoot et al., 2000; Veldkamp et al., 2007). Another difficulty associated with producing functional recombinant chemokines is that in vivo chemokines are secreted proteins and the mature N-terminus that results from removal of the signal sequence of the chemokine is essential for activity. Hence, care must be taken so that the removal of any fusion protein or purification tags used in the production of recombinant chemokines yields a native, mature N-terminus (Lu et al., 2009; Proudfoot et al., 2000; Veldkamp et al., 2007).
For example, we have previously produced both XCL1 and CXCL12 using a system in which an N-terminal hexahistidine tag used to purify the chemokine was removed using a TEV protease. In the case of XCL1, this protocol produced XCL1 without its N-terminal valine residue, a change that rendered the folded protein completely inactive. In the instance of CXCL12, this system left a gly-ser dipeptide preceding the mature, native N-terminal Lys residue producing a protein that was much less active. This is not unexpected, as the sequence within the N-terminus and particularly the N-terminal lysine residue of CXCL12 were shown by Crump et al. to be essential for chemokine activity (Crump, Gong, Loetscher, Rajarathnam et al., 1997). In fact, proteases that act on CXCL12 in vivo, like CD26/ dipeptidyl peptidase, matrix metalloprotease 2, or exopeptidases, cleave the N-terminus of mature, native CXCL12 to yield inactive proteins (De La Luz Sierra, Yang, Narazaki, Salvucci et al., 2004; Segers, Tokunou, Higgins, MacGillivray et al., 2007; Sierra, Yang, Narazaki, Salvucci et al., 2003). CXCL12 variants that are resistant to proteolysis while retaining agonist properties have been developed as potentially useful tools (Segers et al., 2007). Some chemokines, like CXCL5, become more active agonist upon proteolysis of their mature N-terminus (Mortier, Loos, Gouwy, Ronsse et al., 2010; Nufer, Corbett, & Walz, 1999). Thus, there may be reasons for producing recombinant chemokines with mature, native N-termini or varying N-terminal lengths and sequences. We and others have adopted the protein SUMO (SMT3) as a fusion protein for chemokine production in order to take advantage of the SUMO-specific protease ULP1 (Figure 2B). As ULP1 recognizes the SMT3 fold and cleaves the amide linking the SMT3 C-terminus to the chemokine in the SMT3-chemokine fusion (Lu et al., 2009), this system allows for the production of chemokines with native or altered N-termini.
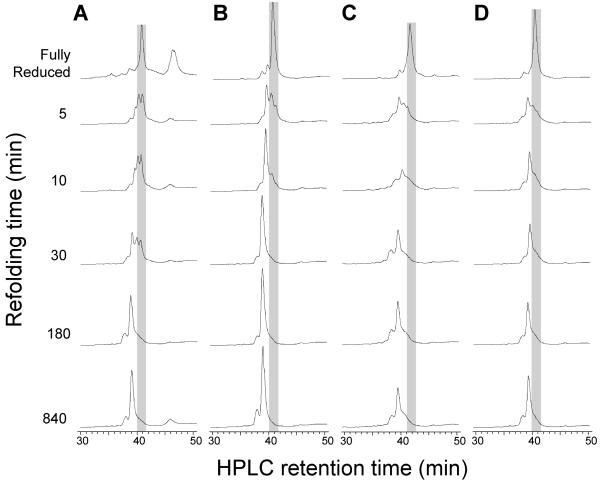
Figure 2.
Kinetics of CXCL12 refolding in different redox buffers. Refolding of fully reduced CXCL12 (gray shading) was initiated by infinite dilution of the IMAC elution fractions into the refolding buffer and quenched by acidification. Disulfide formation was monitored by the shift to an earlier reverse phase HPLC retention time. Rates of refolding varied significantly for CXCL12 in (A) the absence of a redox agent or the presence of (B) cysteine/cystine, (C) β-mercaptoethanol/2-hydroxyethyl disulfide or (D) reduced/oxidized glutathione (GSH/GSSG).
There are many approaches to producing functional recombinant chemokines from E. coli in addition to the ones referenced or presented here. The goal of this work is to describe a robust protocol we employ for many different chemokines, which draw from a variety of previously described approaches. We illustrate the impact of disulfide shuffling cocktails on the efficiency and yield of chemokine refolding and oxidation. Importantly, protocols used to assess the quality of the chemokines produced are also described. Finally, our recent observations from planning the development of cGMP protocols for chemokines are presented.
2. METHODS
This is a general protocol for purification of chemokines with expression sequences that include an N-terminal His6 tag and a SMT3 cleavage site. Removal of the His6-SMT3 from the chemokine with ubiquitin like protease-1 (ULP1 or SUMO-protease-1) results in a native, mature chemokine N-terminus that is suitable for all experimental applications (Lu et al., 2009).
2.1 Chemokine expression, refolding and purification
Although many purification tags, fusion proteins and cleavage options exist, we and others have found expression of a His6-SMT3-Chemokine produces insoluble protein of sufficient yields that refolds, in most cases, robustly and can be purified in a straightforward manner (Lu et al., 2009). While an abundance of refolding techniques have been used with chemokines, the infinite dilution refolding approach presented here works well for most chemokines and we present data on how solution conditions were optimized for refolding speed.
2.1.1 Required materials
Synthetic gene coding for a SMT3-Chemokine fusion protein cloned into the BsaI and HindIII/PstI of either pQE30 or pET28a.
Competent BL21 [pREP4] E. coli (for pQE30 expression plasmids) or BL21 (DE3) E. coli (for pET28a expression plasmids)
Buffer A: 50 mM sodium phosphate, 300 mM sodium chloride, 10 mM imidazole, 1 mM phenylmethylsulfonyl fluoride, 0.1% 2-mercaptoethanol, pH 8.0
Buffer AD: 6 M guanidine HCl, 50 mM sodium phosphate, 300 mM sodium chloride, 10 mM imidazole, 0.1% 2-mercaptoethanol, pH 8.0
Buffer BD: 6 M guanidine HCl, 100 mM sodium acetate, 300 mM sodium chloride, 10 mM imidazole, pH 4.5
Refolding buffers -
Option 1: 100 mM Tris, 10 mM cysteine, 0.5 mM cystine, pH 8.0
Option 2: 100 mM Tris, 3 mM cysteine, 0.5 mM cystine, pH 8.0
Option 3: 100 mM Tris, 10 mM cysteine, 0.5 mM cystine, 10% glycerol pH 8.0
Option 4: 100 mM Tris, 10 mM cysteine, 0.5 mM cystine, 150mM NaCl, pH 8.0
Cation exchange binding: 100 mM Tris, 25 mM sodium chloride, pH 8.0
Cation exchange wash: 100 mM Tris, 50 mM sodium chloride, pH 8.0
Cation exchange elution: 100 mM Tris, 2 M sodium chloride, pH 8.0
RP-HPLC buffer A: Aqueous 0.1% triflouroacetic acid
RP-HPLC buffer B: Aqueous 0.1% triflouroacetic acid, 70% acetonitrile
RP-HPLC column: 218TP510
Lysogeny broth or [U-15N] or [U-15N/13C] M9 minimal media (with 150 μg/mL ampicillin and 50 μg/ml kanamycin for pQE30 expression plasmids or 50 μg/mL kanamycin for pET28a expression plasmids).
2-4 mL of nickel affinity resin
S HyperD F cation exchange resin/column Pall Life Sciences
15 mL disposable column (Qiagen or Thermofisher)
1 M isopropyl-β-D-1-thiogalactopyranoside (IPTG)
French pressure cell or Sonicator
2.1.2 Transformation, expression and cell harvesting
Transform the expression plasmid following the instructions in the QIA expressionist for pQE30 plasmids into BL21 [pREP4] E. coli or the pET system manual for pET28a plasmids into BL21 (DE3) E. coli (Novagen, 2005).
If desired, small scale expression testing can be done at this point to optimize growth conditions (Waltner, Peterson, Lytle, & Volkman, 2005), but the conditions outlined below provide a good starting point.
Grow a 1 L culture at 37°C using either Lysogeny broth or [U-15N] or [U-15N/13C] M9 minimal media with appropriate antibiotics (150 μg/mL ampicillin/50 μg/ml kanamycin for pQE30 expression plasmids or 50 μg/mL kanamycin for pET28a expression plasmids). At an optical density at 600 nm of 0.6 induce expression with 1 mL of 1 M IPTG.
After 5 hours collect the cells by centrifugation at 5,000 × g for 10 minutes and store the cell pellet at −20 to −80 °C until further processing.
2.1.3 Nickel affinity chromatography
The His6-SMT3-chemokine can be isolated from E. coli proteins and cell debris through nickel affinity chromatography.
Completely resuspend the cells by pipetting and repipetting the same 10 mL of buffer A until no cell pellet remains. Vortexing or a rocking incubation are also effective.
Lyse the resuspended cells with three passes through a French pressure cell (desired PSI 10,000) or with sonication pulsed at ~25 % power for 10 seconds on and 10 seconds off for 10-15 minutes.
Collect the His6-SMT3-chemokine inclusion body pellet by centrifugation at 15,000 × g for 30 minutes. The His6-SMT3-chemokine, in most cases so far, is predominately insoluble and in the pellet, but both the supernatant and pellet should be saved if the location of His6-SMT3-chemokine is unknown.
Dissolve the inclusion body pellet in 10 mL of buffer AD and clarify using centrifugation at 15,000 × g for 30 minutes. A 10 mL syringe with a 16-gauge needle can be used to help break up the inclusion body pellet along with incubating and shaking at 37 °C. Membrane fragments should be translucent.
Bind the supernatant containing the His6-SUMO-chemokine to 2-4 mL of nickel affinity resin in batch mode using a 15 mL disposable column with rocking for 30 minutes at room temperature.
Place the column on a ring stand. Collect the flow through and wash the resin with four 10 mL portions of buffer AD.
Elute the His6-SUMO-chemokine from the nickel affinity resin with 10 mL of buffer BD into 15 mL conical vials. Shake the vial, if bubbles form that do not quickly dissipate the fraction contains protein. Three 10 mL portions of buffer BD is usually enough to completely elute the column.
2.1.4 Refolding
Infinite dilution with a disulfide reshuffling cocktail allows protein refolding. Selection of the disulfide reshuffling cocktail will affect refolding efficiency and total time because proper refolding is only achieved when cysteines are oxidized and in the correct disulfide pairs. Figure 2 shows the effect of various disulfide-reshuffling cocktails on CXCL12 refolding.
In our experience with a number of chemokines, cysteine/cystine provides optimal refolding kinetics, higher yields and lower reagent cost than the alternatives.
Pool the eluted fractions that contain the His6-SUMO-chemokine and determine the volume.
Refold by infinite dilution into a volume of refolding buffer that is twelve times the volume of the pooled elutions. Add the pooled elutions dropwise (45 drops per minute) with stirring to refolding buffer option 1, 2, 3, or 4. In most cases, refolding buffer option 1 is optimal.
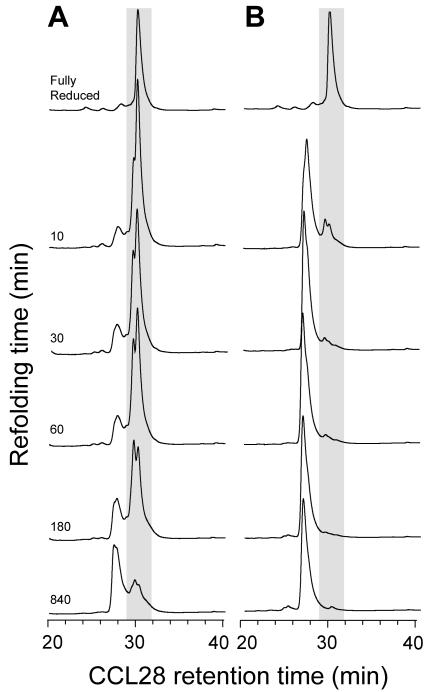
Figure 3 shows that the cysteine/cystine redox pair can accelerate and improve the refolding of complex disulfide pairings.
Incubate with stirring overnight at 4 °C.
Figure 3.
Kinetics of CCL28 folding. (A) In the absence of a disulfide shuffling redox pair, folding of CCL28 is slow and inefficient. (B) Refolding with cysteine/cystine accelerates the formation of both conserved disulfides as well as an additional disulfide linking Cys 30 to Cys 80.
2.1.5 ULP1 digestion
ULP1 digestion separates the His6-SMT3 from the chemokine.
Double the volume of the refolding mixture with 100 mM Tris pH 8.0. The concentration of the Guanidine HCl must be below 250 mM for the ULP1 digestion to be effective.
Add ULP1 and incubate with stirring at 4 °C.
Complete digestion should be observed through comparison of undigested and digested samples using SDS-PAGE.
Clarify the digestion through centrifugation at 5,000 × g for 10 minutes or filtration (10,000 Dalton cut off).
2.1.6 Cation exchange chromatography
Isolation of the chemokine from the His6-SMT3 protein is accomplished by cation exchange chromatography. This chromatography technique is especially useful due to the fact that most chemokines are positively charged. Under basic buffer conditions, the more positively charged chemokine will bind to the negatively charged cation exchange resin, while the negatively charged and slightly acidic His6-SMT3 (theoretical pI 5.7) will not.
Equilibrate the column with cation exchange binding buffer.
Bind the clarified digestion to the column with a peristaltic pump at a flow rate of 2.5 mL/minute.
Wash the column with 5 times the column volume of cation exchange wash buffer.
Elute the column with 4 times the column volume of cation exchange elution buffer.
Filter the elution using a 0.22 Fm syringe filter.
2.1.7 Reverse phase HPLC
Reverse phase HPLC (RP-HPLC) is the final cleanup step and leaves the protein in a volatile solvent suitable for lyophilization. Chemokines are functional when refolded and refolding involves cysteine oxidation and correct pairing in disulfide bonds. First RP-HPLC is used to check for cysteine oxidation and disulfide bond formation. Then RP-HPLC is used to cleanup the sample by separating the major refolded species that has the correct disulfide pairings from small amounts of misfolded chemokine containing either incorrectly paired disulfide bonds or chemokine that is partially or completely reduced.
- Disulfide formation check.
- Two 5 μL samples from the cation exchange elution are diluted by adding 745 μL of water. To one sample 7.5 μL of 1 M dithiothreitol is added (final concentration ~ 10 mM) and 7.5 uL of water is added to the other. Both are incubated at 37 °C for 2 minutes and then acidified with a drop of 6 N HCl.
- These samples are run separately using a linear 30-60 % RP-HPLC buffer B gradient (see Table 1). A difference in retention time for the major peaks in the samples with and without dithiothreitol indicates disulfide bond formation.
The refolded chemokine is isolated using RP-HPLC using a linear 30-60 % buffer B gradient. See Table 1 for the gradient scheme and time program. A fraction collector that is programmable aids in collecting the refolded chemokine. In general, the major peak will have the correct disulfide pairings and will be functionally active in activity assays. The smaller peaks are misfolded chemokine or chemokine that is only partially or completely reduced.
Table 1.
Reverse Phase HPLC gradient scheme
| Time (minutes) | % RP-HPLC buffer A | % RP-HPLC buffer B |
|---|---|---|
| 0 | 90 | 10 |
| 5 | 70 | 30 |
| 35 | 40 | 60 |
| 40 | 0 | 100 |
| 45 | 0 | 100 |
| 50 | 90 | 10 |
| 65 | 90 | 10 |
RP-HPLC buffer A: Aqueous 0.1% triflouroacetic acid
RP-HPLC buffer B: Aqueous 0.1% triflouroacetic acid, 70% acetonitrile
2.1.8 Lyophilization
The elutions from the RP-HPLC are pooled and frozen at −80 °C and dried on a lyophilizer until dry (about 24 hours) and stored at −20 °C.
2.2 Mass spectrometry
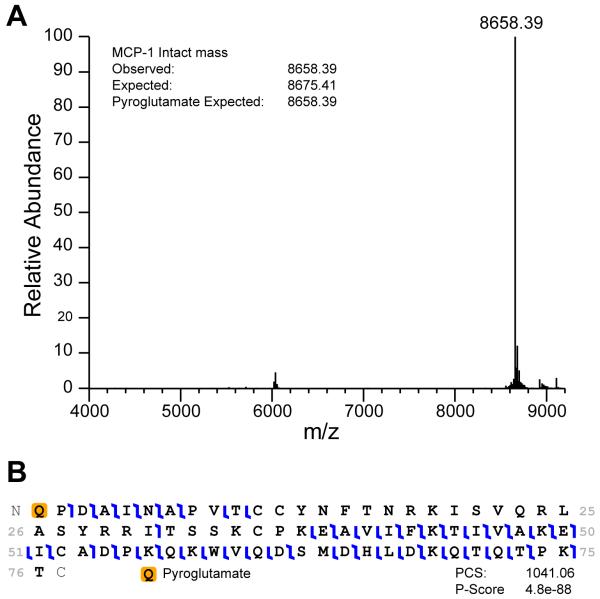
Mass spectrometry, in addition to RP-HPLC, serves as a powerful technique for assessing disulfide bond formation. Additionally, tandem mass spectrometry (MS-MS) can in some instances be used to define the pairing of cysteines, like the non-conserved disulfide bond in CCL28 that links C30 to C80 (Thomas et al., 2015), or confirm the locations of posttranslational modifications, like pyroglutamate formation in CCL2 as shown in Figures 4 and 5, respectively.
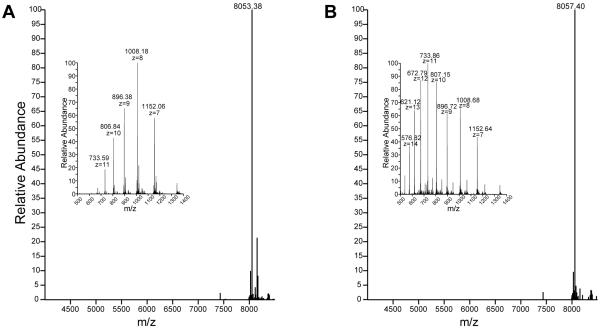
Figure 4.
Using mass spectrometry to confirm identify and disulfide bond formation for a CXCL12 mutant. A) The intact mass spectrum of folded and oxidized CXCL12 S-1 S4V with the charge state envelope inset. B) The denatured and reduced protein, compared to the oxidized and refolded protein the mass increased in size by 4 Daltons with all 4 cysteines being reduced and the charge state envelope has shifted to a lower m/z.
Figure 5.
Top down mass spectrometry analysis of CCL2. A) Intact mass of purified recombinant CCL2. Pyroglutamate at the amino terminus explains the mass discrepancy. B) Fragmentation of CCL2 shows amino terminal pyroglutamate ions with a p-value of 10e-88.
2.2.1 Required materials
Q Exactive™ Hybrid Quadrupole-Orbitrap mass spectrometer from ThermoFisher Scientific or comparable mass spectrometer
LC/MS grade acetonitrile, 0.1 % formic acid
LC/MS grade H20, 0.1% formic acid
Macro spin C4 tips, Nest group
Xtract and Xcalibur™ in QualBrowser from ThermoFisher Scientific or comparable software
ProSightLite (Fellers, Greer, Early, Yu et al., 2015)
2.2.2 General method for detecting disulfide oxidation and identity
For most chemokines, which contain four cysteines that form two conserved disulfide bonds, a measured mass to charge ratio will be four mass units less than the mass predicted based on protein sequence. Hence, measurement of a recombinant chemokines mass to charge ratio using a sensitive and accurate mass spectrometer can confirm not only protein identity but also disulfide oxidation as seen in Figure 4.
Dissolve lyophilized chemokine to a concentration of 1 μM in aqueous 50% acetonitrile and 0.1 % formic acid.
Analyze by direct infusion using a Q Exactive™ Hybrid Quadrupole-Orbitrap mass spectrometer (ThermoFisher Scientific). All analyses are performed with resolving power set to 140,000, four microscans for 30 seconds.
Deconvolute and deisotope with Xtract and Xcalibur™ in QualBrowser (ThermoFisher Scientific)
Compare measured mass to charge ratio to that predicted based on sequence.
2.2.3 Top down approaches for the analysis of chemokines with additional disulfides or posttranslational modifications
In cases where the predicted mass calculated from the amino acid sequence does not match the observed mass, top down sequencing is used to determine sites of posttranslational modifications as seen in Figure 5.
Chemokine is dissolved or diluted into the denaturing/reducing buffer to reduce the mature chemokines disulfide bonds. This is required for efficient fragmentation of the entire molecule.
After 10 minutes protein is desalted by applying to the Macro spin C4 tip, washing five times with 200 μL H20, 0.1% formic acid and eluting in 100uL acetonitrile, 0.1% formic acid.
Protein concentration is determined and diluted to 1 μM in 50% H2O/Acetonitrile, 0.1% formic acid.
Intact folded and oxidized protein was analyzed by direct infusion using a Q Exactive™ Hybrid Quadrupole-Orbitrap mass spectrometer (ThermoFisher Scientific) as before.
Top down sequencing of the denatured and reduced protein was analyzed by direct infusion using a Exactive™ Hybrid Quadrupole-Orbitrap mass spectrometer with a resolving power of 70,000.
For CCL2/MCP1, fragmentation of the 1,293 m/z peak was accomplished in the HCD cell at a power of 20eV.
Top down sequencing used ProSightLite to determine the pyroglutamate on the N-terminus.
2.3 Protein NMR spectroscopy
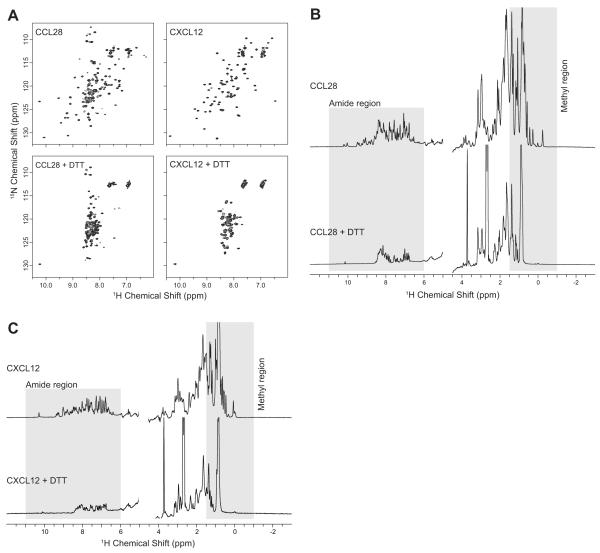
NMR spectroscopy is a robust technique for evaluating the folding state of chemokines and proteins in general. Chemokines are properly folded when the cysteines present are oxidized and form properly paired disulfide bonds, a feature that is essential to maintain the chemokine fold. Properly folded chemokines generate an NMR spectrum with well dispersed chemical shift values (Figure 6, top row) while unfolded chemokines display a collapsed NMR spectrum (Figure 6, bottom, row). A two–dimensional 1H–15N heteronuclear single quantum coherence (HSQC) or 1H–15N heteronuclear multiple–quantum coherence (HMQC) spectrum provides a “finger print” of the chemokine that contains a resonance for every backbone and side chain amide group. Comparison with a validated “finger print” or a prediction based on primary sequence allows for a rapid assessment of a chemokines folding state. However, 1H-15N HSQC or HMQC spectroscopy requires high-field NMR instrumentation that may not be readily available and specialized growth media to enrich recombinant chemokines with the 15N isotopic label. Alternatively, one-dimensional proton NMR spectroscopy can be utilized to evaluate the folding state of a chemokine without the requirement for specialized growth conditions or high-field NMR instrumentation.
Figure 6.
Using NMR to confirm chemokine folding. A) 1H-15N HSQC spectra of CCL28 and CXCL12 with cysteines fully oxidized and properly paired (top row) or fully reduced using DTT (bottom row). One-dimensional 1H spectra of CCL28 (B) and CXCL12 (C) with correct disulfide parings (top) or fully reduced using DTT (bottom).
2.3.1 Required materials
Bruker high-field (≥ 500 MHz) NMR spectrometer running TopSpin or a comparable spectrometer equipped with a 1H/13C/15N probe suitable for proteins
1D (p3919gp) and 2D HSQC (hsqcf3gpph19) or 2D HMQC (sfhmqcf3gpph) pulse sequences from the Bruker library or comparable pulse sequences
3 mm NMR tubes
Buffer: 25 mM deuterated 2-(N_morpholino)ethanesulfonic acid (MES) pH 6.8 containing 10% deuterium oxide
1 M dithiothreitol (DTT)
100 – 300 micrograms of lyophilized chemokine
2.3.2 Confirmation of folding by protein NMR
Oxidized chemokines
Dissolve chemokine in 25 mM MES pH 6.8 containing 10% deuterium oxide to a final concentration of 100-200 μM. A minimum sample volume of 200 μL is required.
Evaluate the folding state by collection 2D or 1D NMR spectra using a Bruker high-field NMR spectrometer. All data will be collected at 25 °C with an appropriate number of scans.
Process the resulting spectrum with TopSpin (Bruker) or comparable software package.
- Compare the spectrum with a validated “finger print” spectrum or with results predicted based on the primary sequence.
- Two-dimensional 1H-15N HSQC or HMQC spectra will contain a set of resonances equivalent to the number of backbone and sidechain amide groups present in the chemokine. The folding state is evaluated based on the number of resonances present, and the uniformity of the shape and intensity of the resonances. Chemokines that are well behaved should display greater than >90% of the expected resonances (Figure 6A, top).
- One-dimensional 1H spectra are also useful for evaluating the folding state of chemokines but suffer from spectral overlap because of reduced dimensionality. To evaluate the folding we look at the peaks dispersion in the amide region and the methyl region of the one-dimensional 1H spectrum (Figure 6B & C, top).
Reduced chemokines
Reduce the chemokine disulfide bonds by adding DTT to the sample to a final concentration of 10 mM and incubating for 30 minutes at room temperature.
Evaluate the folding state of the reduced chemokine by collecting 2D or 1D NMR spectra as in step 2.
Compare the oxidized and reduced chemokine spectra to confirm that disulfide bond reduction caused the NMR signals to collapse (Figure 6, bottom).
2.4 Verifying biological activity
There are numerous approaches for verifying biological activity of chemokines, an essential step prior to using recombinant chemokines for structural or functional research studies. Classic in vitro assays for biological activity can include calcium flux assays or chemotaxis assays (Kar, Srivastava, Andersson, Baratelli et al., 2011; Love et al., 2012; Miller & Krangel, 1992; Neote, DiGregorio, Mak, Horuk et al., 1993; Thomas et al., 2015). Calcium flux assays are those that detect the increase in the cytosol of the secondary messenger calcium that is released as a result of chemokine receptor activation (Love et al., 2012; Miller et al., 1992; Neote et al., 1993). Chemotaxis assays monitor chemokine recruitment of cells expressing chemokine receptor across a barrier that is impermeable to cells without the chemokine functioning as an attractant (Kar et al., 2011; Miller et al., 1992; Thomas et al., 2015). An example chemotaxis assay protocol for CCL21 is presented here.
2.4.1 Required materials for a general chemotaxis assay
T2 cells (ATCC; 174 × CEM.T2 cells Cat. No. CRL-1992)(Salter, Howell, & Cresswell, 1985)
L-glutamine (200 mM) (Invitrogen; Cat. No. 25030-081)
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (1 M) (Invitrogen; Cat. No. 15630-080)
Sodium pyruvate (100 mM) (Invitrogen; Cat. No. 11360)
Penicillin Streptomycin (10,000 U/ml penicillin and 10,000 μg/ml streptomycin) (Invitrogen; Cat. No. 15140-122)
Minimum Essential Medium (MEM) Non-Essential Amino Acids (100X) (Invitrogen; Cat. No. 11140-050)
CO2 tissue culture incubator (set at 37°C, 5% CO2)
CCL21 produced as described above, or for use as controls purchased from a commercial source
Ultrapure water (such as can be produced by a Barnstead Nanopure system or several other equivalent water purification systems)
Microscope with digital imaging capability (Nikon Instruments Inc.; Eclipse 90i)
Iscove’s Modified Dulbecco’s Medium (IMDM) (Life Technologies; Cat. No. 12440)
Fetal bovine serum (FBS) (Atlantic Biologicals; Cat. No. S11150)
Sterile (autoclaved) 1X phosphate-buffered saline (PBS), pH 7.4 (prepared by dilution of a 10X solution purchased from Life Technologies; Cat. No. 70011-044)
Thincert tissue culture inserts for 24-well plates, 3-micron pore size (Greiner Bio One; Cat. No. 662630)
24-well flat-bottom plates (Corning; Cat. No. 3524)
Hema 3 STAT Pack Hemotology Staining [Fixative, Solution I, Solution II] (Thermo Fisher Scientific; Cat. No. 123-869)
VectaMount™ Mounting Medium (Vector Laboratories; Cat. No. H-5000)
Microscope slides (3” × 1” × 1 mm) (Fisher Scientific; Cat. No. 12-544-3)
Glass coverslips (Fisher Scientific; Cat. No. 12-545-88)
Cotton-tipped applicators (Allegiance; Cat. No. C15053-006)
Scalpel (Personna Medical; Cat. No. 73-0111)
Tweezers
2.4.2 General chemotaxis assay for CCL21
Culture T2 cells to 70% confluency in IMDM with 15% fetal bovine serum, 2 nM L-glutamine, 10 mM HEPES buffer, 1 mM sodium pyruvate, 100 units per mL penicillin, 100 μg per mL streptomycin and non-essential amino acids (used at 1x final concentration).
Resuspend CCL21 in ultrapure water (at 600 ng/ml concentration for controls).
Collect and count the T2 cells and resuspend them in serum-free IMDM.
Fill 5 wells of a 24-well plate with 500 μl of the following (as the bottom chamber contents): 1 well with serum-free IMDM (negative control), 1 well with IMDM containing 15% vol/vol FBS and all the other media supplements mentioned in #1 above (positive control), and 3 wells with 600 ng/ml CCL21 suspended in serum-free IMDM (experimental triplicates).
Using tweezers, place tissue culture inserts into filled wells.
Seed 5×105 T2 cells (suspended in 180 μl of serum free IMDM) on top of the tissue culture inserts (top chambers).
Incubate in a 37°C, 5% CO2incubator for 24 hours
Remove the plate from the incubator and wash the inserts with 1x PBS (handling the inserts by using tweezers).
Fill 3 wells in a 24-well plate with 700 μl of each solution from the Hema 3 STAT pack (i.e. 1 well of Fixative, 1 well of Solution I and 1 well of Solution II).
Label slides with the name of the cell line and the identity of the bottom chamber contents for each well.
Quickly dip insert in Fixative 30 times using tweezers.
Quickly dip insert in Solution I 30 times using tweezers.
Quickly dip insert in Solution II 30 times using tweezers.
Use a cotton-tipped applicator to very gently wipe the non-invaded cells off the top of the insert.
Put one drop of mounting media onto a labeled slide.
Carefully cut the insert out using a scalpel.
Place insert face down onto the drop of mounting media.
Cover insert with a glass coverslip.
Take 3 pictures of different fields of each insert using a camera-enabled microscope at a magnification of 100X.
Count cells and calculate the means, ranges, and standard deviations.
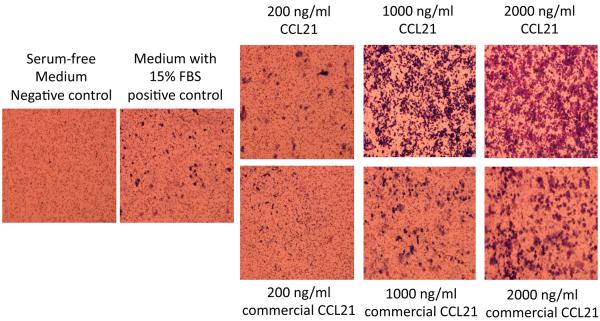
Example results from a CCL21 chemotaxis assay (performed as described above) are shown in Figure 6. In this example, CCL21 prepared at the University of Nebraska Biological Process Development Facility was compared to commercially available CCL21.
3. CAVEATS AND LIMITATIONS
Here a general protocol for producing functional recombinant chemokines is presented that draws from many previously published approaches. The chemokine family has over 50 members and no one-protocol can be expected to fit each chemokine, but this approach has worked for numerous chemokines. For example, XCL1, XCL2, CCL2, CCL11, CCL17, CCL20, CCL28, CXCL5, CXCL11, and CXCL12 have been prepared using the above protocol. CCL19 and CCL21 are examples where small modifications to the above protocol have resulted in functional chemokines. CCL19 requires the inclusion of 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM ethylenediaminetetraacetic acid in the refolding buffer as protease inhibitors and cysteine and cystine during the ULP1 digestion to ensure PMSF does not inhibit ULP1. CCL21 contains an extra disulfide bond in its extended C-terminus. If cysteine and cystine are used in the refolding buffer for CCL21 one must be on the look out for evidence of cystinylation as a side reaction that competes with formation of the third disulfide bond. Alternatively, for CCL21 cysteine and cystine can be left out of the refolding buffer. Other chemokines will likely present similar challenges requiring adaptation of the protocol to the specific situation. Nevertheless, we have found the approach described here to be robust and widely applicable.
4. PERSPECTIVES
4.1 Applications
A thorough review of recombinant chemokines is beyond the scope of this paper. However, many studies have indicated that chemokines themselves or chemokine variants may have therapeutic potential for a multitude of human diseases spanning from cardiovascular health to oncology. For example, CXCL12 and its variants have been proposed, based on animal models of human disease, to be a potentially rejuvenating therapy for myocardial infarction (Abbott, Huang, Liu, Hickey et al., 2004; Askari, Unzek, Popovic, Goldman et al., 2003; Koch, Schaefer, Liehn, Rammos et al., 2006; Saxena, Fish, White, Yu et al., 2008; Segers et al., 2007; Veldkamp, Ziarek, Su, Basnet et al., 2009) or as antimetastatic agents for colon or melanoma cancers (Drury, Ziarek, Gravel, Veldkamp et al., 2011; Takekoshi, Ziarek, Volkman, & Hwang, 2012). Additionally, CCL21 is an effective surgical neoadjuvant for treatment of mammary tumors in mouse models and has shown antitumor activity in other malignancies. (Ashour, Lin, Wang, Turnquist et al., 2007; Dubinett, Lee, Sharma, & Mule, 2010; Kar et al., 2011; Kirk, Hartigan-O'Connor, Nickoloff, Chamberlain et al., 2001; Nomura & Hasegawa, 2000; Sharma, Zhu, Srivastava, Harris-White et al., 2013; Turnquist, Lin, Ashour, Hollingsworth et al., 2007). Incorporating therapeutic chemokines and other cytokines as adjuncts in immunotherapy protocols for cancer treatment has been efficacious in alleviating tumor immune evasion and increasing survival (Ardolino, Hsu, & Raulet, 2015; Wennerberg, Kremer, Childs, & Lundqvist, 2015).
The recurrent success of chemokines or chemokine variants in several animal models of human disease supports the potential of chemokines or chemokine-derived molecules for clinical use in medicine. Continued access to high quality recombinant chemokines that are produced for medicinal use is paramount to the advancement of their therapeutic uses. Additionally, clinical administration of chemokines would require that they be manufactured and formulated in facilities and according to protocols that satisfy the Current Good Manufacturing Practices set by the U.S. Food and Drug Administration.
4.2 Adaptation to a U.S. Food and Drug Administration Current Good Manufacturing Practices (cGMP) system
To adapt the protocols described here for use in a cGMP setting, many additional steps must be taken, which are briefly summarized here. Minimum specifications for all raw materials to be used in the process must be defined and met by the materials to be used. The reagents and raw materials should all be of United States Pharmacopoeia (USP)-grade or meet multi-compendial criteria. (For testing of raw materials, multi-compendial means that the quality control test results on the material have met the acceptance criteria set by multiple regulatory groups, such as USP, Japan Pharma, and equivalent agencies in the European Union and Canada.) Vendors normally will provide a Certificate of Analysis or Master Drug File number. If any products such as microbiological media are of animal origin, they must be certified as Bovine Spongiform Encephalopathy (BSE)-free.
Not only must the raw materials be cGMP compliant, but the equipment, laboratory space, and personnel all must comply with cGMP requirement to ensure the safety of the material being produced. All processing, upstream (microbial growth) and downstream (purification), must be conducted in cGMP-compliant space which requires environmental monitoring, periodic decontamination, and calibration and validation of the equipment. Personnel must periodically receive training for cGMP compliance and they must be individually qualified for each piece of equipment they use.
The developed process should be robust and shown to consistently produce acceptable quality material. Due to variability of expression, even within the same bacterial strain, an initial screen for high expressing isolates should be conducted. After introduction in the host strain, 50-100 colonies should be screened to identify the highest producer of the protein. Before beginning a cGMP production process, master cell banks must be established. At minimum, the master cell bank should consist of ~150-200 glycerol stock vials. An even better policy is to establish a working cell bank containing a similar number of vials (i.e., ~150-200), in addition to the master cell bank. Thorough characterization of the clone that will be used to express a biologic is required by FDA regulations. Therefore, both the master cell bank and the working cell bank should be tested for purity, identity, viability, plasmid retention, and sequence fidelity. Sequence fidelity should be confirmed by DNA sequencing. Using current DNA sequencing techniques, the complete sequence of the vector and insert can be determined with a high degree of accuracy. Restriction endonuclease mapping of the molecule can serve to quickly confirm the correct plasmid is harbored by the production strain. Though not a required test, plasmid copy number may also be determined. After preliminary small-scale analysis of chemokine production has been conducted, further process development and scale-up to optimize conditions in a batch fermentor will be required to move to the cGMP level; conditions in a large fermentor are different from those in shake flasks or small fermentation vessels (e.g., mixing rate, dissolved oxygen, thermal control, pH control, etc.). In bench-top chemokine production dialysis tubing may be used, but not when moving to a larger scale (e.g., a 60-liter fermentor). Cross-flow filtration can serve the same function and is recommended to achieve buffer exchange and concentration. Although this method exposes the protein to shear conditions as it is being circulated across a flat membrane sheet under pressure, no deleterious effects have been noted with small proteins or peptides.
When common resins and aqueous solvents were used to develop a purification process at the research bench, the purification methods can usually be scaled-up with minimal problems. However, if organic solvents have been used, scaling up to a cGMP process may be more complicated. For example, if acetonitrile is used for purification and there are no issues in the acute toxicity studies required by the FDA, then use of acetonitrile is not a problem and scale-up is straightforward. However, if toxicity is observed, it will not be clear if the observed toxicity is due to CCL21 or to acetonitrile residue. Thus, the risk/benefit of including acetonitrile in the purification process should be carefully weighed.
In order to establish that a process results in consistent product, analytical tests should be established and qualified by using them to test a reference standard. These tests are developed as in-process test methods to track the quantity and quality of product during optimization of the purification process. In-process analyses often involve using ion exchange HPLC, reverse phase HPLC, SDS-PAGE, Western Blot, and/or ELISA assays.
Under cGMP guidelines, biotherapeutics destined for clinical trials require meeting stringent release testing criteria. For most products, purity should exceed 98% and there is some flexibility in selecting the specific set of release tests that characterize the identity, purity, function (usually), and important physical attributes of the product. For CCL21, release testing should include assays to confirm identity and function such as reducing and non-reducing SDS-PAGE, Western blot, N-terminal sequencing, potency, capillary isoelectric focusing (pI), amino acid analysis, intact mass spectroscopy, and peptide mapping. Release testing for safety must include an endotoxin assay (e.g., limulus amebocyte lysate, LAL), host cell protein, residual host DNA, and bioburden or sterility. Release assays to characterize physical attributes include color and appearance, pH, and conductivity. Many of the assays used in-process can also be used for release testing if they are qualified or compendial (i.e., follow a USP protocol). A subset of release tests are often also used to analyze stability of the purified product. Typically stability studies are performed at the expected storage temperature and over the expected storage time of the product, e.g., −80°C, 2 years. In some cases, forced degradation studies are conducted for shorter times at elevated temperatures. The analytical methods selected for stability studies must be able to detect low-level degradation of the product.
In adapting a chemokine production protocol to cGMP, it is also important to take into account the requirements for submitting an Investigational New Drug application to the Food and Drug Administration. Complete detailed records must be kept describing every step of the production and purification processes, and final formulation. This includes all the information regarding 1) raw materials and reagents (Certificate of Analysis or Master Drug File number, inventory and storage records, all product label information), 2) manufacturing (all related protocols must be written as Master Batch Records (MBRs) that are approved by Quality Assurance (QA) and followed precisely; deviations from the MBRs must be investigated by QA and the potential impact on the product explained), 3) quality control (all data on development and qualifications of analytical assays or verification of compendial methods must be included) and lastly, 4) equipment maintenance (records of equipment maintenance, qualification, and calibration).
4.2 Concluding Remarks
In conclusion, the supply of pure, natively folded chemokine proteins is vital for a variety of basic, translation and clinical research programs. Our structure-function studies and drug development efforts have motivated the development of the robust protocol for recombinant chemokine expression, refolding, purification and validation presented here. Finally, many additional factors must be considered when manufacturing a product under cGMP guidelines that may not be obvious to personnel in research settings.
Figure 7.
Comparison of T2 cell chemotactic response elicited by CCL21 produced at the University of Nebraska - Lincoln Biological Process Development Facility (top right panels) or by commercial CCL21 (bottom right panels) using the chemotaxis procedure outlined in Section 2.4. Fetal bovine serum (FBS), which contains a mixture of chemotactic components, was used as a positive control. The CCL21 wells contained no FBS.
ACKNOWLEDGEMENTS
Many approaches for preparing functional chemokines have been presented in the literature either as reviews or in individual research articles; we sincerely apologize to the numerous individuals whose work could not be discussed and/or cited due to space limitations. This work was supported by NIH grant 1R15CA159202-01 to C.T.V., NIH grants R01AI058072 and R01GM09738 to B.F.V., a grant to J.C.S. and W.B. from the Nebraska Research Initiative, and other funding to J.C.S. (LB506, SPORE Pilot Grant, the State of Nebraska through the Pediatric Cancer Research Group, and the Fred & Pamela Buffett Cancer Center Support Grant P30CA036727). We wish to thank Professor Rebekah Gundry for assistance and training in the mass spectrometry methods presented here.
REFERENCES
- Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- Ardolino M, Hsu J, Raulet DH. Cytokine treatment in cancer immunotherapy. Oncotarget. 2015;6(23):19346–19347. doi: 10.18632/oncotarget.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour AE, Lin X, Wang X, Turnquist HR, Burns NM, Tuli A, Solheim JC. CCL21 is an effective surgical neoadjuvant for treatment of mammary tumors. Cancer Biol Ther. 2007;6(8):1206–1210. doi: 10.4161/cbt.6.8.4405. [DOI] [PubMed] [Google Scholar]
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250(2):91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I. Synthesis of chemokines. Methods Mol Biol. 2000;138:47–63. doi: 10.1385/1-59259-058-6:47. [DOI] [PubMed] [Google Scholar]
- Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell- derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. Embo J. 1997;16(23):6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, Tosato G. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004;103(7):2452–2459. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- Drury LJ, Ziarek JJ, Gravel S, Veldkamp CT, Takekoshi T, Hwang ST, Dwinell MB. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(43):17655–17660. doi: 10.1073/pnas.1101133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinett SM, Lee JM, Sharma S, Mule JJ. Chemokines: can effector cells be redirected to the site of the tumor? Cancer J. 2010;16(4):325–335. doi: 10.1097/PPO.0b013e3181eb33bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton MD, Gerlach LO, Boesen TP, Allet B. Expression of chemokines in Escherichia coli. Methods Mol Biol. 2000;138:33–40. doi: 10.1385/1-59259-058-6:33. [DOI] [PubMed] [Google Scholar]
- Fellers RT, Greer JB, Early BP, Yu X, LeDuc RD, Kelleher NL, Thomas PM. ProSight Lite: graphical software to analyze top-down mass spectrometry data. Proteomics. 2015;15(7):1235–1238. doi: 10.1002/pmic.201570050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- Kar UK, Srivastava MK, Andersson A, Baratelli F, Huang M, Kickhoefer VA, Sharma S. Novel CCL21-vault nanocapsule intratumoral delivery inhibits lung cancer growth. PLoS One. 2011;6(5):e18758. doi: 10.1371/journal.pone.0018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk CJ, Hartigan-O'Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, Mule JJ. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61(5):2062–2070. [PubMed] [Google Scholar]
- Koch KC, Schaefer WM, Liehn EA, Rammos C, Mueller D, Schroeder J, Weber C. Effect of catheter-based transendocardial delivery of stromal cell-derived factor 1alpha on left ventricular function and perfusion in a porcine model of myocardial infarction. Basic Res Cardiol. 2006;101(1):69–77. doi: 10.1007/s00395-005-0570-3. [DOI] [PubMed] [Google Scholar]
- Kufareva I, Salanga CL, Handel TM. Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies. Immunol Cell Biol. 2015;93(4):372–383. doi: 10.1038/icb.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler DF, Uetz-von Allmen E, Hauser MA. CCR7: roles in cancer cell dissemination, migration and metastasis formation. Int J Biochem Cell Biol. 2014;54:78–82. doi: 10.1016/j.biocel.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Love M, Sandberg JL, Ziarek JJ, Gerarden KP, Rode RR, Jensen DR, Veldkamp CT. Solution structure of CCL21 and identification of a putative CCR7 binding site. Biochemistry. 2012;51(3):733–735. doi: 10.1021/bi201601k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Burns MC, McDevitt PJ, Graham TL, Sukman AJ, Fornwald JA, Johanson KO. Optimized procedures for producing biologically active chemokines. Protein expression and purification. 2009;62(2):251–260. doi: 10.1016/j.pep.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Miller MD, Krangel MS. The human cytokine I-309 is a monocyte chemoattractant. Proc Natl Acad Sci U S A. 1992;89(7):2950–2954. doi: 10.1073/pnas.89.7.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier A, Loos T, Gouwy M, Ronsse I, Van Damme J, Proost P. Posttranslational modification of the NH2-terminal region of CXCL5 by proteases or peptidylarginine Deiminases (PAD) differently affects its biological activity. The Journal of biological chemistry. 2010;285(39):29750–29759. doi: 10.1074/jbc.M110.119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neote K, DiGregorio D, Mak JY, Horuk R, Schall TJ. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993;72(3):415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- Nomura T, Hasegawa H. Chemokines and anti-cancer immunotherapy: anti-tumor effect of EBI1-ligand chemokine (ELC) and secondary lymphoid tissue chemokine (SLC) Anticancer Res. 2000;20(6A):4073–4080. [PubMed] [Google Scholar]
- Novagen . The pET System Manual. 11th ed 2005. [Google Scholar]
- Nufer O, Corbett M, Walz A. Amino-terminal processing of chemokine ENA-78 regulates biological activity. Biochemistry. 1999;38(2):636–642. doi: 10.1021/bi981294s. [DOI] [PubMed] [Google Scholar]
- O'Hayre M, Salanga CL, Handel TM, Hamel DJ. Emerging concepts and approaches for chemokine-receptor drug discovery. Expert opinion on drug discovery. 2010;5(11):1109–1122. doi: 10.1517/17460441.2010.525633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot AE, Borlat F. Purification of recombinant chemokines from E. coli. Methods Mol Biol. 2000;138:75–87. doi: 10.1385/1-59259-058-6:75. [DOI] [PubMed] [Google Scholar]
- Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21(3):235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117(17):2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. doi: CIRCULATIONAHA.107.694992 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116(15):1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- Sharma S, Zhu L, Srivastava MK, Harris-White M, Huang M, Lee JM, Dubinett S. CCL21 Chemokine Therapy for Lung Cancer. Int Trends Immun. 2013;1(1):10–15. [PMC free article] [PubMed] [Google Scholar]
- Sierra MD, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, Tosato G. Differential processing of stromal-derived factor-1 {alpha} and {beta} explains functional diversity. Blood. 2003;103(7):2452–2459. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- Takekoshi T, Ziarek JJ, Volkman BF, Hwang ST. A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability. Molecular cancer therapeutics. 2012;11(11):2516–2525. doi: 10.1158/1535-7163.MCT-12-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Buelow BJ, Nevins AM, Jones SE, Peterson FC, Gundry RL, Volkman BF. Structure-function analysis of CCL28 in the development of post-viral asthma. J Biol Chem. 2015;290(7):4528–4536. doi: 10.1074/jbc.M114.627786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnquist HR, Lin X, Ashour AE, Hollingsworth MA, Singh RK, Talmadge JE, Solheim JC. CCL21 induces extensive intratumoral immune cell infiltration and specific anti-tumor cellular immunity. Int J Oncol. 2007;30(3):631–639. [PubMed] [Google Scholar]
- Tyler RC, Murray NJ, Peterson FC, Volkman BF. Native-state interconversion of a metamorphic protein requires global unfolding. Biochemistry. 2011;50(33):7077–7079. doi: 10.1021/bi200750k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp CT, Peterson FC, Hayes PL, Mattmiller JE, Haugner JC, 3rd, de la Cruz N, Volkman BF. On-column refolding of recombinant chemokines for NMR studies and biological assays. Protein Expr Purif. 2007;52(1):202–209. doi: 10.1016/j.pep.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp CT, Ziarek JJ, Su J, Basnet H, Lennertz R, Weiner JJ, Volkman BF. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci. 2009;18(7):1359–1369. doi: 10.1002/pro.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltner JK, Peterson FC, Lytle BL, Volkman BF. Structure of the B3 domain from Arabidopsis thaliana protein At1g16640. Protein Sci. 2005;14(9):2478–2483. doi: 10.1110/ps.051606305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother. 2015;64(2):225–235. doi: 10.1007/s00262-014-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]