Abstract
Purpose of review
Inflammatory cells involved in the allergic response, including eosinophils, mast cells, basophils, and neutrophils, express sialoglycan-binding proteins such as Siglec-8 and Siglec-9, which inhibit cell function and survival. The purpose of this review is to briefly discuss the biology of these siglecs and their ligands and consider their potential impact in pathology and treatment of chronic rhinosinusitis (CRS).
Recent findings
Recent studies demonstrate the presence of ligands for Siglec-8 and Siglec-9 in sinonasal tissue from patients with CRS as well as healthy patients, suggesting that the immunoregulatory functions of siglecs may be triggered in sinus tissue in health and disease.
Summary
Ligands for Siglec-8 and Siglec-9 may regulate the function of eosinophils, mast cells, neutrophils, and other cells in sinus mucosa. Therapeutic strategies that activate the anti-inflammatory effects of siglecs may dampen inflammation and disease in CRS patients.
Keywords: chronic rhinosinusitis, eosinophil, glycan, mast cell, siglec
INTRODUCTION
Chronic rhinosinusitis as a model inflammatory disease of the human sinuses and upper airways
Chronic rhinosinusitis (CRS) is defined as inflammation of the nose and paranasal sinuses of at least 12 weeks’ duration. CRS affects nearly 10% of the population and the two classes of drugs most used to manage it, antibiotics and corticosteroids, are generally not effective, necessitating 200 000 surgeries/year in the United States and countless procedures worldwide [1,2]. Owing to the frequent failure of medical management and requirements for surgical intervention, inflamed tissue from CRS patients is often removed and available for scientific investigation in the laboratory. Although there is heterogeneity of disease, and there are variations in pathophysiology of CRS from continent to continent, there is general agreement to functionally divide it into a form without nasal polyps (CRSsNP) and a form with nasal polyps (CRSwNP) [3]. CRSwNP is more likely to be associated with anosmia, airflow limitation, and a feeling of facial pressure, whereas CRSsNP is more likely to be associated with facial pain. Nasal polyp tissue is highly inflammatory and is characterized by significant edema, deposition of fibrin, and infiltration of inflammatory cells, whereas CRSsNP does not have a particular cellular inflammatory cause [4,5].
The roles of eosinophils, basophils, neutrophils, and mast cells in chronic rhinosinusitis
Inflammatory cell types elevated in nasal polyp tissues, include lymphocytes (CD4+ and CD8+ T cells, B cells, plasmablasts, and plasma cells), granulocytes (neutrophils, eosinophils, and basophils), other myeloid cells (monocytes, macrophages, and innate lymphoid cells [ILC2] in particular), andresident inflammatory cells such as mast cells and dendritic cells [6]. Although there is evidence that many of these cell types are activated (e.g. Th2 cells and ILC2 produce type 2 cytokines and B cells and plasma cells produce antibodies), the focus of this brief review is the role of Siglec-8 and 9 in regulation of granulocytes in inflammation. Only the eosinophils, basophils, and mast cells are known to express Siglec-8. Although monocytes, dendritic cells, natural killer cells, and neutrophils all express Siglec-9, we will limit our comments primarily to neutrophils in this review.
Eosinophils have been associated with CRS, and nasal polyps in particular, for nearly a century [7]. They are highly elevated in most nasal polyps, although many Asian populations, especially those living in rural areas, have polyps that are primarily infiltrated by neutrophils rather than eosinophils [8]. Interestingly, even second generation Asians born and raised in the United States have polyps that are largely neutrophilic [9]. Eosinophil infiltration is associated with anosmia, or the loss of the sense of smell, and recent evidence shows that large numbers of eosinophils infiltrate the olfactory centers that are found deep in the superior meatus in patients with CRSwNP (Lavine, Tan et al. unpublished observations). One hypothesis to explain this link suggests that eosinophil cationic proteins interfere with survival or function of sensory nerves or the sustentacular cells that sustain them in the olfactory zone. Anti-IL-5 antibody (mepolizumab) has been shown to have benefit in CRSwNP, including improved sense of smell, suggesting that eosinophils play a pathophysiological role in the disease [10]. Mast cells have been found to be highly elevated in CRSwNP and can be localized both within the mucosal epithelium as well as in submucosal glands [11]. Basophils have not been studied extensively in CRS, but a recent study [12] shows that they are present in elevated numbers in nasal polyps. Anti-IgE antibody (omalizumab, Xolair) has been shown to have some efficacy in CRS, suggesting that mast cells and/or basophils are involved [13■]. Mast cells, basophils, and eosinophils can all produce mediators that induce vascular leak, mucus secretion, and nerve activation, all responses that may mediate the pathophysiological effects of these cells in CRS.
Expression, function, and ligands for Siglec-8/Siglec-F and Siglec-9/Siglec-E
The term siglecs, which stands for sialic acid-binding immunoglobulin-like lectins, was coined in 1998 to describe a subset of the immunoglobulin (Ig) gene superfamily [14]. These surface proteins contain extracellular N-terminal lectin domains that bind different forms of sialic acid, a prominent terminal sugar on many cell surface glycoproteins and glycolipids. Their membrane-proximal cytoplasmic domains usually, but not always, contain certain conserved amino acid sequences found in other groups of inhibitory receptors that are referred to as immunoreceptor tyrosine-based inhibitory motifs or immunoreceptor tyrosine-based switch motifs. The former deliver inhibitory signals, usually through activation of downstream phosphatases, whereas the latter can either be activating or inhibitory via activation of kinases and/or phosphatases, hence the use of the term ‘switch’ for these domains. There are 14 known human siglecs, and each possesses a characteristic number of Ig-like domains varying in number from two to 17, but most commonly two to four, especially those belonging to the CD33 subfamily of siglecs [15,16■]. Mice have fewer siglecs than humans, but relevant to Siglec-8 and Siglec-9, mouse Siglec-F and human Siglec-8 are functionally convergent paralogs, whereas human Siglec-9 and mouse Siglec-E are true orthologs, and thus Siglec-E expression on cells mirrors that for Siglec-9 mentioned above [17-19]. Like Siglec-8, Siglec-F is expressed predominantly on mouse eosinophils, but unlike Siglec-8 it is also found a high level on alveolar macrophages, and it is not expressed on mouse mast cells or basophils [17,20].
A variety of in-vitro experiments have shown that engagement of Siglec-8 or Siglec-9 with antibodies causes eosinophil and neutrophil death, respectively, especially in cytokine-primed cells [21■,22-27]. Cell death in primed cells requires the generation of reactive oxygen species (ROS), and for eosinophils involves mitogen-activated protein kinases and others [28], whereas in non-activated cells, death is less pronounced and is mainly caspase dependent [24]. For mast cells, Siglec-8 engagement does not induce cell death, but does inhibit the ability of FcεRI triggering to release mediators such as histamine and prostaglandin D2 by shifting their response to stimulation by over 10-fold [29]. Siglec-9 also mediates inhibitory activities. For example, in a macrophage cell line, Siglec-9 engagement enhanced IL-10 production while reducing TNFα release [30]. Siglec-9 has also been shown to bind broadly to many different toll-like receptor (TLR) agonists [31].
Data on Siglec-F and Siglec-E function are more limited. It has been shown that engagement of Siglec-F on mouse eosinophils induces apoptosis, albeit to a much more modest degree, and is unaffected by cytokine priming; nor is there any role for ROS, NADPH, or SHP-1 in this process [32,33]. Regarding the function of Siglec-E, one study [34] using bone marrow-derived mouse macrophages showed that Siglec-E was induced by several TLR ligands, including LPS, and negatively regulated TLR-dependent responses such as production of IFN-β and CCL5, suggesting that Siglec-E on macrophages may dampen antiviral responses via TLRs. Subsequent studies showed that dendritic cells missing Siglec-E exhibited enhanced responses to many different TLR ligands, and in particular, TLR4 ligands trigger lysosomal sialidases that then interrupt the interactions between TLR4 and Siglec-E, suggesting that TLR functional responses can be regulated by siglecs and specific sialylated glycans [31]. Siglec-E null mice displayed increased killing of malignant cells in vivo, and enhanced protection against autologous cancers, but established tumors actually grew faster in Siglec-E-deficient mice [35,36], although this is probably because of an effect on Siglec-9 expressing natural killer cells. Finally, a correlation was observed between the number of CD33-related genes and lifespan in mammals. Mice deficient in Siglec-E had reduced survival and manifested multiple characteristics of aging that were attributed to altered ROS metabolism [37].
A major advance in our understanding of glycan–lectin binding occurred with glycan arrays developed by the NIH-funded Consortium for Functional Glycomics (http://www.functionalglycomics.org). Using this approach, it was determined that both Siglec-8 and Siglec-F preferentially bind the sialoside glycans 6′-sulfo-sialyl-Lewis X (6′-S-Sialyl-Lex) and 6′-sulfo-sialyl-N-acetyl-D-lactosamine (6′-S-Sialyl-LacNAc) [38■,39■]. Although human Siglec-8 shows high selectivity for these sulfated and sialylated glycans, mouse Siglec-F is somewhat more promiscuous, recognizing additional nonsulfated, multivalent, and sialylated LacNAc structures [39■]. Indeed, a polyacrylamide polymer decorated with 6′-S-Sialyl-Lex selectively binds to eosinophils among leukocytes in human blood in a Siglec-8 dependent manner and also causes apoptosis [26]. The specificity of Siglec-8 and Siglec-F ligand binding contrasts with Siglec-9 and Siglec-E ligands, which instead bind a related but different sulfated glycan, namely 6-sulfo sialyl Lewis X [15].
Siglec-F and Siglec-E function in vivo in lung inflammation
Siglec-F and Siglec-E function have been explored in vivo using antibodies, knockout mice, and models where expression of specific ligands has been altered. For example, administration of Siglec-F antibodies in mouse models of chronic allergic asthma nearly normalizes eosinophilic pulmonary inflammatory responses and virtually eliminates lung remodeling [32,40■]. Siglec-F deficient mice, as well as mice deficient in a key enzyme required to synthesize its sialylated glycan ligands, namely the α2–3 sialyltransferase ST3Gal-III, display a selective enhancement of allergic eosinophilic inflammation [41-45], suggesting that the loss of specific sialic acids from lung glycoproteins allows the augmented accumulation and survival of eosinophils in the airways of these mice. Proteomic analysis of Siglec-F binding material enriched from mouse lungs or mouse airway cells grown in vitro identified Muc5b and Muc4 as carriers of sialylated glycan ligands for Siglec-F. Muc5b-deficient mice exhibited decreased Siglec-F-Fc binding to their airways, most consistently in airway submucosal glands. Purified mucin preparations carried sialylated and sulfated glycans, bound to mouse eosinophils and induced their death in vitro, and mice conditionally deficient in Muc5b displayed exaggerated eosinophilic inflammation in response to intratracheal installation of IL-13 [39■]. These data define an innate anti-inflammatory property of specific airway mucins by which their glycan composition can control lung eosinophilia through engagement of Siglec-F.
Given this newly identified role of mucins in dampening eosinophilic inflammation, it is worth briefly discussing which mucins are found in the airway under normal or inflammatory conditions. Among the five secreted human gel-forming mucins, MUC5AC and MUC5B, are the main ones produced in the lower airways [46]. Although MUC5B and Muc5b are the predominant gel-forming mucins constitutively present in the airways of humans and mice respectively [47,48], MUC5AC and Muc5ac are the predominant gel-forming mucins found at increased levels in allergic airways [46,49]. Interestingly, MUC5B expression was decreased by 90% in asthmatic airways compared with normals, and was associated with increased airway eosinophils and worse airway hyperreactivity[50,51]. In contrast, diseases associated with tissue eosinophilia and increased expression of MUC5B, include COPD [52] and CRS [53].
With the development and study of Siglec-E null mice, its biology could be explored in vivo in models of lung disease. It was shown in an LPS-induced lung inflammation model that Siglec-E-deficient mice exhibited exaggerated neutrophil recruitment compared with normal mice, a finding reminiscent of the exaggerated eosinophilic lung inflammation seen in asthma models using Siglec-F null mice [54]. Altered β2 integrin function plays a role in this response, because administration of an antibody to CD11b abrogated this exaggerated neutrophil recruitment response. Furthermore, Siglec-E-deficient neutrophils showed enhanced outside-in signaling when adhering to fibrinogen in vitro, and sialidase treatment of fibrinogen reversed the suppressive effect of Siglec-E on CD11b signaling in normal neutrophils. These data suggest that the inhibitory effects of Siglec-E on integrin function in normal neutrophils required sialic acid recognition [54]. Furthermore, the inhibitory function of Siglec-E in neutrophils was dependent on ROS generation because an NADPH oxidase inhibitor blocked both neutrophil ROS production and Siglec-E-mediated suppression of neutrophil recruitment [55]. Therefore, Siglec-E on neutrophils, like Siglec-F on eosinophils, functions as an inhibitory receptor, although the inhibitory effects mediated via Siglec-F, such as eosinophil apoptosis, are independent of ROS [33].
Evidence of Siglec-8 and Siglec-9 ligand expression in chronic rhinosinusitis
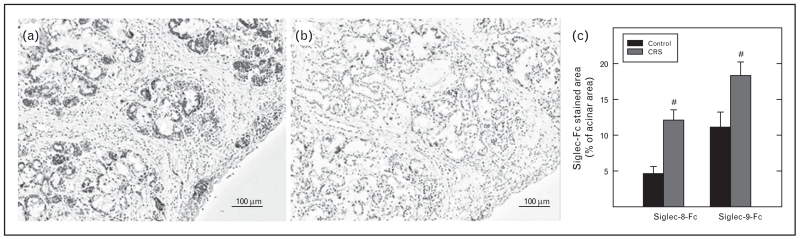
Recent studies have investigated the expression of ligands for Siglec-8 and Siglec-9 in normal human turbinate tissue, as well as tissues derived from patients with CRS [56■]. The ligands were detected with fusion proteins bearing the extracellular domain, which includes the lectin domain of the siglec, as a fusion protein with human IgG1 heavy chain. In the normal turbinate, Siglec-8 ligand was prominently expressed in submucosal gland epithelial cells. Based on the morphology of the Siglec-8-expressing cells, they were serous cells. Serous cells produce the thin secretions found constitutively in airways, and their secretion is induced upon exposure to cold or chemical or biological stimuli. No expression of Siglec-8 ligand was detected in mucous cells. Mucous cells are the cells that produce copious quantities of mucinous mucosal fluids, especially after stimulation with innate activating proteins from bacteria, fungi, or viruses. A different pattern of expression of Siglec-9 ligand was observed in the normal turbinate, with the ligand being detected in both serous and mucous cells within the submucosal glands as well as broadly in connective tissue (Fig. 1). All of the staining was eliminated when the tissue was treated with sialidase, suggesting that it was because of sialic acid moieties in the tissue sites [56■].
FIGURE 1.
Expression of ligands for Siglec-8 and Siglec-9 in human sinonasal tissue is elevated in patients with CRS. (a, b) Inferior turbinate tissue was stained with Siglec-8-Fc, which detects the Siglec-8 ligand. (b) Pretreatment of the tissue with sialidase eliminated the binding activity. Similar staining results were obtained with Siglec-9-Fc (not shown). (c) Quantitation of binding of Siglec-8-Fc and Siglec-9-Fc show that ligand levels were increased in tissue from patients with CRS. Adapted with permission from [56■]. CRS, chronic rhinosinusitis.
Evaluation of turbinate tissue sections from patients with CRS revealed a marked increase in the level of staining of both Siglec-8 ligand and Siglec-9 ligand (Fig. 1), suggesting that inflammatory cells or mediators involved in the disease have activated the submucosal gland cells to increase their expression of the ligands [56■]. Companion studies with a cultured epithelial cell line demonstrated that inflammatory cytokines indeed increase expression of ligands. Additional studies indicated that the Siglec-9 ligand was present on MUC5B, a highly glycosylated protein that is increased in CRS; the molecule that carries Siglec-8 ligand is yet unknown, so it appears that while Muc5B is a ligand for Siglec-F, MUC5B is not a ligand for Siglec-8 [56■]. Previous studies [57,58] had shown that Siglec-9 also recognizes glycans on other mucins such as MUC1. Although the increased levels of siglec ligands in CRS are in agreement with the increased levels in the in-vitro studies, it is difficult to reconcile these findings with the disease process itself. Owing to the fact that siglec ligands should downregulate both the viability and the activation of mast cells, eosinophils, and basophils [59], one might have expected lower levels of these regulators in disease. However, it is unknown at present whether the ligands are being released into the tissue or into the lumen of the upper airways and sinuses. The information that we have merely shows that ligands sequestered within what appear to be serous (Siglec-8 and Siglec-9 ligands) and mucous (Siglec-9 ligands) cells presumably have insufficient access to or effect on the accumulated tissue inflammatory cells that they are known to regulate. It is possible in disease that the ligands are produced in glandular epithelial cells but not secreted, for example. In addition, it is not clear whether the ligands for either Siglec-8 or Siglec-9 found in turbinate tissue are oriented in such a way that they can crosslink siglecs and activate signaling on the surface of cells. Presumably the valency and spacing of the glycan ligands can play an important role in determining the intrinsic activity of the endogenous ligands on receptor-bearing cells. Future studies evaluating the extracellular presence of these ligands and the biological activity of endogenous siglec ligands in triggering the anti-inflammatory effects will be necessary to determine whether these findings support or contradict a model in which the ligands regulate disease activity.
Potential therapeutic applications of siglecs in chronic rhinosinusitis and other type 2 diseases
A considerable body of literature now suggests that Siglec-8 and Siglec-9 are important regulators of inflammation and disease [16■,45,59]. As described above, mice lacking the mouse versions of these receptors have increased inflammatory responses, and treatment of mice with drugs that activate the siglec-mediated anti-inflammatory pathways can suppress inflammation in mouse models of type 2 diseases and acute lung inflammation. Based on the prominent role of the three Siglec-8 expressing cell types, mast cells, eosinophils, and basophils, in CRSwNP, it is reasonable to expect that Siglec-8-activating therapeutics will be more beneficial in CRSwNP than Siglec-9-activating therapeutics, at least in patients that are not of Asian descent. Though perhaps less obvious, Siglec-9 therapeutics are still worthy of consideration in CRSwNP. Neutrophils are highly elevated in both forms of CRS, and recent studies have shown elevated levels of other Siglec-9 ligand bearing cells, including monocytes, macrophages, and dendritic cells, in nasal polyps.
The potential approaches to exploiting siglec biology for therapeutic gain, include antibodies and synthetic ligands that crosslink siglecs. Synthetic ligands that crosslink siglecs could include low-molecular weight compounds that contain at least two ligands, enabling them to crosslink larger molecules with many repeating ligands and even liposomes or nanoparticles that have on their surface siglec-binding ligands [26,60]. In all cases, the drugs should be highly specific for the desired siglec. There are reports showing that commercial intravenous immunoglobulin replacement preparations contain antibodies to Siglec-8 and Siglec-9 [61]. Although it is tempting to attribute some of the beneficial effects of intravenous immunoglobulin in allergic or neutrophilic diseases to this component, there is no direct evidence to support this theory.
CONCLUSION
The potential benefits of Siglec-8 activating drugs are both profound and subtle. The ability of drugs that crosslink Siglec-8 to turn off mast cell mediator release [29] is of obvious potential value in CRSwNP and other diseases with a strong mast cell component. As discussed above, the biology of Siglec-8 crosslinking on eosinophils, and Siglec-9 cross-linking on neutrophils indicates that primed, or cytokine-activated cells are selectively responsive to the apoptosis-inducing effects of Siglec-8 and Siglec-9 crosslinking, respectively. This suggests that Siglec-8 or Siglec-9 binding drugs will selectively kill activated eosinophils or neutrophils and leave undisturbed resting eosinophils and neutrophils. If this is the case, then such drugs may be more effective in aspirin-exacerbated respiratory disease (i.e. CRSwNP plus asthma plus aspirin intolerance), where the level of activation of eosinophils (as well as mast cells and presumably basophils) is considerably higher. It will be of great interest as drugs that engage the siglec mechanisms make their way to clinical trials in patients with CRSwNP.
KEY POINTS.
Cells that express the immunoregulatory cell surface molecules Siglec-8 (eosinophils, mast cells, and basophils) and Siglec-9 (neutrophils, monocytes/macrophages etc.) are important in CRS.
Ligands that have the potential to engage and activate Siglec-8 and Siglec-9 are expressed constitutively in normal nasal tissue and expression is increased in tissue from patients with CRS.
The potential therapeutic usefulness of crosslinking and activation of Siglec-8 and Siglec-9 in CRS and other airways diseases should be explored.
Acknowledgements
We would like to thank members of the Schnaar and Bochner laboratories and members of the Northwestern Sinus Center for collaborations and intellectual contributions.
Financial support and sponsorship
R.P.S. has received research support from the NIH (R37HL068546 and U19AI106683 (Chronic Rhinosinusitis Integrative Studies Program (CRISP)), and from the Ernest S. Bazley Foundation. R.L.S. has received research support from the NIH (HL107151). B.S.B. has received research support from the NIH (AI72265, HL107151).
Footnotes
Conflicts of interest
R.P.S. and B.S.B. have received consultancy fees from, and own stock in, Allakos, Inc. R.L.S. has no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011;144:440–445. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 2.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006;118:S17–S61. doi: 10.1016/j.jaci.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takabayashi T, Kato A, Peters AT, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187::49–57. doi: 10.1164/rccm.201207-1292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–346. doi: 10.1111/cea.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schleimer RP, Kato A, Kern RC. Eosinophils and chronic rhinosinusitis. In: Rosenberg HF, Lee JJ, editors. Eosinophils in health and disease. Elsevier; Amsterdam: 2012. pp. 508–518. [Google Scholar]
- 8.Zhang N, Holtappels G, Claeys C, et al. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2006;20:445–450. doi: 10.2500/ajr.2006.20.2887. [DOI] [PubMed] [Google Scholar]
- 9.Mahdavinia M, Suh LA, Carter RG, et al. Increased noneosinophilic nasal polyps in chronic rhinosinusitis in US second-generation Asians suggest genetic regulation of eosinophilia. J Allergy Clin Immunol. 2015;135:576–579. doi: 10.1016/j.jaci.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–995. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Takabayashi T, Kato A, Peters AT, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130:410–420. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahdavinia M, Carter RG, Ocampo CJ, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014;133:1759–1763. doi: 10.1016/j.jaci.2013.12.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13■.Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–116. doi: 10.1016/j.jaci.2012.07.047. The study demonstrates clinical benefit in CRS from anti-IgE, suggesting a role for mast cells in disease.
- 14.Crocker PR, Clark EA, Filbin M, et al. Siglecs: a family of sialic-acid binding lectins. Glycobiology. 1998;8:v. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 15.Bochner BS. Novel therapies for eosinophilic disorders. Immunol Allergy Clin North Am. 2015;35:577–598. doi: 10.1016/j.iac.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16■.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. doi: 10.1038/nri3737. An outstanding recent review on siglecs, their ligands, and their functions, including Siglec-8 and Siglec-9.
- 17.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 18.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 20.Stevens WW, Kim TS, Pujanauski LM, et al. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21■.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. The study demonstrates that crosslinking of Siglec-8 leads to eosinophil apoptosis.
- 22.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–924. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 23.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–124. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Gunten S, Yousefi S, Seitz M, et al. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 2005;106:1423–1431. doi: 10.1182/blood-2004-10-4112. [DOI] [PubMed] [Google Scholar]
- 25.von Gunten S, Schaub A, Vogel M, et al. Immunologic and functional evidence for anti-Siglec-9 autoantibodies in intravenous immunoglobulin preparations. Blood. 2006;108:4255–4259. doi: 10.1182/blood-2006-05-021568. [DOI] [PubMed] [Google Scholar]
- 26.Hudson SA, Bovin N, Schnaar RL, et al. Eosinophil-selective binding and pro-apoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis X. J Pharmacol Exp Ther. 2009;330:608–612. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Na HJ, Hudson SA, Bochner BS. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine. 2012;57:169–174. doi: 10.1016/j.cyto.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kano G, Almanan M, Bochner BS, Zimmermann N. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation. J Allergy Clin Immunol. 2013;132:437–445. doi: 10.1016/j.jaci.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokoi H, Choi OH, Hubbard W, et al. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by Siglec-8 engagement. J Allergy Clin Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Ando M, Tu W, Nishijima K, Iijima S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem Biophys Res Commun. 2008;369:878–883. doi: 10.1016/j.bbrc.2008.02.111. [DOI] [PubMed] [Google Scholar]
- 31.Chen GY, Brown NK, Wu W, et al. Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. Elife. 2014;3:e04066. doi: 10.7554/eLife.04066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann N, McBride ML, Yamada Y, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao H, Kano G, Hudson SA, et al. Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS One. 2013;8:e68143. doi: 10.1371/journal.pone.0068143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyd CR, Orr SJ, Spence S, et al. Siglec-E is up-regulated and phosphorylated following lipopolysaccharide stimulation in order to limit TLR-driven cytokine production. J Immunol. 2009;183:7703–7709. doi: 10.4049/jimmunol.0902780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laubli H, Pearce OM, Schwarz F, et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci U S A. 2014;111:14211–14216. doi: 10.1073/pnas.1409580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jandus C, Boligan KF, Chijioke O, et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014;124:1810–1820. doi: 10.1172/JCI65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz F, Pearce OM, Wang X, et al. Siglec receptors impact mammalian lifespan by modulating oxidative stress. Elife. 2015;4:e06184. doi: 10.7554/eLife.06184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38■.Bochner BS, Alvarez RA, Mehta P, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. The study was the first to identify a unique and specific glycan ligand for Siglec-8.
- 39■.Kiwamoto T, Katoh T, Evans CM, et al. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2015;135:1329–1340. doi: 10.1016/j.jaci.2014.10.027. The study shows that mucins can carry ligands for Siglec-F.
- 40■.Song DJ, Cho JY, Lee SY, et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. The study demonstrates anti-inflammatory effects from engaging Siglec-F in vivo in mice.
- 41.Guo JP, Brummet ME, Myers AC, et al. Characterization of expression of glycan ligands for Siglec-F in normal mouse lungs. Am J Respir Cell Mol Biol. 2011;44:238–243. doi: 10.1165/rcmb.2010-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Angata T, Cho JY, et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzukawa M, Miller M, Rosenthal P, et al. Sialyltransferase ST3Gal-III regulates Siglec-F ligand formation and eosinophilic lung inflammation in mice. J Immunol. 2013;190:5939–5948. doi: 10.4049/jimmunol.1203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiwamoto T, Brummet ME, Wu F, et al. Mice deficient in the St3gal3 gene product α2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J Allergy Clin Immunol. 2014;133:240–247. doi: 10.1016/j.jaci.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiwamoto T, Katoh T, Tiemeyer M, Bochner BS. The role of lung epithelial ligands for Siglec-8 and Siglec-F in eosinophilic inflammation. Curr Opin Allergy Clin Immunol. 2013;13:106–111. doi: 10.1097/ACI.0b013e32835b594a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wickstrom C, Davies JR, Eriksen GV, et al. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334:685–693. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young HW, Williams OW, Chandra D, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henke MO, Renner A, Huber RM, et al. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. Am J Respir Cell Mol Biol. 2004;31:86–91. doi: 10.1165/rcmb.2003-0345OC. [DOI] [PubMed] [Google Scholar]
- 51.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkham S, Kolsum U, Rousseau K, et al. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1033–1039. doi: 10.1164/rccm.200803-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DH, Chu HS, Lee JY, et al. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004;130:747–752. doi: 10.1001/archotol.130.6.747. [DOI] [PubMed] [Google Scholar]
- 54.McMillan SJ, Sharma RS, McKenzie EJ, et al. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling. Blood. 2013;121:2084–2094. doi: 10.1182/blood-2012-08-449983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMillan SJ, Sharma RS, Richards HE, et al. Siglec-E promotes β2-integrin-dependent NADPH oxidase activation to suppress neutrophil recruitment to the lung. J Biol Chem. 2014;289:20370–20376. doi: 10.1074/jbc.M114.574624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56■.Jia Y, Yu H, Fernandes SM, et al. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 2015;135:799–810. doi: 10.1016/j.jaci.2015.01.004. The study demonstrates expression of ligands for Siglec-8 and Siglec-9 in human sinonasal tissues. The ligands were increased in patients with CRS.
- 57.Tomioka Y, Morimatsu M, Nishijima K, et al. A soluble form of Siglec-9 provides an antitumor benefit against mammary tumor cells expressing MUC1 in transgenic mice. Biochem Biophys Res Commun. 2014;450:532–537. doi: 10.1016/j.bbrc.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Tanida S, Akita K, Ishida A, et al. Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth. J Biol Chem. 2013;288:31842–31852. doi: 10.1074/jbc.M113.471318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135:327–336. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spence S, Greene MK, Fay F, et al. Targeting Siglecs with a sialic aciddecorated nanoparticle abrogates inflammation. Sci Transl Med. 2015;7:303ra140. doi: 10.1126/scitranslmed.aab3459. [DOI] [PubMed] [Google Scholar]
- 61.von Gunten S, Simon HU. Natural anti-Siglec autoantibodies mediate potential immunoregulatory mechanisms: implications for the clinical use of intravenous immunoglobulins (IVIg) Autoimmun Rev. 2008;7:453–456. doi: 10.1016/j.autrev.2008.03.015. [DOI] [PubMed] [Google Scholar]