Highlights
-
•
We compared the hyperglycemia prevalence in Asian Indians to other ethnic groups.
-
•
The prevalence of diabetes was higher in Indians compared to other ethnic groups.
-
•
The prevalence of prediabetes was lower in Indians compared to other ethnic groups.
-
•
These differences may be driven by impaired β-cell function.
Keywords: Type 2 diabetes, Ethnicity, Asian Indian
Abstract
Aims
It is unclear how the prevalence of diabetes in Asian Indians in urban India compares to that of race/ethnic groups in the US that may have different underlying susceptibilities. Therefore, we examined ethnic variations in the prevalence of type 2 diabetes, iIFG, iIGT, IFG + IGT, and the associated risk factors in Asian Indians in Chennai, India, and Whites, Blacks, and Hispanics in the United States.
Methods
Cross-sectional analyses, using representative samples of 4867 Asian Indians aged 20–74 years from Chennai, India, in the Centre for Cardiometabolic Risk Reduction in South-Asia study (CARRS) (2010–2011) and 6512 US Whites, Blacks, and Hispanics aged 20–74 years from the National Health and Nutrition Examination Survey (NHANES) (2007–2012).
Results
The age-adjusted prevalence of type 2 diabetes was highest in Asian Indians (men: 28.4, 95% CI: 25.9, 31.0; women: 30.6, 95% CI, 27.5, 33.9) and lowest in Caucasians (men: 12.2, 95% CI, 10.3, 14.4, women: 9.5, 95% CI, 7.9, 11.5). Asian Indians had the lowest prediabetes prevalence (men: 19.0, 95% CI, 17.2, 20.8; women: 27.2, 95% CI, 22.8, 32.1) and Caucasians had the highest (men; 46.5, 95% CI, 43.5, 49.6, women: 34.4, 95% CI, 31.7, 37.3). However, there were differences in prediabetes prevalence by gender and prediabetes state. The inclusion of HOMA-β in standardized polytomous logistic regression models resulted in a greater odds of diabetes in Blacks and Hispanics compared to Asian Indians.
Conclusions
The high prevalence of diabetes in Asian Indians may be due to innate susceptibilities for β-cell dysfunction in this high risk population.
Introduction
Type 2 diabetes mellitus (diabetes) is a complex metabolic disorder that involves both impaired insulin action and impaired insulin secretion. Traditionally, the pathophysiology has been described as age- or obesity-induced insulin resistance followed by a decrease in compensatory pancreatic β-cell response, eventually leading to overt hyperglycemia [1], [2]. For the last three decades, India has experienced rapid increases in the prevalence of diabetes [3], [4] that have occurred alongside concurrent economic, epidemiological, and nutritional transitions [5], [6], [7]. While some of the high diabetes burden in India can likely be attributed to urbanization and the consequent obesogenic changes in patterns of food consumption and physical inactivity [6], it is also possible that Asian Indians experience unique biological susceptibilities to diabetes development, such as impaired pancreatic insulin secretion early in the natural history of disease [8], [9], [10]. These unique susceptibilities, coupled with factors related to the changing landscape in urban India, may be the driving factors behind the high risk in this race/ethnic group. However, it is unclear as to how the prevalence of diabetes in Asian Indians living in rapidly transitioning urban India currently compares to that of other race/ethnic groups in a developed country such as the United States who are also at high risk but may develop diabetes through different physiological mechanisms such as obesity-driven insulin resistance. We, therefore, examined the age-specific prevalence of diabetes and its precursor states of isolated impaired fasting glucose (iIFG), isolated impaired glucose tolerance (iIGT) and combined impaired fasting glucose and impaired glucose tolerance (IFG + IGT), and the associated risk factors in a population-based sample of Asian Indians living in Chennai, India, and compared them to several race/ethnic groups living the United States.
Materials and methods
In brief, The Center for Cardiometabolic Risk Reduction in South Asia study (CARRS) is a multi-site, cross-sectional surveillance study consisting of two urban cities in India and one in Pakistan. Recruitment and data collection were conducted between 2010 and 2011 [11]. For the purposes of this study, data were analyzed from the Chennai, India, site only, as this was the only site to collect both fasting and two hour plasma glucose samples. Chennai is a metropolitan city located in the South Indian state of Tamil Nadu with a population of approximately 4.68 million people [12]. Households were selected for participation using multi-stage random sampling technique in order to be representative of Chennai [11]. A total of 6920 individuals aged ≥20 were screened for participation, of which 6906 (99%) provided questionnaire data and 876 (13%) reported a previous diabetes diagnosis. Fasting plasma glucose was obtained from 5952 participants (86%). In those not reporting a previous diabetes diagnosis (6030), two hour post-challenge glucose was obtained from 4051 participants (67%). For this study we limited our population to the 4867 (70%) participants who were either previously diagnosed with diabetes or who provided fasting and two hour post-challenge glucose measurements. All participants in CARRS-Chennai were considered Asian Indian.
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional complex sample survey conducted by the US Centers for Disease Control and Prevention's National Center for Health Statistics. The survey is designed to be representative of the US civilian, non-institutionalized population on the basis of a complex multi-stage, biennial probability sample [13]. After completing an in home questionnaire, participants attended a mobile examination clinic where they received a questionnaire as well as physical and laboratory measurements. Cycles 2007–2008, 2009–2010, and 2011–2012 were combined for analysis. A total of 24,731 participants aged ≥20 were screened for participation. Of those, 17,713 (72%) provided questionnaire data, and 17,085 (69%) participated in the mobile examination. Participants who self-reported as “other ethnicity” (1542 (9%)) or who were currently pregnant (116 (0.7%)) were excluded from analysis. We also excluded 1776 (10%) participants who were over the age of 75 to remain in concordance with the upper age group included in CARRS. Of the remaining 14,279 participants, 1749 participants (12%) were previously diagnosed with diabetes. Fasting plasma glucose values were obtained from 6399 and two hour post-challenge glucose values were obtained from 4763 participants. We thus limited our population to the 6512 individuals who met inclusion criteria and had either a previous diabetes diagnosis or gave both fasting and two hour post challenge glucose measurements, and self-identified as either Mexican American (Hispanic), Other Hispanic (Hispanic), Non-Hispanic Caucasian (White), or Non-Hispanic Black (Black). Details regarding the eligibility criteria, questionnaire, and examination components in NHANES and CARRS are listed in Table 1. Additional details of each study have been previously published [11], [13].
Table 1.
Eligibility, questionnaire and exam components in NHANES and CARRS used for analysis
| NHANES | CARRS | |
|---|---|---|
| Eligibility criteria | ||
| Inclusion criteria |
|
|
| Exclusion criteria |
|
|
| Questionnaires |
|
|
| Weight |
|
|
| Height |
|
|
| Waist circumference |
|
|
| Phlebotomy |
|
|
| Glucose |
|
|
NHANES = National Health and Nutrition Examination Survey; CARRS = Center for Cardiometabolic Risk Reduction Survey; OGTT = Oral Glucose Tolerance Test.
In both the CARRS and NHANES studies, type 2 diabetes was defined by previous physician diagnosis, the use of glucose lowering medication, or fasting plasma glucose ≥7.0 mmol/L and/or two hour post-challenge glucose ≥11.1 mmol/L [14]. Both individuals with previously diagnosed diabetes or diabetes that was newly detected were included in the analysis. Given that the precursor states of diabetes, iIFG, iIGT and IFG + IGT have different etiological and pathophysiological mechanisms [15], [16], each state was analyzed separately. iIFG was defined by fasting plasma glucose of 5.6–6.9 mmol/L with normal two hour-post challenge glucose. iIGT was defined as normal fasting plasma glucose with two hour post-challenge glucose of 7.8–11.0 mmol/L. Combined IFG + IGT was defined as both fasting plasma glucose of 5.6–6.9 mmol/L and two hour post-challenge glucose of 7.8–11.0 mmol/L [14]. Given the small numbers of individuals with iIGT in lower age categories, when stratifying by age, all states of prediabetes were combined and defined as fasting plasma glucose of 5.6–6.9 mmol/L and/or two hour post-challenge glucose of 7.8–11.0 mmol/L. All stages of prediabetes were defined by plasma glucose measurements and were not self-reported. Normal glucose tolerance was defined as both fasting plasma glucose <5.6 mmol/L and a two hour post-challenge glucose <7.8 mmol/L [14]. Plasma glucose was analyzed using the hexokinase method in both studies.
The HOMA model has been widely used to estimate insulin resistance and β-cell function and reflects the balance between glucose and insulin in the fasting state as maintained by a feedback loop between the liver and pancreatic β-cells [15]. Therefore, HOMA modeling was used to generate estimates of inherent insulin resistance and β-cell function in participants [15]. HOMA-β was used to measure β-cell function and was calculated as [20 × I0(µIU/mL)/G0 (mmol/L) − 3.5]. HOMA-IR was used to measure insulin resistance and was calculated as [I0(µIU/mL) × G0 (mmol/L)/22.5] [17].
Statistical analysis
All analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC) or SAS callable SUDAAN (version 9, Research Triangle Institute) software. Data from NHANES and CARRS were combined into a single dataset for analysis. Sampling weights for each survey were created independently in order to maximize the representativeness of each sample and were maintained upon combined analysis. Participant characteristics were stratified by sex and were compared using conditional marginal distributions and Wald chi-squared tests. A p-value of <0.05 was considered statistically significant. Weighted crude prevalence values and 95% confidence intervals were estimated by study site, sex, and age group. To obtain plots of the percent of the population in intervals of fasting plasma glucose, two hour post challenge glucose, and fasting insulin, we used the 2.5 and 97.5 percentiles of the distributions of each measure as end points to define the lowest and highest groups. We then divided the population into twelve groups of equal increments. Polytomous logistic regression was used to estimate the age- and sex-adjusted probability of classification into each group and to obtain the predicted percentages of study population [18]. Additionally, multivariable logistic regression models were used to determine the adjusted prevalence of diabetes, total prediabetes, iIFG, iIGT, and IFG + IGT. The models were adjusted for age, sex, BMI, and waist circumference. Standardized polytomous logistic regression was used to compare the odds of iIFG, iIGT, IFG + IGT and diabetes compared to normal glucose tolerance by race/ethnic group.
Results
In total, 11,379 participants were included in the analysis from four race/ethnic groups. Table 2 describes the weighted mean age, anthropometric, and physiological characteristics of participants by race/ethnic group and sex. In men, Hispanics were on average younger than Asian Indians, Blacks, and Whites (p > 0.0001). In women, Asian Indians were on average younger than Blacks and Whites (p > 0.0001); however, there were no significant differences in age between Asian Indians and Hispanics (p = 0.40). In both sexes Asian Indians had the lowest mean height, weight, BMI, and waist circumference measurements (p > 0.0001). In men, there were no significant differences in fasting glucose between Asian Indians and Hispanics (p = 0.07) or Blacks (p = 0.34). However, Asian Indian men had a higher mean fasting glucose than White men (p = 0.01). In women, Asian Indians had the highest mean level of fasting glucose (p > 0.01). Both Hispanic men and women had the highest mean two hour glucose values compared to Whites and Blacks (p > 0.0001); however, this was not significantly different compared to Asian Indians (men, p = 0.22; women, p = 0.67). In both sexes, Asian Indians had the lowest measures of log fasting insulin, and log HOMA-IR (p > 0.0001). In men, Asian Indians had the lowest mean levels of log HOMA-β compared to Whites and Hispanics (p > 0.0001); however, this was not significantly different compared to Blacks (p = 0.22). Asian Indian women had the lowest values of log HOMA-β (p > 0.0001).
Table 2.
Weighted characteristics of participants aged 20–75 years by race/ethnicitya
| Men | NHANES White | NHANES Black | NHANES Hispanic | CARRS Asian Indian |
|---|---|---|---|---|
| N | 1481 | 736 | 994 | 2067 |
| Age (mean year) | 46.0 ± 0.6 | 43.0 ± 0.6 | 39.5 ± 0.5 | 42.4 ± 0.5 |
| (44.9,47.2) | (41.9,44.1) | (38.5,40.5) | (41.6,43.8) | |
| Height (mean cm)b | 178.3 ± 0.3 | 176.7 ± 0.3 | 171.3 ± 0.3 | 164.5 ± 0.2 |
| (177.8,178.9) | (176.1,177.1) | (170.5,171.7) | (163.9,164.7) | |
| Weight (mean kg)b | 91.4 ± 0.8 | 89.9 ± 0.9 | 85.9 ± 0.8 | 65.5 ± 0.4 |
| (90.3,92.6) | (88.0,91.7) | (84.2,87.5) | (64.8,66.2) | |
| BMI (mean kg/m2)b | 28.8 ± 0.2 | 28.6 ± 0.3 | 29.0 ± 0.2 | 24.2 ± 0.1 |
| (28.4,29.1) | (28.1,29.4) | (28.8,29.7) | (24.0,24.5) | |
| Waist circumference (mean cm)b | 102.6 ± 0.6 | 97.5 ± 0.7 | 101.4 ± 0.6 | 87.0 ± 0.4 |
| (101.3,103.2) | (96⋅7,99⋅5) | (100.2,102.6) | (86.7,88.1) | |
| Fasting glucose (mmol/L)b | 5.9 ± 0.1 | 6.0 ± 0.1 | 6.3 ± 0.1 | 6.1 ± 0.1 |
| (5.8,6.0) | (5.8,6.2) | (6.1,6.5) | (6.0,6.3) | |
| 2-hr glucose (mmol/L)b | 6.1 ± 0.1 | 6.2 ± 0.1 | 6.9 ± 0.1 | 6.7 ± 0.1 |
| (5.9,6.3) | (6.0,6.5) | (6.6,7.2) | (6.4,6.9) | |
| Fasting insulin (pmol/L)b | 2.5 ± 0.3 | 2.4 ± 0.4 | 2.7 ± 0.4 | 2.1 ± 0.2 |
| (2.4,2.6) | (2.3,2.5) | (2.6,2.8) | (2.0. 2.1) | |
| Log HOMA-IR (µIU/mL × mmol/L)b | 1.1 ± 0.4 | 1.0 ± 0.5 | 1.3 ± 0.6 | 0.6 ± 0.2 |
| (1.0,1.2) | (0.9,1.1) | (1.2,1.5) | (0.6,0.7) | |
| Log HOMA-β (µIU/mL/mmol/L)b | 4.8 ± 0.3 | 4.6 ± 0.5 | 4.8 ± 0.3 | 4.5 ± 0.3 |
| (4.7,4.8) | (4.5,4.7) | (4.7,4.8) | (4.5,4.6) |
| Women | NHANES White | NHANES Black | NHANES Hispanic | CARRS Asian Indian |
|---|---|---|---|---|
| N | 1418 | 818 | 1065 | 2800 |
| Age (mean year) | 46.8 ± 0.4 | 43.7 ± 0.6 | 41.1 ± 0.6 | 40.4 ± 0.4 |
| (45.9,47.7) | (42.5,44.9) | (39.9,42.2) | (39.6,41.3) | |
| Height (mean cm)b | 164.0 ± 0.3 | 164.0 ± 0.3 | 157.8 ± 0.3 | 150.6 ± 0.1 |
| (163.6,164.6) | (163.3,164.4) | (156.9,158.0) | (150.3,150.9) | |
| Weight (mean kg)b | 76.4 ± 0.6 | 86.3 ± 0.9 | 73.5 ± 0.6 | 62.0 ± 0.4 |
| (75.2,77.5) | (84.6,88.1) | (72.2,74.7) | (61.3,62.7) | |
| BMI (mean kg/m2)b | 28.5 ± 0.2 | 32.0 ± 0.3 | 29.3 ± 0.3 | 27.3 ± 0.1 |
| (27.9,28.8) | (31.4,32.7) | (29.1,30.2) | (27.0,27.6) | |
| Waist circumference (mean cm)b | 95.5 ± 0.5 | 100.7 ± 0.7 | 95.6 ± 0.6 | 83.7 ± 0.4 |
| (94.2,96.3) | (99.6,102.5) | (95.3,98.0) | (83.0,84.5) | |
| Fasting glucose (mmol/L)b | 5.5 ± 0.0 | 5.9 ± 1.0 | 5.9 ± 1.0 | 6.3 ± 1.0 |
| (5.4,5.6) | (5.7,6.1) | (5.7,6.0) | (6.1,6.4) | |
| 2-hr glucose (mmol/L)b | 6.2 ± 1.0 | 6.3 ± 0.1 | 6.9 ± 1.0 | 6.9 ± 1.0 |
| (6.1,6.4) | (6.1,6.5) | (6.7,7.2) | (6.7,7.1) | |
| Fasting insulin (pmol/L)b | 2.4 ± 0.4 | 2.7 ± 0.5 | 2.6 ± 0.5 | 2.2 ± 0.2 |
| (2.4,2.5) | (2.6,2.8) | (2.5,2.7) | (2.1,2.2) | |
| Log HOMA-IR (µIU/mL × mmol/L)b | 1.0 ± 0.5 | 1.3 ± 0.6 | 1.2 ± 0.5 | 0.8 ± 0.2 |
| (0.9,1.1) | (1.2,1.4) | (1.1,1.3) | (0.7,0.8) | |
| Log HOMA-β (µIU/mL/mmol/L)b | 4.8 ± 0.4 | 5.0 ± 0.5 | 4.9 ± 0.6 | 4.5 ± 0.3 |
| (4.7,4.9) | (4.9,5.0) | (4.7,4.9) | (4.4,4.5) |
Values represent mean, ± SE,and 95% CI.
Values are adjusted for age.
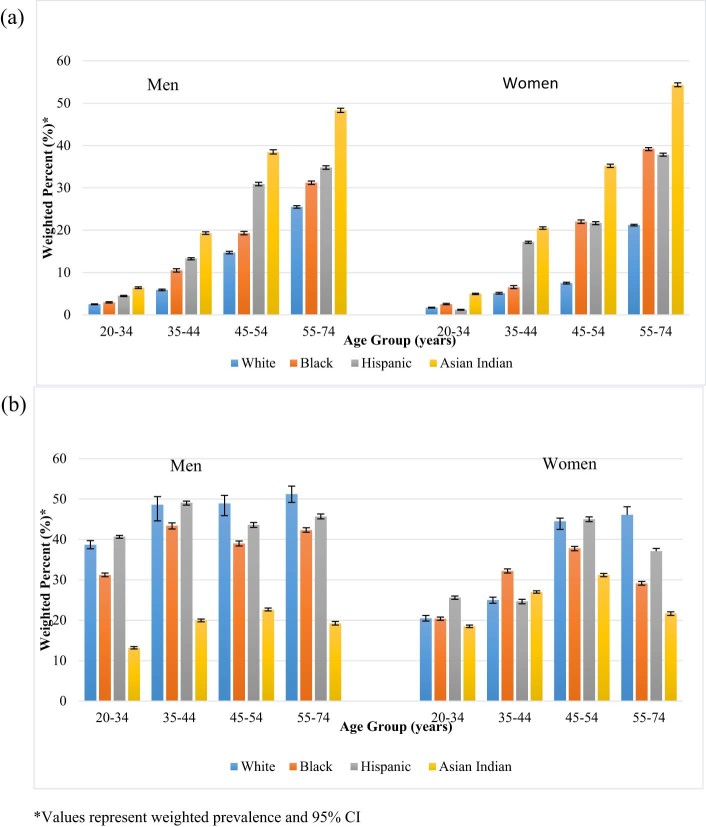
The crude prevalence of diabetes was highest in Asian Indians and lowest in Whites (Table 3). Adjustment for age resulted in a greater difference in diabetes prevalence between Asian Indians and Whites. Additional adjustment for age, BMI, and waist circumference resulted in a diabetes prevalence that was approximately 3 times as high among Asian Indians than Whites in both sexes. When stratified by age, in both sexes, Asian Indian participants had the highest diabetes prevalence in all age categories (Fig. 1a).
Table 3.
Weighted crude and adjusted prevalence of diabetes and prediabetes by sex and race/ethnicity
| NHANES White | NHANES Black | NHANES Hispanic | CARRS Asian Indian | |
|---|---|---|---|---|
| Men | ||||
| Crude type 2 diabetes prevalence | 13.1(11.1,15.5) | 15.1(12.1,18.6) | 16.2(13.4,19.3) | 25.2(22.4,28.2) |
| Type 2 diabetes prevalence adjusted for age,body mass index,and waist circumference | 11.9(10.0,14.1) | 17.3(14.1,21.0) | 21.1(17.7,24.8) | 39.0(34.7,43.8) |
| Crude prediabetes prevalence | 46.9(43.8,50.0) | 37.8(34.6,41.0) | 44.2(40.1,48.3) | 18.6(16.9,20.4) |
| Prediabetes prevalence adjusted for age,body mass index,and waist circumference | 46.2(43.3,49.3) | 39.5(35.8,43.4) | 46.0(41.8,50.3) | 23.0(20.1,26.2) |
| Crude iIFG prevalence | 35.0(32.3,37.8) | 27.6(24.1,31.4) | 33.6(29.9,37.5) | 10.3(8.3,12.8) |
| iIFG prevalence adjusted for age,body mass index,and waist circumference | 35.1(32.4,37.9) | 27.8(24.1,31.7) | 33.5(29.9,37.4) | 11.7(9.1,14.9) |
| Crude iIGT prevalence | 2.3(1.3,3.8) | 3.5(2.2,5.4) | 2.2(1.4,3.6) | 5.1(4.0,6.5) |
| iIGT prevalence adjusted for age,body mass index,and waist circumference | 2.1(1.2,3.6) | 3.7(2.4,5.4) | 2.5(1.5,4.1) | 5.2(3.4,7.8) |
| Crude IFG + IGT prevalence | 9.7(8.3,11.3) | 6.6(5.3,8.4) | 8.3(6.5,10.6) | 3.2(2.4,4.1) |
| IFG + IGT prevalence adjusted for age,body mass index,and waist circumference | 9.0(7.8,10.5) | 7.8(6.0,10.1) | 10.4(8.3,13.0) | 5.2(3.8,7.2) |
| Women | ||||
| Crude type 2 diabetes prevalence | 10.3(8.5,12.3) | 16.9(14.0,20.3) | 15.7(13.1,18.8) | 22.9(20.2,25.9) |
| Type 2 diabetes prevalence adjusted for age,body mass index,and waist circumference | 9.6(7.9,11.6) | 15.3(12.6,18.3) | 19.6(16.7,22.9) | 41.4(37.3,45.7) |
| Crude prediabetes prevalence | 35.2(32.5,38.1) | 29.1(25.6,32.9) | 31.4(26.8,36.5) | 24.2(20.2,28.6) |
| Prediabetes prevalence adjusted for age,body mass index,and waist circumference | 35.0(32.0,38.1) | 28.1(24.4,32.1) | 34.4(29.6,39.6) | 32.8(26.8,39.5) |
| Crude iIFG prevalence | 21.4(19.0,24.1) | 19.8(16.4,23.7) | 18.0(14.6,21.8) | 17.3(14.1,21.0) |
| iIFG prevalence adjusted for age,body mass index,and waist circumference | 21.4(18.9,24.2) | 19.5(15.8,23.8) | 19.6(16.0,23.8) | 24.0(18.6,30.4) |
| Crude iIGT prevalence | 5.7(4.3,7.5) | 2.8(1.8,4.4) | 6.8(5.1,9.0) | 2.4(1.9,3.0) |
| iIGT prevalence adjusted for age,body mass index,and waist circumference | 5.8(4.3,7.7) | 2.7(1.6,4.5) | 7.0(5.2,9.4) | 2.2(1.3,3.5) |
| Crude IFG + IGT prevalence | 8.1(6.5,10.1) | 6.5(4.9,8.5) | 6.7(5.2,8.6) | 4.5(3.6,5.5) |
| IFG + IGT prevalence adjusted for age,body mass index,and waist circumference | 7.7(6.1,9.7) | 6.1(4.5,8.1) | 7.9(6.3,1.0) | 7.2(5.3,9.7) |
Figure 1.
Weighted age-specific diabetes and prediabetes prevalence by sex and race/ethnicity. a) Weighted age-specific type 2 diabetes prevalence. b) Weighted age-specific prediabetes prevalence.
Asian Indians had the lowest crude prevalence of prediabetes, followed by Blacks, Hispanics, and Whites. After adjustment for age, BMI, and waist circumference, the difference in prediabetes prevalence between Asian Indians and other race/ethnic groups was attenuated, especially in women. In age-specific analyses, the prevalence of prediabetes was the lowest in Asian Indians in all age groups and both sexes (Fig. 1b). When prediabetes was assessed by distinct state, Asian Indian men had the lowest crude prevalence of iIFG, followed by Blacks, Hispanics, and Whites. This remained the case after adjustment for age, BMI, and waist circumference. Compared to White, Black, and Hispanic men, Asian Indian men had the highest prevalence of iIGT. This result persisted after adjustment for age, BMI, and waist circumference. Asian Indian men also had the lowest prevalence of IFG + IGT compared to White, Black, and Hispanic men. While adjustment for age, BMI, and waist circumference attenuated IFG + IGT prevalence in White men, it increased IFG + IGT prevalence in Black, Hispanic and Asian Indian men. However, this difference was only significant in Asian Indian men.
Asian Indian women had a slightly, but not significantly, lower crude prevalence of iIFG compared to White, Black, and Hispanic women. After adjustment for age, BMI, and waist circumference, Asian Indian women had the highest prevalence of iIFG compared to White, Black, and Hispanic women. However, this difference was not significant. Compared to White, Black and Hispanic women, Asian Indian women had the lowest crude prevalence of iIGT and IFG + IGT. This remained the case after iIFG was adjusted for age, BMI, and waist circumference. Similar adjustments resulted in a decreased prevalence of IFG + IGT in White and Black women and an increased prevalence in Hispanic and Asian Indian women; however, these differences were not significant.
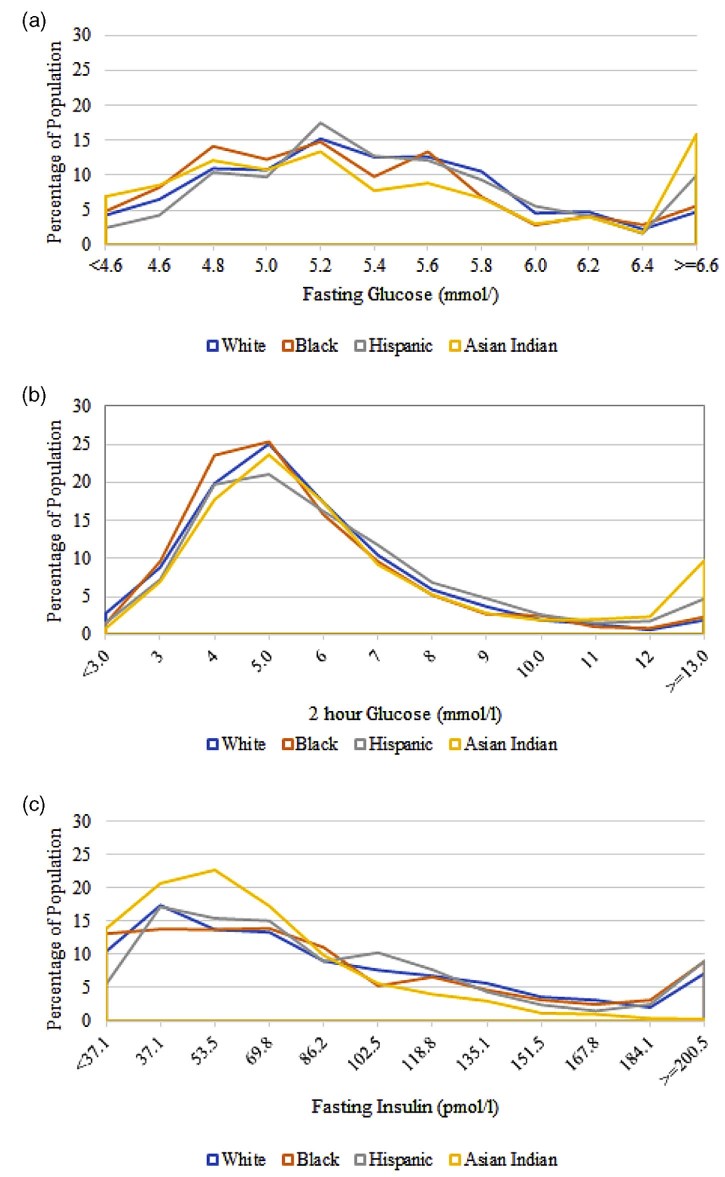
In examining the distributions of fasting plasma glucose (Fig. 2a), compared to Whites, Asian Indians had a higher probability of being classified in the <4.6 mmol/L, 4.6 mmol/L (p < 0.01) and 4.8 mmol/L (p = 0.03) ranges and had a significantly lower probability of being classified in the 5.4–6.0 mmol/L ranges (p ≤ 0.01). When compared to Blacks, Asian Indians had a higher probability of being classified in the <4.6 mmol/L (p ≤ 0.01) range and a significantly lower probability of being classified in the 5.4 mmol/L (p = 0.03) and 5.6 mmol/L (p ≤ 0.01) as well as the 6.4 and ≥6.6 mmol/L (p ≥ 0.01) ranges. In comparison to Hispanics, Asian Indians had a higher probability of being classified in the <4.6–5.0 mmol/L (p ≤ 0.01) ranges and a lower probability of being classified in the 5.2 mmol/L (p = 0.02) and the 5.4–6.0 mmol/L (p ≤ 0.01) ranges of fasting glucose.
Figure 2.
Distribution of fasting glucose, 2 hour glucose, and fasting insulin by race/ethnicity. a) Distribution of fasting glucose (mmol/L). b) Distribution of 2 hour glucose (mmol/L). c) Distribution of fasting insulin (pmol/L).
For two hour post challenge glucose distributions (Fig. 2b), compared to Whites, Asian Indians had a significantly lower probability of being classified at the lowest point of the distribution and a significantly higher probability of being classified at the highest end (p ≤ 0.01). When compared to Blacks, Asian Indians were more likely to be classified at the 12.0 mmol/L (p = 0.01) and the ≥13.0 mmol/L (p ≥ 0.01) ranges of the distribution, and in comparison to Hispanics, Asian Indians were more likely to be classified between the 5.0–6.0 mmol/L and at the highest end of the distribution (p = 0.02) and less likely to be classified between the 7.0 mmol/L (p = 0.02) and the 9 mmol/L (p ≤ 0.01) ranges of two hour glucose.
In comparing the distribution of fasting insulin (Fig. 2c), compared to Whites, Asian Indians had a significantly higher probability of being classified in the lowest three categories (p ≥ 0.01, 0.01 and >0.01 respectively) and a significantly lower probability of being classified in the 102.5–167.8 pmol/L (p > 0.01), the 184.1 pmol/L (p = 0.03) and the ≥200.5 pmol/L (p = 0.01) ranges. In comparison to Blacks, Asian Indians had a significantly higher probability of being classified in the <37.1–53.5 mmol/L (p > 0.01) range and a significantly lower probability of being classified at 86.2 pmol/L (p = 0.02) and between 118.8–≥200.5 pmol/L (p ≤ 0.01, 0.02, 0.01, 0.03, <0.01 and <0.01, respectively) ranges. When compared to Hispanics, Asian Indians again had a significantly higher probability of being classified in the <37.1–53.5 pmol/L range (p < 0.01) and a significantly lower probability of being classified in the 102.5–151.5 pmol/L (p = 0.01, >0.01, 0.02, and 0.02 respectively) as well as the 184.1–≥200.5 (p = 0.01 and <0.01) ranges of fasting insulin.
Table 4 details the odds of iIFG, iIGT, IFG + IGT and diabetes in Whites, Blacks and Hispanics compared to Asian Indians. After adjusting for age and sex, Whites, Blacks and Hispanics were 70%, 49% and 20% less likely than Asian Indians to have diabetes, respectively, and were 69%, 45%, and 150% more likely to have IFG + IGT. Compared to Asian Indians, Whites and Hispanics were 13% and 57% more likely to have iIGT, respectively; however, Blacks were 18% less likely. The adjustment for BMI resulted in an increase in the odds of diabetes in all race/ethnic groups compared to Asian Indians. The adjustment for BMI also resulted in and attenuation of the odds of IFG + IGT, iIGT, and iIFG in all race/ethnic groups compared to Asian Indians, as well as a reversal of the point estimate comparing the odds of IFG + IGT in Blacks to Asian Indians. The inclusion of waist circumference in the model instead of BMI resulted in a further attenuation of the odds of diabetes compared to Asian Indians in all race/ethnic groups, as well as a greater attenuation in the odds of IFG + IGT, iIGT, and iIFG in all race/ethnic compared to Asian Indians, with Blacks having a comparatively lower odds of all prediabetes states. The inclusion of Log HOMA-β in the model severely attenuated the odds of diabetes in Whites and reversed the direction of the point estimate, resulting in increased odds of diabetes in Blacks and Hispanics compared to Asian Indians. When compared to the model adjusting for waist circumference, the inclusion of Log HOMA-β increased the odds of IFG + IGT, iIGT, and iIFG in all race/ethnic groups compared to Asian Indians aside from the odds of iIGT in Whites compared to Asian Indians, which remained the same in the two models. Compared to the model including Log HOMA-β, when Log HOMA-IR was added to the model the odds of diabetes between Asian Indians and Blacks, Whites, and Hispanics were decreased. The odds of all prediabetes states in Blacks, Whites, and Hispanics compared to Asian Indians were also decreased aside from the odds of iIGT in Whites compared to Asian Indians, which was slightly but not significantly increased.
Table 4.
Weighted risk factors associated with prediabetes and diabetes
| Model | IFG | IGT | IFG/IGT | T2DM | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age group,a sex, race/ethnicity | ||||||||
| Age group | 1.52 | (1.52, 1.52) | 1.51 | (1.51, 1.51) | 2.20 | (2.20, 2.20) | 3.10 | (3.10, 3.10) |
| Sex | 0.42 | (0.42, 0.42) | 1.52 | (1.52, 1.52) | 0.57 | (0.57, 0.58) | 0.54 | (0.54, 0.54) |
| White vs. Asian Indian | 1.76 | (1.74, 1.77) | 1.13 | (1.11, 1.14) | 1.69 | (1.67, 1.72) | 0.30 | (0.30, 0.31) |
| Black vs. Asian Indian | 1.50 | (1.48, 1.51) | 0.82 | (0.81, 0.84) | 1.45 | (1.43, 1.48) | 0.51 | (0.51, 0.51) |
| Hispanic vs. Asian Indian | 2.19 | (2.17, 2.21) | 1.57 | (1.54, 1.59) | 2.50 | (2.46, 2.54) | 0.80 | (0.79, 0.81) |
| Age group, sex, BMI, race/ethnicity | ||||||||
| Age group | 1.45 | (1.45, 1.45) | 1.44 | (1.44, 1.44) | 2.12 | (2.12, 2.12) | 3.12 | (3.12, 3.12) |
| Sex | 0.41 | (0.41, 0.41) | 1.51 | (1.51, 1.51) | 0.53 | (0.53, 0.53) | 0.45 | (0.45, 0.45) |
| BMI | 1.01 | (1.01, 1.01) | 1.05 | (1.05, 1.05) | 1.12 | (1.12, 1.12) | 1.17 | (1.17, 1.17) |
| White vs. Asian Indian | 1.47 | (1.45, 1.48) | 1.07 | (1.05, 1.09) | 1.24 | (1.22, 1.26) | 0.19 | (0.18, 0.19) |
| Black vs. Asian Indian | 1.09 | (1.08, 1.10) | 0.71 | (0.70, 0.73) | 0.88 | (0.86, 0.89) | 0.24 | (0.24, 0.25) |
| Hispanic vs. Asian Indian | 1.74 | (1.72, 1.75) | 1.42 | (1.40, 1.44) | 1.73 | (1.70, 1.76) | 0.46 | (0.46, 0.47) |
| Age group, sex, waist circumference, race/ethnicity | ||||||||
| Age group | 1.37 | (1.37, 1.37) | 1.40 | (1.40, 1.40) | 1.96 | (1.96, 1.96) | 2.77 | (2.77, 2.77) |
| Sex | 0.48 | (0.48, 0.48) | 1.65 | (1.64, 1.65) | 0.69 | (0.69, 0.69) | 0.70 | (0.70, 0.70) |
| Waist circumference | 1.03 | (1.03, 1.03) | 1.02 | (1.02, 1.02) | 1.05 | (1.05, 1.05) | 1.07 | (1.07, 1.07) |
| White vs. Asian Indian | 1.20 | (1.19, 1.21) | 0.92 | (0.91, 0.94) | 0.86 | (0.84, 0.87) | 0.10 | (0.10, 0.10) |
| Black vs. Asian Indian | 0.99 | (0.98, 0.99) | 0.64 | (0.63, 0.65) | 0.69 | (0.68, 0.70) | 0.16 | (0.16, 0.17) |
| Hispanic vs. Asian Indian | 1.49 | (1.48, 1.50) | 1.26 | (1.24, 1.28) | 1.31 | (1.29, 1.34) | 0.30 | (0.30, 0.30) |
| Age group, sex, HOMA-β, race/ethnicity | ||||||||
| Age group | 1.50 | (1.50, 1.50) | 1.48 | (1.48, 1.49) | 2.23 | (2.23, 2.23) | 3.10 | (3.09, 3.10) |
| Sex | 0.54 | (0.54, 0.54) | 1.24 | (1.23, 1.24) | 0.50 | (0.49, 0.50) | 0.73 | (0.73, 0.73) |
| Log HOMA-β | 0.91 | (0.91, 0.91) | 1.15 | (1.15, 1.16) | 1.39 | (1.39, 1.39) | 0.65 | (0.65, 0.65) |
| White vs. Asian Indian | 1.78 | (1.76, 1.80) | 0.92 | (0.90, 0.93) | 1.19 | (1.17, 1.21) | 0.85 | (0.84, 0.86) |
| Black vs. Asian Indian | 1.28 | (1.27, 1.29) | 0.75 | (0.74, 0.76) | 1.26 | (1.24, 1.28) | 1.71 | (1.68, 1.72) |
| Hispanic vs. Asian Indian | 1.97 | (1.95, 1.99) | 1.07 | (1.05, 1.09) | 1.49 | (1.46, 1.51) | 2.94 | (2.90, 3.0) |
| Age group, sex, HOMA-IR, race/ethnicity | ||||||||
| Age group | 1.62 | (1.62, 1.62) | 1.47 | (1.46, 1.47) | 2.53 | (2.53, 2.53) | 4.33 | (4.32, 4.33) |
| Sex | 0.50 | (0.50, 0.50) | 1.3 | (1.2, 1.3) | 0.46 | (0.46, 0.46) | 0.66 | (0.66, 0.66) |
| Log HOMA-IR | 2.49 | (2.49, 2.49) | 1.16 | (1.16, 1.16) | 4.55 | (4.55, 4.56) | 7.61 | (7.60, 7.61) |
| White vs. Asian Indian | 1.06 | (1.05, 1.07) | 0.93 | (0.91, 0.94) | 0.53 | (0.52, 0.54) | 0.16 | (0.16, 0.17) |
| Black vs. Asian Indian | 0.70 | (0.70, 0.71) | 0.76 | (0.75, 0.77) | 0.55 | (0.55, 0.56) | 0.33 | (0.32, 0.33) |
| Hispanic vs. Asian Indian | 0.16 | (0.16, 0.17) | 1.05 | (1.03, 1.07) | 0.58 | (0.57, 0.59) | 0.49 | (0.48, 0.49) |
Age was defined as a categorical variable. The age groups represented were 20–34, 35–44, 45–54, 55–74, with 20–34 used as the referent group.
Discussion
We found an overall higher prevalence of diabetes in Asian Indians living in India than in Whites, Blacks, and Hispanics living in the US, despite lower levels of adiposity. Interestingly, we found the prevalence of prediabetes to be lower in Asian Indians compared to other ethnic groups. After adjustment for age, BMI, and waist circumference, the differences in diabetes prevalence became more evident; however, the difference in prediabetes prevalence was attenuated, especially in women.
It is possible that some of the increased diabetes risk in Asian Indians is due to innate susceptibilities for impaired pancreatic β-cell function [8], [9], [10], while in other race/ethnic groups, obesity driven insulin resistance might be the driving factor behind increased hyperglycemia. This notion was further evidenced in our study, in that the inclusion of Log HOMA-β in polytomous regression models resulted in a greater odds of diabetes in Blacks and Hispanics compared to Asian Indians, whereas the inclusion of Log HOMA-IR did not. However, when Log HOMA-IR was included in polytomous logistic regression models, the odds of all prediabetes states were severely attenuated in Blacks, Whites, and Hispanics compared to Asian Indians, and in some instances the point estimates were reversed, resulting in a greater odds of iIFG in Asian Indians compared to Blacks and Hispanics, a greater odds of iIGT in Asian Indians compared to Whites and Blacks, and a greater odds of IFG + IGT in Asian Indians compared to all other race/ethnic groups. Results of our study also indicated that amongst all race/ethnic groups, Asian Indians had the highest probability of being classified in the lowest ranges of fasting insulin, thereby suggesting differences in insulin sensitivity compared to Whites, Blacks, and Hispanics.
While the findings of our study point to a lower overall prevalence of prediabetes in Asian Indians living in India compared to White, Black, and Hispanic ethnic groups in the US, we noted differences in the prediabetes prevalence by prediabetes state and sex. In particular, Asian Indian men had a lower prevalence of iIFG and IFG + IGT compared to White, Black and Hispanic men, but a higher prevalence of iIGT. Additionally, Asian Indian women had a similar prevalence of iIFG compared to White, Black, and Hispanic women; however, they had a lower prevalence of iIGT and IFG + IGT. Previous studies have noted a higher prevalence of iIFG in men compared to women [16], [19] as was the case in our study when assessing Whites, Blacks, and Hispanics. However, in the case of Asian Indians, the prevalence of iIFG was 7% higher in women than in men. These results are in concordance with another study assessing the prevalence of impaired fasting glucose and impaired glucose tolerance in individuals living in urban India that similarly reported a higher prevalence of IFG in women compared to men [20]. Therefore, there may be ethnic differences in the distribution of impaired fasting glucose by sex; however, the mechanisms behind this are not known and further studies in this area are warranted.
Our study directly compared the prevalence of diabetes and prediabetes four race/ethnic groups using two large population-based surveys using both self-report and laboratory measures. While glucose and insulin were analyzed in different laboratories, both used the same assays for analysis, thereby reducing intra-laboratory bias. Additionally, assays from the laboratory in Chennai have been run against a reference laboratory in the US and show a high concordance of r = 0.945. Furthermore, while there were differences in the sampling frames, both studies are large population-based samples that are representative either of the US, or an urban city in India. While they cannot be generalized to the entire Indian population, results from our study mirror those of other population-based studies in urban India that have noted an especially high diabetes prevalence [3], [21], [22], [23]. Furthermore, many rural areas of India are now starting to urbanize and experience their own increases in diabetes prevalence as well [4], [24], [25].
In conclusion, we found that compared to Whites, Blacks and Hispanics in the US, Asian Indians in India have a higher age-specific prevalence of diabetes in both sexes and in all adult age groups that exists despite Asian Indians having lower adiposity measurements. This suggests the contribution of non-obesity driven factors to the disproportionate burden of disease. The results of this study point to impaired pancreatic β-cell function as the driving force behind the high diabetes prevalence in Asian Indians, and suggest that Asian Indians are at high risk even at lower levels of age and adiposity, and interventions and treatments aimed solely at improving insulin resistance may not be optimal in this ethnic group. Additionally, the higher prevalence of type 2 diabetes in Asian Indians even in the youngest age group of 20–34 years points to the need for population based prevention strategies that start early in life, possibly even during youth and early adolescence. Further studies aimed at both the pathophysiological and the socio-environmental mechanisms of β-cell preservation early in the natural history of disease in groups who experience type 2 diabetes even in the absence of obesity-driven factors are therefore warranted.
Funding
The CARRS study is funded in whole or in part by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), U.S. Department of Health and Human Services, under Contract No. HHSN268200900026C, and the United Health Group, Minneapolis, MN, USA.
Conflict of interest
The authors declare they have no conflicts of interest.
Parts of this study were presented as part of a doctoral dissertation entitled “Type 2 Diabetes in Asian Indians on Two Continents: Insights into the Epidemic and Pathophysiology,” Emory University, Atlanta, GA, May 2015.
Author contributions
U.P.G. analyzed data, wrote the manuscript, drafted tables and figures, and revised the manuscript and approved the final manuscript for submission. V.M. contributed to the design of the CARRS study, contributed to the discussion and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript for submission. R.P. and M.D oversaw the CARRS research operations and contributed to the design, and data collection of the CARRS study. R.M.A contributed to the discussion and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript for submission. N.K.M. and E.W.G contributed to the discussion and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript for submission. K.M.V.N. contributed to concept, design, analysis, and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript for submission. U.P.G. is the guarantor of this work and has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors would like to thank Dr. Donna Brogan for her insights into survey sampling weights and methodology.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Kasuga M. Insulin resistance and pancreatic cell failure. J Clin Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saad M.F., Knowler W.C., Pettitt D.J., Nelson R.G., Charles M.A., Bennett P.H. A two-step model for development of non-insulin-dependent diabetes. Am J Med. 1991;90:229–235. [PubMed] [Google Scholar]
- 3.Mohan V., Deepa M., Deepa R., Shanthirani C.S., Farooq S., Ganesan A. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India – the Chennai Urban Rural Epidemiology Study (CURES-17) Diabetologia. 2006;49:1175–1178. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 4.Hwang C.K., Han P.V., Zabetian A., Ali M.K., Narayan K.M. Rural diabetes prevalence quintuples over twenty-five years in low- and middle-income countries: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;96:271–285. doi: 10.1016/j.diabres.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths P.L., Bentley M.E. The nutrition transition is underway in India. J Nutr. 2001;131:2692–2700. doi: 10.1093/jn/131.10.2692. [DOI] [PubMed] [Google Scholar]
- 6.Shetty P.S. Nutrition transition in India. Public Health Nutr. 2002;5:175–182. doi: 10.1079/PHN2001291. [DOI] [PubMed] [Google Scholar]
- 7.Misra A., Singhal N., Sivakumar B., Bhagat N., Jaiswal A., Khurana L. Nutrition transition in India: secular trends in dietary intake and their relationship to diet-related non-communicable diseases. J Diabetes. 2011;3:278–292. doi: 10.1111/j.1753-0407.2011.00139.x. [DOI] [PubMed] [Google Scholar]
- 8.Gujral U.P., Narayan K.M., Kahn S.E., Kanaya A.M. The relative associations of β-cell function and insulin sensitivity with glycemic status and incident glycemic progression in migrant Asian Indians in the United States: the MASALA study. J Diabetes Complications. 2014;28:45–50. doi: 10.1016/j.jdiacomp.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staimez L.R., Weber M.B., Ranjani H., Ali M.K., Echouffo-Tcheugui J.B., Phillips L.S. Evidence of reduced β-cell function in Asian Indians with mild dysglycemia. Diabetes Care. 2013;36:2772–2778. doi: 10.2337/dc12-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan V., Amutha A., Ranjani H., Unnikrishnan R., Datta M., Anjana R.M. Associations of β-cell function and insulin resistance with youth-onset type 2 diabetes and prediabetes among Asian Indians. Diabetes Technol Ther. 2013;15:315–322. doi: 10.1089/dia.2012.0259. [DOI] [PubMed] [Google Scholar]
- 11.Nair M., Ali M.K., Ajay V.S., Shivashankar R., Mohan V., Pradeepa R. CARRS surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health. 2012;12:701. doi: 10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandramouli C., General R. Office of the Registrar General Census Commissioner, India, Indian Census Bureau; 2011. Census of India 2011. Provisional population tools – India data sheet. [Google Scholar]
- 13.NHANES – survey methods and analytic guidelines. 2014. http://www.cdc.gov/nchs/nhanes/survey_methods.htm accessed 18.02.16.
- 14.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Færch K., Borch-Johnsen K., Holst J.J., Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52:1714–1723. doi: 10.1007/s00125-009-1443-3. [DOI] [PubMed] [Google Scholar]
- 16.Færch K., Vaag A., Holst J.J., Hansen T., Jørgensen T., Borch-Johnsen K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care. 2009;32:439–444. doi: 10.2337/dc08-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y.J., Kahn H.S., Gregg E.W., Imperatore G., Geiss L.S. Recent population changes in HbA1c and fasting insulin concentrations among US adults with preserved glucose homeostasis. Diabetologia. 2010;53:1890–1893. doi: 10.1007/s00125-010-1800-2. [DOI] [PubMed] [Google Scholar]
- 19.Williams J.W., Zimmet P.Z., Shaw J.E., De Courten M.P., Cameron A.J., Chitson P. Gender differences in the prevalence of impaired fasting glycaemia and impaired glucose tolerance in Mauritius. Does sex matter? Diabet Med. 2003;20:915–920. doi: 10.1046/j.1464-5491.2003.01059.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran A., Snehalatha C., Satyavani K., Vijay V. Impaired fasting glucose and impaired glucose tolerance in urban population in India. Diabet Med. 2003;20:220–224. doi: 10.1046/j.1464-5491.2003.00904.x. [DOI] [PubMed] [Google Scholar]
- 21.Anjana R.M., Pradeepa R., Deepa M., Datta M., Sudha V., Unnikrishnan R. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research–INdia DIABetes (ICMR–INDIAB) study. Diabetologia. 2011;54:3022–3027. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A., Gupta R., Sarna M., Rastogi S., Gupta V.P., Kothari K. Prevalence of diabetes, impaired fasting glucose and insulin resistance syndrome in an urban Indian population. Diabetes Res Clin Pract. 2003;61:69–76. doi: 10.1016/s0168-8227(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 23.Deepa M., Anjana R.M., Manjula D., Narayan K.V., Mohan V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10-year follow-up of the Chennai Urban Population Study. J Diabetes Sci Technol. 2011;5:918–927. doi: 10.1177/193229681100500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramachandran A., Snehalatha C., Mary S., Mukesh B., Bhaskar A.D., Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 25.Misra P., Upadhyay R.P., Misra A., Anand K. A review of the epidemiology of diabetes in rural India. Diabetes Res Clin Pract. 2011;92:303–311. doi: 10.1016/j.diabres.2011.02.032. [DOI] [PubMed] [Google Scholar]