Abstract
Sanguisorbae Radix (SR) is well known as herbal medicine named “Zi-Yu” in Korea, which is the dried roots of Sanguisorba officinalis L. (Rosacease). We investigated the underlying mechanism on the inhibition of atopic dermatitis (AD) of an ethanol extract of SR (ESR) using 2,4-dinitrochlorobenzene- (DNCB-) induced AD mice model. Oral administration of ESR significantly suppressed DNCB-induced AD-like symptoms such as scratching behavior, ear thickness, epidermal thickness, and IgE levels. To investigate the effects of ESR treatment on degranulation of IgE/Ag-activated mouse bone marrow-derived mast cells (BMMCs), we measured the release of β-hexosaminidase (β-HEX, degranulation marker). ESR decreased the infiltration of eosinophils and mast cells into the AD skin lesions. Furthermore, ESR significantly inhibited degranulation of IgE/Ag-activated BMMCs. We have demonstrated that ESR decreased AD symptoms in mice and inhibits degranulation of IgE/Ag-activated mast cells. Our study suggests that ESR may serve as a potential therapeutic candidate for the treatment of AD symptoms.
1. Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease. AD causes epidermal thickness with cutaneous hypersensitivity associated with increased serum immunoglobulin E (IgE) levels and infiltration of inflammatory cell types including mast cells and eosinophils [1, 2]. AD is a complex interaction of innate and adaptive immune responses based on an individual's genetic, environmental, pharmacological, and psychological conditions.
IgE secretion is an important characteristic of AD, with elevated levels related to disease severity in AD patients. Previous studies report that high serum IgE levels induce activation of mast cells and cause an allergic reaction [2, 3]. Because mast cells aggregate high-affinity IgE receptors (FcεRI) on their surfaces, which is important in the proinflammatory/allergic response, mast cells are believed to play an important role in the induction of AD [4, 5]. An association between mast cell activation and AD is suggested by the increase in mast cell counts and activation in AD lesions [6]. In addition, mast cells produce inflammatory mediators such as prostaglandin D2 (PGD2) and induce eosinophil chemotaxis to inflammatory sites in the skin of AD patients [7].
Sanguisorbae Radix (SR) is well known as herbal medicine named “Zi-Yu” in Korea, which is the dried roots of Sanguisorba officinalis L. (Rosacease) [8]. SR has been used as a traditional herbal medicine to treat diarrhea, chronic intestinal infections, duodenal ulcers, internal hemorrhage, and burns [8–10]. SR includes saponin glycosides and ellagitannins (i.e., pomolic acid, sanguisorbic acid dilactone, and ziyuglycoside I). SR and its active components have been demonstrated to have biological activity in vivo and in vitro. SR inhibits the renal dysfunction induced by lipopolysaccharide (LPS) endotoxin in vivo by suppressing the serum nitrite/nitrate levels and the activity of inducible nitric oxide synthase (iNOS) [11]. In addition, SR was reported to have antioxidative stress and antiaging activity via suppressing of nitric oxide (NO) production and activity of iNOS [10, 12, 13]. SR blocked H2O2-induced ROS generation in vitro and MCAO-induced ischemic brain damage in vivo [14]. Additional reported properties of SR include anticancer [15], antiasthma [16], anticoronavirus [17], and antiwrinkle activity [18]. Many researchers reported anti-inflammatory activities of SR. Nonetheless, antiatopic dermatitis effects of SR are not reported. Recently, studies reported inhibitory effect of SR on contact dermatitis [19], and we reported anti-inflammatory mechanism of ESR in human keratinocyte by suppressing the expression of TNF-α/IFN-γ-stimulated chemokines and proinflammatory molecules via blockade of NF-κB, STAT-1, and ERK activation. However, the biological and pharmacological actions of SR are not fully understood in atopic dermatitis.
In this study, we examined the effects of ESR on 2,4-dinitrochlorobenzene- (DNCB-) induced AD mouse skin lesions and mouse bone marrow-derived mast cells (BMMCs) to determine its therapeutic potential for the treatment of AD.
2. Materials and Methods
2.1. Preparation of ESR
SOL roots were obtained from Yeongcheon Oriental Herbal Market (Yeongcheon, Korea). All samples were deposited in the herbal bank of KM Application Center, Korea Institute of Oriental Medicine (KIOM; Daejeon, Republic of Korea). To prepare the ESR, dried SR pieces (50.0 g) were extracted using 390 mL 70% ethanol in a 40°C shaking incubator for 24 h. We obtained 9.5 g, giving us a yield of 19.1%.
2.2. Animals
Male BALB/c mice (5 weeks old) were purchased from Samtako Bio Korea (Osan, Korea). Mice were observed every day for one week during quarantine and acclimation. Mice were divided into six groups (n = 5 per group): (1) negative control (vehicle), (2) DNCB + vehicle (control), (3) DNCB + 50 mg/kg ESR, (4) DNCB + 100 mg/kg ESR, (5) DNCB + 200 mg/kg ESR, and (6) and 1 mg/kg dexamethasone (Dexa.). All groups were maintained under standard conditions of temperature (22.5 ± 0.5°C), humidity (42.6 ± 1.7%), 12 h lighting (8:00 AM–8:00 PM, 290 lx), ventilation (10–15 times per hour), and diet (Teklad Global Diets, Harlan Laboratories Inc., USA). This study was conducted according to the guidelines listed in the Pharmaceutical Affairs Act of Korea Food and Drug Association (KFDA) and approved by the Institutional Animal Care and Use Committee of Korea Conformity Laboratories (IA11-00920).
2.3. Induction of AD and Drug Treatment
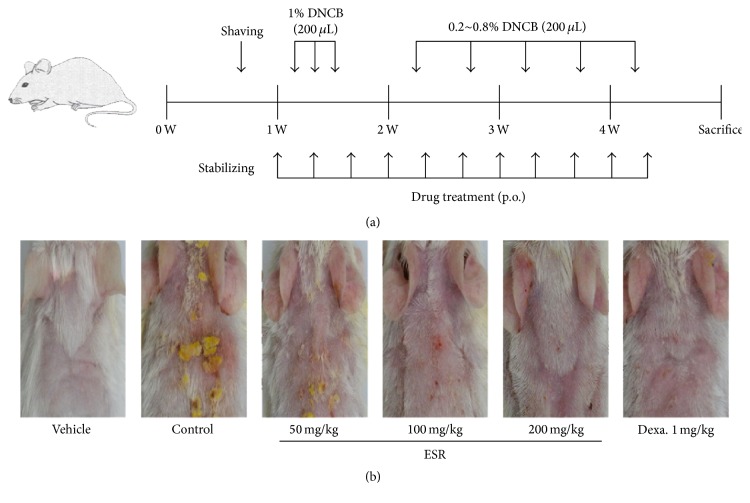
After 1 week of acclimation, DNCB was applied to the dorsal skin and both ears of BALB/c mice to induce AD-like symptoms and skin lesions. One day after complete dorsal hair removal, 200 μL 1% DNCB dissolved in an acetone : olive oil mixture (3 : 1 vol/vol) was applied to the dorsal skin and 20 μL was applied to both ears (days 2–4). Five days after dorsal hair removal, 0.2–0.8% DNCB dissolved in an acetone : olive oil mixture (3 : 1 vol/vol) was applied to challenge the dorsal skin (200 μL) and both ears (20 μL each) two times a week for 2 weeks. Similarly, 1% DNCB solution was applied one day prior to sacrifice. ESR dissolved in saline (10 mL/kg body weight) was orally administered by gavage at 50, 100, and 200 mg/kg or using dexamethasone (Sigma-Aldrich, St. Louis, MO, USA; 1 mg/kg) three times a week for 4 weeks (days 0–24). The experimental scheme is summarized in Figure 1(a).
Figure 1.
Effects of ESR on the development of DNCB-induced AD mouse skin lesions in BALB/c mice. (a) Experimental schedule for the induction of dermatitis. (b) Effects of ESR on clinical features of DNCB-induced AD skin lesions. Vehicle, negative control; control, DNCB + vehicle; ESR, DNCB + ESR treated group (50, 100, and 200 mg/kg); Dexa., DNCB + 1 mg/kg dexamethasone treated group.
2.4. Scratching Behavior and Ear Thickness Measurements
Scratching behavior was measured by placing each mouse into a cage once a week for 10 min and observing and recording behavior [20]. For each mouse, ear thickness was measured and recorded with a micrometer (Mitutoyo, Kawasaki, Japan). To minimize variation, a single investigator performed all measurements [21].
2.5. Histopathological Analysis
At the end of the study period, the dorsal skin lesions of each mouse were removed, fixed with 10% neutral-buffered formalin, and embedded in paraffin. 4 μm thick sections were stained with hematoxylin and eosin (H&E) and toluidine blue to detect epidermal thickness and inflammatory cells (i.e., eosinophils and mast cells), respectively. Histopathological evaluation of all skin sections occurred in a blind fashion [1]. All samples were observed using an inverted microscope and data are representative of five observations (Nikon Eclipse Ti, Nikon, Tokyo, Japan).
2.6. Serum IgE Measurements
At the end of the study period, mice were sacrificed and whole blood was collected. Blood samples were centrifuged at 2,000 ×g for 20 min at 4°C. Serum obtained from whole blood was stored at −80°C until use. Serum IgE levels were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (BioLegend, San Diego, CA, USA). Inhibitory effects of ESR were determined based on absorbance at 450 nm measured using a multilabel microplate reader (SpectraMax i3, Molecular Devices, Silicon Valley, CA, USA).
2.7. BMMCs
BMMCs isolated from male BALB/c mice were cultured for up to 10 weeks in RPMI-1640 media containing 2 mM L-glutamine, 0.1 mM nonessential amino acids, antibiotics, and 10% fetal bovine serum (FBS) with 20% pokeweed mitogen-stimulated spleen condition medium (PWM-SCM) as a source of interleukin-3 (IL-3). After 3 weeks, more than 98% of the cells were verified as BMMCs according to a previously described procedure [22].
2.8. Cell Viability Assay
Cell cytotoxicity was analyzed using a cell counting kit (CCK) (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). BMMCs (2 × 105 cells/well) were seeded into 96-well plates. After 24 h, ESR was added at concentrations of 10, 50, 100, and 200 μg/mL, and plates were incubated for 24 h at 37°C in a 5% CO2 incubator. CCK solutions were added to each well and cells were incubated for 1 h. Optical density was measured at 570 nm using an ELISA plate reader (Infinite M200, Tecan, Männedorf, Switzerland).
2.9. β-HEX Release Assay
β-hexosaminidase (β-HEX), a marker of mast cell degranulation, was quantified by spectrophotometric analysis of the hydrolysis of p-nitrophenyl-2-acetamido-2-deoxy-β-D-glucopyranoside (4-Nitrophenyl N-acetyl-β-D-glucosaminide (p-NAG), Sigma-Aldrich, St. Louis, MO, USA).
For cell stimulation, BMMCs (5 × 105 cells/mL) were sensitized overnight with 100 ng/mL anti-dinitrophenyl (DNP) antibody and then stimulated for 15 min with 25 ng/mL DNP-human serum albumin (HSA). To investigate the effects of ESR, ESR of varying concentrations was added 1 h prior to the addition of DNP-HSA. After harvesting supernatants according to a previously described procedure [23], the percentage of β-HEX released into the supernatant was calculated using the following formula: [supernatant/(supernatant + pellet)] × 100.
2.10. Statistical Analysis
Data were analyzed using GraphPad Prism software (ver. 5.0 GraphPad Software, San Diego, CA, USA). Results are expressed as the mean ± standard error of the mean (SEM) and were evaluated using Student's t-test or analysis of variance (ANOVA). A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Effects of ESR on the Development of DNCB-Induced AD Mouse Skin Lesions
To investigate the therapeutic effects of ESR on AD mouse lesions, we administered ESR following the induction of AD mouse skin lesions using DNCB. Topical application of DNCB-induced crusting, epidermal thickness, redness, and dryness of skin are shown in Figure 1(b). However, skin conditions significantly improved in ESR-administered groups compared to the control group.
3.2. Effects of ESR on Scratching Behavior and Ear Thickness in AD Mice
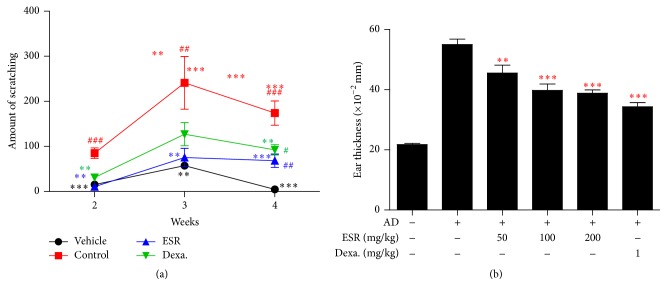
Scratching behavior of the control group was rapidly increased and became significantly different from that observed in the vehicle group at day 14 after DNCB application. In AD mice treated with 200 mg/kg ESR, scratching was inhibited strongly compared to the vehicle group (Figure 2(a)). In addition, ESR significantly reduced DNCB-induced ear thickness in a dose-dependent manner. These results suggest that ESR has a therapeutic effect that can reduce AD symptoms in mice.
Figure 2.
Effects of ESR on scratching behavior and ear thickness in AD mouse skin lesions. (a) Experimental induction of AD mouse skin lesions. (b) Ear thickness was measured with a dial thickness gauge. Results are expressed as the mean ± standard error of the mean (SEM) (n = 5). Vehicle, negative control; control, DNCB + vehicle; ESR, DNCB + 200 mg/kg ESR; Dexa., DNCB + 1 mg/kg dexamethasone treated group. # p < 0.05, ## p < 0.01, and ### p < 0.001 versus vehicle; ∗∗ p < 0.01 and ∗∗∗ p < 0.001 versus control.
3.3. Effects of ESR on Dorsal Skin Thickness and Eosinophil Accumulation in AD Mouse Skin Lesions
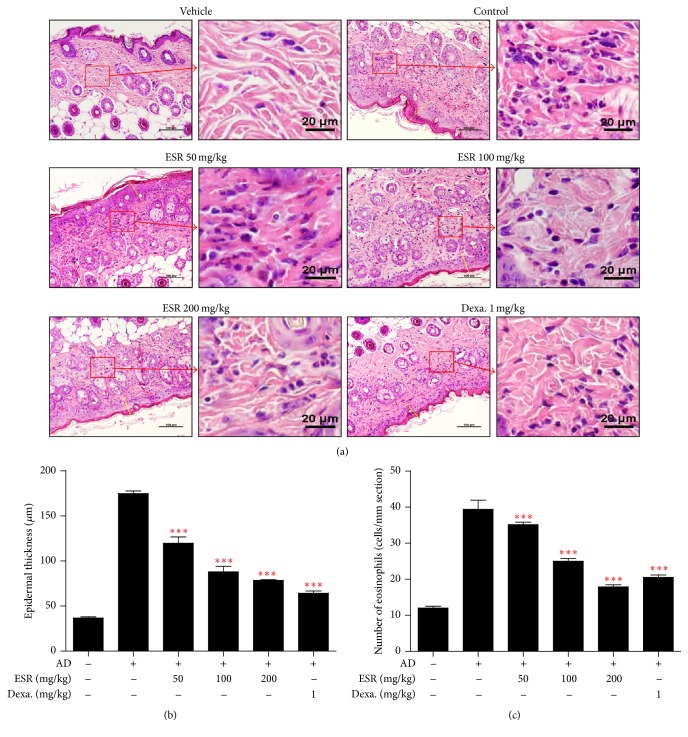
Multiple applications of DNCB-induced infiltration of high levels of inflammatory cells into the skin result in increased dermal thickness [24]. To determine whether ESR treatment decreases eosinophil accumulation in AD mouse skin lesions, we performed H&E staining on the skin following oral administration of ESR and observed the tissue under an optical microscope. Dorsal histopathological results showed thickening of the epidermis and eosinophil accumulation within the dorsal skin lesions of BALB/c mice with AD. The treatment of 100 and 200 mg/kg ESR suppressed eosinophil accumulation and reduced dorsal skin thickness in a dose-dependent manner (Figures 3(a) and 3(b)).
Figure 3.
Effects of ESR on epidermal thickness and eosinophil accumulation in DNCB-induced AD mouse skin lesions. (a) H&E stained AD mouse skin lesions (20x, scale bar = 100 μm) and image of amplification sections (right image of group, scale bar = 20 μm). (b) Determination of epidermal thickness. (c) Number of eosinophils per mm section. Epidermal thickness in H&E stained sections was measured under a microscope and the number of eosinophils accumulations is expressed as the average total count in five fields of 100 μm2. ∗∗∗ p < 0.001 versus control.
3.4. Effects of ESR on Mast Cell Infiltration and Serum IgE Levels in AD Mice
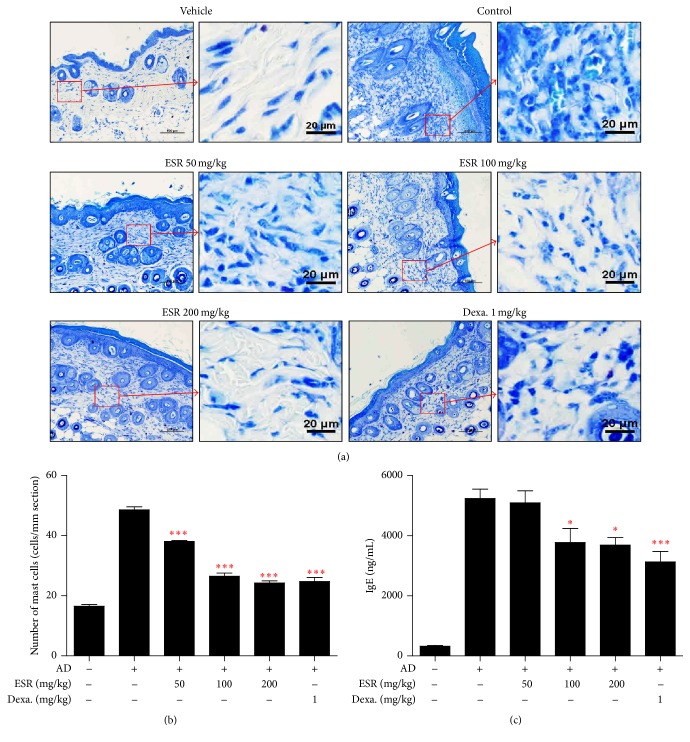
Elevated serum IgE levels and mast cell infiltration are major characteristics of AD and related to disease severity [24]. To assess the effects of ESR on mast cell infiltration, we stained sliced cross sections of skin lesions with toluidine blue. As shown in Figure 4, we found an increase in mast cell infiltration (Figures 4(a) and 4(b)) and IgE levels in DNCB-treated mice compared to the vehicle group (Figure 4(c)). However, ESR significantly decreased the level of IgE elevated by DNCB induction and inhibited mast cell infiltration, in a dose-dependent manner (Figures 4(b) and 4(c)).
Figure 4.
Effects of ESR on mast cell infiltration and serum IgE level in DNCB-induced AD mouse lesions. (a) Toluidine blue stained AD mouse skin lesions (20x, scale bar = 100 μm) and image of amplification sections (right image of group, scale bar = 20 μm). (b) Number of mast cells per mm section. Mast cell infiltration in toluidine blue stained sections is expressed as the average total count in five fields of 100 μm2. ∗ p < 0.05, ∗∗∗ p < 0.001 versus control.
3.5. Effects of ESR on BMMC Cytotoxicity and Mast Cell Degranulation
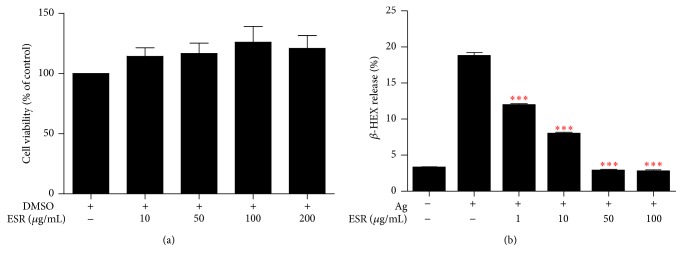
Degranulation of mast cells is correlated with AD severity and the recruitment of immune cells to inflammatory sites in AD patients [2]. To investigate the effect of ESR treatment on degranulation of IgE/Ag-activated BMMCs, we measured the release of β-HEX in the presence or absence of ESR. As shown in Figure 5(b), ESR potently reduced β-HEX release in a dose-dependent manner.
Figure 5.
Effects of ESR on cytotoxic and mast cell degranulation in BMMCs. (a) Cytotoxicity of BMMCs was determined using a CCK assay. BMMCs were seeded into 96-well plates and treated with various concentrations of ESR for 24 h. (b) Inhibitory effects of ESR on β-HEX release in BMMCs. BMMCs were sensitized overnight with anti-DNP IgE and challenged with DNP-HAS (Ag) with or without ESR. The data are presented as the mean ± SEM of three independent experiments. ∗∗∗ p < 0.001 versus control.
To check cytotoxicity of ESR on BMMCs, the cells were incubated with different ESR concentrations (10, 50, 100, and 200 μg/mL) for 18 h and subjected for CCK-8 assay. ESR at 200 μg/mL had no significant cytotoxic effect on BMMCs after 24 h (Figure 5(a)).
4. Discussion
AD is an inflammatory disease characterized by an increase of cells associated with a Th2 response, including monocytes, macrophages, eosinophils, and mast cells. The pathogenesis of AD is primarily driven by Th2 immune responses and increased IgE production [5]. Proliferation, migration, and local activation of eosinophils are common in AD. Eosinophils act as immunoregulatory factors by secreting a variety of cytokines and chemokines attracting more eosinophils to the site of inflammation. Furthermore, eosinophils promote a switch from acute to chronic responses in AD [2]. ESR treatment reduces eosinophil accumulation in AD mouse skin lesions (Figures 3(a) and 3(c)).
High levels of serum IgE represent another characteristic of AD; thus, it is likely that targeting IgE may impact AD disease outcome. Specifically, IgE binding to mast cells affects the development and severity of AD [21]. Mast cells play a key role in allergic reactions via the production and secretion of proinflammatory mediators such as histamine, chemokines, cytokines, and growth factors. On the surface of mast cells, Th2 cells produce IgE, which binds to the FcεRI on the mast cell surface [25]. FcεRI-mediated mast cell activation is triggered by antigen IgE cross-linking and leads to the degranulation and expression of proinflammatory mediators. FcεRI-activated mast cells induce IgE elevation and increase mast cells in a majority of AD patients; therefore, mast cells are hypothesized to contribute to the pathogenesis of AD [3]. Our findings show that ESR treatment suppresses serum IgE levels and mast cell infiltration in a DNCB-induced AD mouse model.
β-HEX, a degranulation marker, is released along with other proinflammatory mediators when mast cells are activated [23, 26]. Therefore, we examined the degranulation by measuring β-HEX release in antigen IgE-activated BMMCs and confirmed that ESR treatment inhibited β-HEX release in a dose-dependent manner.
Active components of SR include phenolic compounds including tannins and flavonoids, saponin glycosides, and ellagitannins (i.e., pomolic acid, sanguisorbic acid dilactone, and ziyuglycoside I) [27]. Ziyuglycoside I and ziyuglycoside II are the major effective ingredients of triterpenoid saponins extracted from Sanguisorba officinalis L., and many studies have focused on their pharmacological activities. In addition, ziyuglycoside I is a major marker of SR to confirm the origin of the medicinal plant in Korean Pharmacopoeia. Thus, we used ziyuglycoside I as a phytochemical marker of SR in the experiment (data not shown).
In this study, we investigated the therapeutic effects of ESR in the treatment of AD using a DNCB-induced AD mouse model and BMMCs. ESR significantly improved scratching behavior, ear thickness, epidermal thickness, and serum IgE levels in DNCB-induced AD mice. We observed a reduction in the infiltration of eosinophils and mast cells into the AD skin lesions following ESR treatment. In addition, ESR significantly inhibited degranulation of IgE/Ag-activated BMMCs. The therapeutic effects of ESR treatment on AD are supported by the beneficial effect of anti-IgE therapy in a number of clinical studies [2, 3].
In conclusion, these results demonstrate that ESR decreases AD symptoms in mice and inhibits degranulation of IgE/Ag-activated mast cells. Our study suggests that ESR may serve as a potential therapeutic candidate for the treatment of AD.
Acknowledgments
This work was supported by Grant no. K16281 awarded to Korea Institute of Oriental Medicine (KIOM) from Ministry of Education, Science and Technology (MEST), Republic of Korea.
Abbreviations
- ESR:
Ethanol extract of Sanguisorbae Radix
- AD:
Atopic dermatitis
- DNCB:
2,4-Dinitrochlorobenzene
- H&E:
Hematoxylin and eosin
- FcεRI:
High-affinity immunoglobulin E receptor
- BMMCs:
Mouse bone marrow-derived mast cells
- β-HEX:
β-Hexosaminidase.
Competing Interests
The authors declare that there are no competing interests.
Authors' Contributions
Ju-Hye Yang designed the study, performed the research, and wrote the paper; Jae-Myung Yoo was involved in experiments; Won-Kyung Cho and Jin Yeul Ma supervised the study.
References
- 1.Karuppagounder V., Arumugam S., Thandavarayan R. A., et al. Tannic acid modulates NFκB signaling pathway and skin inflammation in NC/Nga mice through PPARγ expression. Cytokine. 2015;76(2):206–213. doi: 10.1016/j.cyto.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Liu F.-T., Goodarzi H., Chen H.-Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clinical Reviews in Allergy & Immunology. 2011;41(3):298–310. doi: 10.1007/s12016-011-8252-4. [DOI] [PubMed] [Google Scholar]
- 3.Kawakami T., Ando T., Kimura M., Wilson B. S., Kawakami Y. Mast cells in atopic dermatitis. Current Opinion in Immunology. 2009;21(6):666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nedoszytko B., Sokołowska-Wojdyło M., Ruckemann-Dziurdzińska K., Roszkiewicz J., Nowicki R. J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatologii i Alergologii. 2014;31(2):84–91. doi: 10.5114/pdia.2014.40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt E. B., Sivaprasad U. Th2 cytokines and atopic dermatitis. Journal of Clinical & Cellular Immunology. 2011;2(3, article 110) doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi J. K., Kim S.-H. Inhibitory effect of galangin on atopic dermatitis-like skin lesions. Food and Chemical Toxicology. 2014;68:135–141. doi: 10.1016/j.fct.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Nakano N., Nishiyama C., Yagita H., et al. Notch signaling enhances FcεRI-mediated cytokine production by mast cells through direct and indirect mechanisms. Journal of Immunology. 2015;194(9):4535–4544. doi: 10.4049/jimmunol.1301850. [DOI] [PubMed] [Google Scholar]
- 8.Shin T.-Y., Lee K.-B., Kim S.-H. Anti-allergic effects of Sanguisorba officinalis on animal models of allergic reactions. Immunopharmacology and Immunotoxicology. 2002;24(3):455–468. doi: 10.1081/iph-120014729. [DOI] [PubMed] [Google Scholar]
- 9.Oh H., Hur J., Park G., Kim H. G., Kim Y. O., Oh M. S. Sanguisorbae radix protects against 6-hydroxydopamine-induced neurotoxicity by regulating NADPH oxidase and NF-E2-related factor-2/heme oxygenase-1 expressions. Phytotherapy Research. 2013;27(7):1012–1017. doi: 10.1002/ptr.4802. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S., Liu X., Zhang Z.-L., He L., Wang Z., Wang G.-S. Isolation and identification of the phenolic compounds from the roots of Sanguisorba officinalis L. and their antioxidant activities. Molecules. 2012;17(12):13917–13922. doi: 10.3390/molecules171213917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C. P., Yokozawa T., Kitani K. Beneficial effects of sanguisorbae radix in renal dysfunction caused by endotoxin in vivo. Biological & Pharmaceutical Bulletin. 1999;22(12):1327–1330. doi: 10.1248/bpb.22.1327. [DOI] [PubMed] [Google Scholar]
- 12.Yokozawa T., Chen C. P., Tanaka T., Kitani K. Effects of sanguiin H-6, a component of Sanguisorbae Radix, on lipopolysaccharide-stimulated nitric oxide production. Biochemical Pharmacology. 2002;63(5):853–858. doi: 10.1016/S0006-2952(01)00930-3. [DOI] [PubMed] [Google Scholar]
- 13.Yokozawa T., Chen P. C. Evidence suggesting a nitric oxide-scavenging activity for traditional crude drugs, and action mechanisms of Sanguisorbae Radix against oxidative stress and aging. Journal of the American Aging Association. 2001;24(1):19–30. doi: 10.1007/s11357-001-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen T. T. H., Cho S. O., Ban J. Y., et al. Neuroprotective effect of Sanguisorbae radix against oxidative stress-induced brain damage: in vitro and in vivo . Biological and Pharmaceutical Bulletin. 2008;31(11):2028–2035. doi: 10.1248/bpb.31.2028. [DOI] [PubMed] [Google Scholar]
- 15.Wu Z., Sun H., Li J., et al. A polysaccharide from Sanguisorbae radix induces caspase-dependent apoptosis in human leukemia HL-60 cells. International Journal of Biological Macromolecules. 2014;70:615–620. doi: 10.1016/j.ijbiomac.2014.06.062. [DOI] [PubMed] [Google Scholar]
- 16.Lee N.-H., Lee M.-Y., Lee J.-A., et al. Anti-asthmatic effect of Sanguisorba officinalis L. and potential role of heme oxygenase-1 in an ovalbumin-induced murine asthma model. International Journal of Molecular Medicine. 2010;26(2):201–208. doi: 10.3892/ijmm-00000453. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.-Y., Eo E.-Y., Park H., et al. Medicinal herbal extracts of Sophorae radix, Acanthopanacis cortex, Sanguisorbae radix and Torilis fructus inhibit coronavirus replication in vitro. Antiviral Therapy. 2010;15(5):697–709. doi: 10.3851/imp1615. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y. H., Chung C. B., Kim J. G., et al. Anti-wrinkle activity of ziyuglycoside I isolated from a Sanguisorba officinalis root extract and its application as a cosmeceutical ingredient. Bioscience, Biotechnology and Biochemistry. 2008;72(2):303–311. doi: 10.1271/bbb.70268. [DOI] [PubMed] [Google Scholar]
- 19.Yokozawa T., Chen C. P., Tanaka T., Kitani K. A study on the nitric oxide production-suppressing activity of sanguisorbae radix components. Biological & Pharmaceutical Bulletin. 2000;23(6):717–722. doi: 10.1248/bpb.23.717. [DOI] [PubMed] [Google Scholar]
- 20.Luo Y., Wang H., Yuan Z. Triterpenoid saponins of Sanguisorba officinalis and their anti-inflammatory activity. Chinese Journal of Medicinal Chemistry. 2008;18(2):138–141. [Google Scholar]
- 21.Jo S., Ryu J., Kim H., et al. Anti-inflammatory effects of Sanguisorbae Radix on contact dermatitis induced by dinitrofluorobenzene in mice. Chinese Journal of Integrative Medicine. 2015 doi: 10.1007/s11655-015-2148-8. [DOI] [PubMed] [Google Scholar]
- 22.Choi M. S., Kim E. C., Lee H. S., et al. Inhibitory effects of Saururus chinensis (Lour.) Baill on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Biological and Pharmaceutical Bulletin. 2008;31(1):51–56. doi: 10.1248/bpb.31.51. [DOI] [PubMed] [Google Scholar]
- 23.Choi J. H., Jin S. W., Park B. H., et al. Cultivated ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice and TNF-α/IFN-γ-induced TARC activation in HaCaT cells. Food and Chemical Toxicology. 2013;56:195–203. doi: 10.1016/j.fct.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y., Yang J. H., Li X., et al. Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation. Biochemical Pharmacology. 2011;82(11):1700–1708. doi: 10.1016/j.bcp.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y., Li Y., Jin M., et al. Inula japonica extract inhibits mast cell-mediated allergic reaction and mast cell activation. Journal of Ethnopharmacology. 2012;143(1):151–157. doi: 10.1016/j.jep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Kim S. R., Choi H.-S., Seo H. S., et al. Oral administration of herbal mixture extract inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis in BALB/c mice. Mediators of Inflammation. 2014;2014:10. doi: 10.1155/2014/319438.319438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Je I.-G., Kim D.-S., Kim S.-W., et al. Tyrosol suppresses allergic inflammation by inhibiting the activation of phosphoinositide 3-kinase in mast cells. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0129829.e0129829 [DOI] [PMC free article] [PubMed] [Google Scholar]