Abstract
Purpose
The nitric oxide (NO)–cyclic guanosine-3′,5′-monophosphate (cGMP) pathway regulates aqueous humor outflow and therefore, intraocular pressure. We investigated the pharmacologic effects of the soluble guanylate cyclase (sGC) stimulator IWP-953 on primary human trabecular meshwork (HTM) cells and conventional outflow facility in mouse eyes.
Methods
Cyclic GMP levels were determined in vitro in HEK-293 cells and four HTM cell strains (HTM120/HTM123: predominantly myofibroblast-like phenotype, HTM130/HTM141: predominantly endothelial-like phenotype), and in HTM cell culture supernatants. Conventional outflow facility was measured following intracameral injection of IWP-953 or DETA-NO using a computerized pressure-controlled perfusion system in enucleated mouse eyes ex vivo.
Results
IWP-953 markedly stimulated cGMP production in HEK-293 cells in the presence and absence of DETA-NO (half maximal effective concentrations: 17 nM, 9.5 μM). Similarly, IWP-953 stimulated cGMP production in myofibroblast-like HTM120 and HTM123 cells, an effect that was greatly amplified by the presence of DETA-NO. In contrast, IWP-953 stimulation of cGMP production in endothelial-like HTM130 and HTM141 cells was observed, but was markedly less prominent than in HTM120 and HTM123 cells. Notably, cGMP was found in all HTM culture supernatants, following IWP-953/DETA-NO stimulation. In paired enucleated mouse eyes, IWP-953 at 10, 30, 60, and 100 μM concentration-dependently increased outflow facility. This effect (89.5%) was maximal at 100 μM (P = 0.002) and in magnitude comparable to DETA-NO at 100 μM (97.5% increase, P = 0.030).
Conclusions
These data indicate that IWP-953, via modulation of the sGC–cGMP pathway, increases aqueous outflow facility in mouse eyes, suggesting therapeutic potential for sGC stimulators as novel ocular hypotensive drugs.
Keywords: trabecular meshwork, glaucoma pharmacology, conventional outflow, soluble guanylate cyclase
Glaucoma is a progressive and degenerative optic neuropathy associated with irreversible loss of vision, starting with peripheral visual field loss and moving centrally. Primary open-angle glaucoma (POAG) is the most common form resulting in chronic degeneration of retinal ganglion cell axons at the level of the lamina cribrosa and subsequent apoptotic cell death.1 Chronically elevated intraocular pressure (IOP) is the major modifiable risk factor, with several prospective, randomized multicenter studies showing that effective IOP lowering has neuroprotective effects, delaying or even preventing structural and functional damage to optic nerve axons.2 Intraocular pressure is generated and maintained via the aqueous humor (AH) circulation system in the anterior eye. The conventional (trabecular) outflow pathway, a pressure-driven system, is responsible for generation and maintenance of IOP under physiological conditions and provides resistance to unobstructed AH outflow.3,4
At present, the only proven treatment in the management of glaucoma is directed toward lowering IOP, either by increasing the rate of conventional outflow or reducing AH production. Among the five classes of drugs most commonly used, two of them, prostaglandin analogs and cholinergics, lower IOP by primarily affecting outflow. Prostaglandins increase the outflow of AH through the secondary outflow route, the uveoscleral pathway, and cholinergic agents (generally restricted to acute use) indirectly affect the conventional pathway by contracting the ciliary muscle. The other three classes, carbonic anhydrase inhibitors, adrenergic agonists, and β-blockers, act by decreasing AH production.1 On the horizon, rho-associated kinase (ROCK) inhibitors are in late stage clinical development, emerging as the first pharmacologic class of drugs that decrease IOP by directly increasing conventional outflow. These drugs primarily act by altering the actin cytoskeleton and contractility of trabecular meshwork (TM) cells.5 Additionally, latanoprostene bunod (VESNEO; Baush and Lomb, Rochester, NY, USA), a topically administered nitric oxide (NO)-donating prostaglandin F2-alpha analog, is currently under review by the Food and Drug Administration (FDA) as an IOP-lowering single-agent eye drop. VESNEO is metabolized into the prostaglandin analog latanoprost acid and NO upon instillation in the eye, theoretically targeting both uveoscleral and conventional outflow.
Converging evidence implicates the NO–soluble guanylate cyclase–cyclic guanosine-3′,5′-monophosphate (NO–sGC–cGMP) signaling pathways in the homeostatic processes involved in conventional outflow resistance regulation and thereby IOP, positioning this pathway as a therapeutic target for pharmacologic intervention in POAG. Studies using pharmacologic NO donors, inhibitors of NO synthases (NOS), inhibitors and activators of sGC, and membrane-permeable analogs of cGMP in vivo in several species, including mouse, dog, rabbit, and monkey, have implicated the NO–sGC–cGMP pathway in the regulation of AH outflow and IOP.6–10 The contribution of NO–sGC–cGMP pathway deficiencies in the development of ocular hypertension and glaucomatous optic neuropathy was revealed further in studies using genetically engineered mice, including sGC-α1 knockout mice, transgenic mice expressing human endothelial NOS (eNOS), and NOS3 knockout mice.11–13 Finally, the identification of a single nucleotide polymorphism risk variant located in the locus encoding the sGCα and sGCβ subunits highlights the potential relevance of sGC in the pathogenesis of POAG.11 Despite these findings, the precise molecular mechanisms translating NO–sGC–cGMP effects downstream to IOP regulation in the anterior segment of the eye with a secondary effect impacting the optic neuropathy remain to be elucidated.
Soluble guanylate cyclase is the primary NO receptor in mammals and a central component of the NO–sGC–cGMP pathway. This receptor is expressed by two of the contractile cell types (TM and ciliary smooth muscle cells) that modulate conventional outflow resistance.8,11 Activation of sGC by NO induces production of cGMP regulating various downstream effector systems including cGMP-dependent protein kinases, ion channels, and phosphodiesterases.14,15 IWP-953 is a novel small molecule sGC stimulator, a member of a class of small molecules that directly bind and activate sGC independently of NO and in synergy with NO.
In this study, we report pharmacologic effects of IWP-953, measured by production of the primary sGC downstream effector, cGMP, in vitro in cellular models, and secondly, investigate whether stimulation of the sGC–cGMP pathway by IWP-953 translates to improved aqueous outflow dynamics in mouse eyes by measuring outflow facility. An ex vivo mouse model was used due to its similarity with regard to the outflow anatomy, physiology, and pharmacologic responses of human eyes.
Methods
Reagents
Reagents were obtained from the following vendors: DETA-NONOate (DETA-NO; Enzo Life Sciences, Farmingdale, NY, USA), Amersham cGMP Direct Biotrak EIA (General Electric Healthcare Bio-Sciences, Pittsburgh, PA, USA), 3-isobutyl-1-methylxanthine (IBMX, a phosphodiesterase inhibitor; Sigma-Aldrich Corp., St. Louis, MO, USA), 1-H(1,2,4)oxadiazole[4,3-a]quinoxalin-1-one (ODQ; Tocris, Minneapolis, MN, USA). Poly-D-lysine coated flat-bottom plates were obtained from Corning (Kennebunk, ME, USA). IWP-953 is a member of a group of pharmacologic active sGC stimulators developed at Ironwood Pharmaceuticals (Cambridge, MA, USA) by systematic structure–activity exploration around a central pyrazole-pyrimidine motif.16
Cell Culture
Human embryonic kidney (HEK)-293 cells (CRL-1573) were purchased from ATCC (Manassas, VA, USA) and maintained in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin.
Primary human trabecular meshwork (HTM) cells were isolated and characterized as previously described.17–21 For the current project, cells were cultured under conditions to undergo a differentiation step prior to the assay as described below.19,20,22 Four HTM cell strains (HTM120, HTM123, HTM130, and HTM141) isolated from four pairs of human donor eyes (11 months, 39 years, 2 years, and 38 years old) were used in experiments.
Mouse Eyes
All experiments were conducted in compliance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and under Institutional Animal Care and Use Committee approval for research at Duke University. Eyes were enucleated from culled C57BL/6 mice procured from several different Duke University laboratories, depending upon availability on the day of experiment.
Cyclic Guanosine Monophosphate Assays
Two methods to measure cGMP were used in this study. The amount of cGMP produced in HEK-293 cells was sufficiently high to allow measurement by liquid chromatography–tandem mass spectrometry (LC/MS/MS). In contrast, when lower cGMP levels were expected as in the case of HTM cells, ELISA provided higher sensitivity for measuring cGMP concentrations below the lower level of quantitation for LC/MS/MS.
HEK-293 Cells
Human embryonic kidney-293 cells were seeded at a density of 6 × 104 cells/well in 96-well poly-D-lysine coated flat-bottom plates in 50 μL DMEM and incubated 24 hours at 37°C. Culture medium was removed and the cells were washed once with 80 μL Hank's Balanced Salt Solution (HBSS). Cells were then incubated with 80 μL of a solution containing 0.5 mM IBMX in HBSS for 15 minutes at 37°C. Ten microliters of an ODQ solution (10 μM in HBSS containing 0.5 mM IBMX) or vehicle (0.4% dimethyl sulfoxide) was added to the cells, which were incubated for a further 10 minutes at 37°C. Ten microliters of either IWP-953 (final concentrations: 10−11M − 3 × 10−5M) or vehicle in the presence or absence of DETA-NO (10 μM) was added to the cells, which were further incubated for 20 minutes at 37°C. The selection of this concentration–response curve is based on extensive experience of our laboratory assessing this class of compounds in vitro in potency and other functional assays. Following the incubation step, assay buffer was removed and 50 μL ice cold 10% acetic acid and 150 ng/mL internal standard were added to each well, and samples were incubated on ice for 30 minutes. Following centrifugation (4°C, 5 minutes, 1000g) the amount of cGMP in cell supernatants was determined by LC-MS/MS. Data are expressed as the mean ± standard deviation (SD; N = 2) and are representative of two independent assays.
HTM Cells
All HTM cell strains were seeded at the same density, cultured for the same period of time, and cell confluence was monitored by contact inhibition. These culture conditions have repeatedly and consistently produced similar cell numbers for different HTM strains. Briefly, HTM cells were seeded at a density of 1 × 105 cells/well in 24-well plates and incubated at 37°C in DMEM, containing 10% FBS (Atlanta Biologicals, Flowery Branch, GA, USA). After 1 week, confluent cells were switched to DMEM supplemented with 1.0% FBS for an additional week. The culture medium was then aspirated, the cells washed once with warmed PBS (without calcium and magnesium), and treated with DMEM containing 0.5 mM IBMX for 15 minutes at 37°C. The culture medium was aspirated, and the cells incubated with either vehicle (0.5% DMSO) or IWP-953 (concentration–response curve ranging from 0.01 μM to 10 μM, prepared in 3-fold serial dilution steps in DMEM containing IBMX) in the presence or absence of 10 μM DETA-NO for 15 minutes at 37°C. The incubation was stopped by addition of 500 μL ice cold 0.1 M HCl in distilled H2O, incubated for 30 minutes at 4°C. Cells were scraped from bottom of wells and lysed by repeated up-and-down pipetting. The mixture was centrifuged and the supernatant was transferred to a fresh tube. Both cell lysates and cell culture supernatants were frozen. The concentration of cGMP in HTM cell lysates and HTM culture supernatants was measured using a cGMP enzyme immunoassay. Briefly, 20 μL cell lysate was added to 180 μL assay buffer (4.5 μL 1 M Tris-HCl, pH 8 and 175.5 μL buffer 50 mM sodium acetate, pH 6 containing 0.002% BSA and 0.01% preservative) and similarly, 20 μL cell supernatant was added to 180 μL assay buffer (50 mM sodium acetate, pH 6 containing 0.002% BSA and 0.01% preservative). Cyclic GMP standards (2-512 fmol, prepared fresh in 200 μL assay buffer) and diluted cell lysates and cell culture supernatants were acetylated with 20 μL acetylation reagent (one volume acetic anhydride, two volumes of triethanolamine), and the amount of cGMP was measured using a cGMP enzyme immunoassay following the manufacturer's instructions. Data are expressed as the mean ± SD (N = 3).
Mouse Eye Perfusions In Situ
A previously established mouse eye perfusion protocol was modified slightly for the present study.12,23,24 Briefly, paired eyes from C57BL/6 mice were enucleated and randomized to drug (IWP-953 or DETA-NO) or vehicle perfusions. Working solutions of drug and vehicle were prepared from stock solutions (20 mM) diluted in Dulbecco's phosphate-buffered saline (PBS) plus 5.5 mM D-glucose (DBG) just prior to use. Four different concentrations of IWP-953 (10, 30, 60, and 100 μM) were tested and compared to a positive control, DETA-NO (100 μM). IWP-953 was dissolved in DMSO (final concentration of 0.5%) while DETA-NO was dissolved in DBG. The contralateral eye was stored in DBG at 4°C during the first perfusion (approximately 3 hours).
Enucleated eyes were affixed to a post in the perfusion chamber using cyanoacrylate gel and were then cannulated using a 33-gauge needle (World Precision Instruments, Sarasota, FL, USA), guided by a micro manipulator and stereo microscope. During the cannulation procedure, eyes were held stable using a 0.5-mm curret. The inserted needle was connected by pressure tubing to a 50-μL glass syringe (Hamilton Robotics, Reno, NV, USA), which was mounted in a Harvard minipump (model 33; Harvard Apparatus, Holliston, MA, USA) and controlled by custom software written in Labview (National Instruments, Newbury, UK). The software monitored pressure readings from a Honeywell transducer (model 142PC01G; Honeywell, Fort Washington, PA, USA) that was in communication with eye interior via a three-way stop cock connecting tubing between syringe pump and eye. Desired pressure steps were maintained by the computer controlled pump, adjusting flow rate. After a 45-minute equilibration period at 8 mm Hg to allow drug/vehicle to access outflow tissues, eyes were perfused for approximately 20 minutes at each of four pressure steps (4, 8, 12, and 16 mm Hg). To calculate flow rate at each pressure step, we used a minimum of 10 minutes of stable data. Stable flow rate for at least three of the four pressures was a requirement in order to be included in the final analysis.
Outflow Facility Analysis
In order to calculate outflow facility (C), we used the modified Goldmann equation, F = C(IOP) + Fu where F represents the flow rate at each pressure step (IOP) and Fu is an estimate of the pressure–independent outflow rate. Note that this equation is valid only when episcleral venous pressure and AH inflow is zero (as with ex vivo perfusion of enucleated eyes), F reaches equilibrium at each value of IOP, and C and Fu are independent of IOP. The value of C is calculated from the slope of the best-fit linear regression of flow data at each pressure step.
Statistical Analysis
Data from HEK-293 cell assays were fit using a four-parameter fit (log [agonist] versus response − variable slope) using GraphPad Prism Software version 5 (GraphPad Software, San Diego, CA, USA) and the EC50 was interpolated from the curve fit, and cGMP concentrations were analyzed using GraphPad Prism Software version 5. Statistical analysis of cGMP concentrations in HTM cell lysates and HTM culture supernatants was performed using the unpaired (two-tailed) Student's t-test, compared to control. P < 0.05 is considered statistically significant.
Due to recent findings that pressures greater than 18 mm Hg cause artificial deepening of the anterior chamber (and a subsequent increase in outflow), only data from pressures between 4 to 16 mm Hg were used in the final analysis.25 A Student's t-test (paired, two-tailed) was used to compare data sets, using imputed averages for a single data set to replace missing data when one eye of a pair failed to pass the stability criteria. P < 0.05 is considered statistically significant.
Results
IWP-953 Stimulates cGMP Production in HEK-293 Cells
We determined the in vitro pharmacologic potency of IWP-953 in HEK-293 cells, which are known to express high levels of endogenous sGC and to contain high basal cGMP levels. A concentration–response curve ranging from 10−11 M to 3 × 10−5 M was generated for IWP-953 in the presence and absence of the NO donor, DETA-NO (10 μM). As shown in Figure 1, IWP-953 in the presence of DETA-NO (10 μM) potently stimulated the production of cGMP in HEK-293 cells in a concentration-dependent manner, with a calculated half maximal effective concentration (EC50) of 17 nM. The sGC inhibitor, ODQ (10 μM), potently inhibited IWP-953-stimulated cGMP production in the presence of DETA-NO (IWP-953 EC50: 2.2 μM in the presence of ODQ). In the absence of exogenous DETA-NO, IWP-953 stimulated cGMP production but less potent with an EC50: 9.5 μM (Fig. 1), and was further decreased in the presence of ODQ (data not shown).
Figure 1.
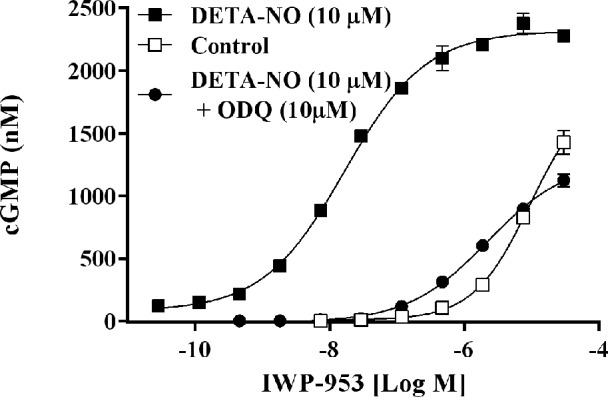
IWP-953 stimulates cGMP production in HEK-293 cells. Human embryonic kidney-293 cells were seeded at a density of 6 × 104 cells/well in 96-well plates and incubated with a concentration–response curve of IWP-953 ranging from 10−11 M to 3 × 10−5 M in the presence or absence of fixed concentration of DETA-NO (10 μM), and in the presence or absence of a fixed concentration of ODQ (10 μM), and IBMX (0.5 mM) for 20 minutes at 37°C. Following cell lysis (50 μL ice cold 10% acetic acid), 150 ng/mL internal cGMP standard was added to each well, and samples were further incubated on ice for 30 minutes. Subsequent to centrifugation, cell supernatants were analyzed for cGMP content by LC/MS/MS. Data are expressed as the mean ± SD (N = 2) and are representative of two independent assys.
IWP-953 Stimulates cGMP Production in HTM Cells
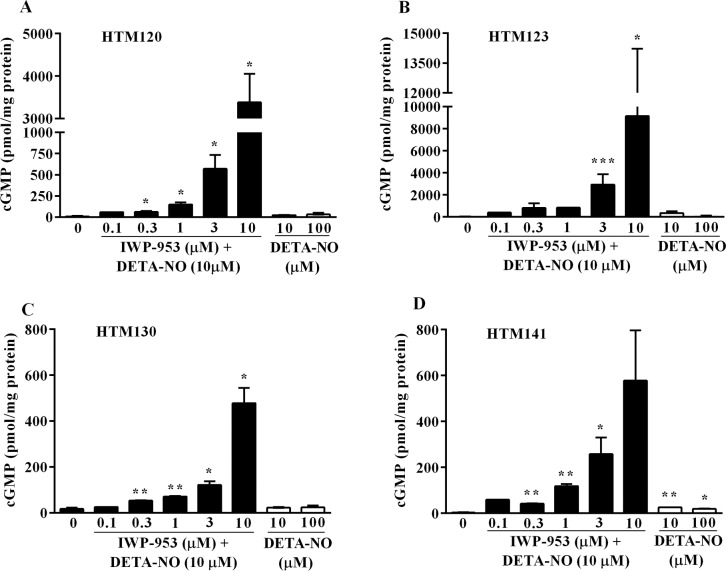
The NO–sGC–cGMP signaling pathway is implicated in conventional outflow and thereby, the regulation of IOP. We investigated the in vitro pharmacologic effects of IWP-953 on intracellular cGMP production in four HTM cell strains: two of which (HTM120 and HTM123) have predominantly a myofibroblast-like phenotype, while the other two (HTM130 and HTM141) display an endothelial-like phenotype. A concentration–response curve for IWP-953 (ranging from 0.1–10 μM) was generated to determine effects on cGMP accumulation, either in the presence or absence of DETA-NO (10 μM). In the presence of DETA-NO, IWP-953 elicited significant, concentration-dependent effects on cGMP production in both HTM120 (P < 0.05) (Fig. 2A) and HTM123 (P < 0.05, P < 0.001) cell strains (Fig. 2B). Concentration-dependent elevations in cGMP levels were also found in HTM130 (P < 0.01; Fig. 2C) and HTM141 (P < 0.05, P < 0.01; Fig. 2D) cell strains, but these increases were markedly less robust than observed in the other two cell strains (maximal cGMP levels reaching 478 ± 47 and 578 ± 154 pmol/mg protein for HTM130 and HTM141 versus 3381 ± 473 and 9148 ± 3593 pmol/mg protein for HTM120 and HTM123 cultures). In the absence of DETA-NO, cGMP concentrations following IWP-953 stimulation were generally less than 100 pmol/mg protein in all HTM cultures at all concentrations tested (Supplementary Table S1). This reveals a considerably higher magnitude of the synergistic effect of DETA-NO on cGMP production in myofibroblast-like HTM cultures, compared to endothelial-like HTM cultures. Interestingly, the effect of DETA-NO (10 μM, 100 μM) stimulation of cGMP production alone was generally trivial in all HTM cultures (Fig. 2).
Figure 2.
Cyclic GMP production in primary cultures of HTM cells stimulated by IWP-953 in the presence of DETA-NO and by DETA-NO alone. Myofibroblast-like HTM120 (A) and HTM123 (B) cultures and endothelial-like HTM130 (C) and HTM141 (D) cell cultures were seeded at a density of 1 × 105 cells/well in 24-well plates and incubated with DMEM containing IBMX (0.5 mM) for 15 minutes at 37°C. Human trabecular meshwork cell cultures were then incubated with increasing concentrations of IWP-953 ranging from 0.1 μM to 10 μM in the presence of a fixed concentration of DETA-NO (10 μM) or vehicle (0.5% DMSO), or DETA-NO (10 μM, 100 μM) for 15 minutes at 37°C. Following cell lysis, cleared HTM cell supernatants were acetylated and the amount of cGMP was measured using a cGMP enzyme immunoassay. Data are expressed as the mean ± SD (N = 3; except for HTM120, HTM130 cultures at IWP-953 [0.1 μM] with DETA-NO [10 μM]: N = 1). *P < 0.05; **P < 0.01, ***P < 0.001.
IWP-953 Stimulates cGMP Secretion in Supernatants of Human TM Cells
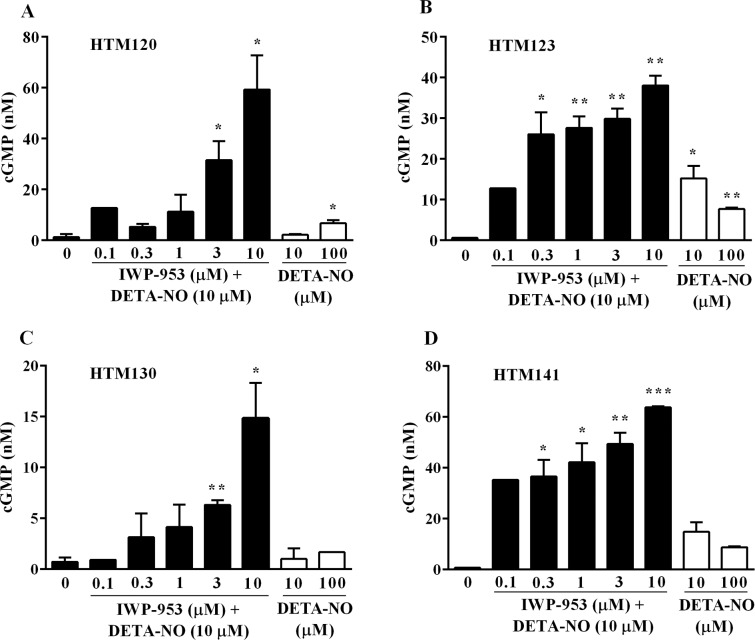
To further investigate whether cGMP produced by HTM cells is secreted, we measured cGMP in cell culture supernatants by ELISA. IWP-953 treatment (0.1–10 μM) in the presence of DETA-NO (10 μM) significantly increased cGMP (P < 0.05, P < 0.01, P < 0.001) in all HTM culture supernatants (Fig. 3). Interestingly, unlike the measurements of intracellular cGMP, the levels of extracellular cGMP were similar in both the myofibroblast-like and endothelial-like HTM cultures. Maximal levels of cGMP in the supernatants of the myofibroblast-like HTM120 (Fig. 3A) and HTM123 (Fig. 3B) were 59 ± 9 nM and 38 ± 2 nM, respectively. By comparison, maximal levels of cGMP in the supernatants of the endothelial-like HTM130 (Fig. 3C) and HTM141 (Fig. 3D) cell culture supernatants were 15 ± 2 nM and 64 ± 1 nM, respectively. DETA-NO stimulation alone resulted in small amounts of cGMP in all HTM culture supernatants (Fig. 3). IWP-953 (0.1–10 μM) stimulation alone (in the absence of 10 μM DETA-NO) had very little effect on supernatant cGMP levels with all values below 10 nM, except for HTM141 culture supernatants (17.2 nM) at 10 μM IWP-953 (Supplementary Table S2).
Figure 3.
Cyclic GMP secretion by primary cultures of HTM cells, following treatment with IWP-953 in the presence of DETA-NO and DETA-NO alone. Myofibroblast-like HTM120 (A) and HTM123 (B) cell cultures and endothelial-like HTM130 (C) and HTM141 (D) cell cultures were treated with increasing concentrations of IWP-953 (0.1–10 μM) in the presence of a fixed concentration of DETA-NO (10 μM) or vehicle (0.5% DMSO), or DETA-NO (10 μM, 100 μM). HTM cell culture supernatants were acetylated, and cGMP content was measured using a cGMP enzyme immunoassay. Data are expressed as the mean ± SD (N = 3; except for supernatants from HTM120, HTM130 cell cultures at IWP-953 [0.1 μM] with DETA-NO [10 μM]: N = 1). *P < 0.05; **P < 0.01; ***P < 0.001.
Ex Vivo Mouse Eye Perfusions With IWP-953
In order to examine the impact of IWP-953 on conventional outflow cell function in an intact model, we perfused paired enucleated mouse eyes with drug (10–100 μM) or vehicle in the contralateral eye as a control. This model functionally isolates pressure-dependent conventional outflow from other physiological contributors to IOP like inflow, uveoscleral outflow, and episcleral venous pressure. Anterior chambers were cannulated and eyes were subjected to sequential pressure steps, while flow was continuously measured. Shown in Figure 4 are perfusion data from a representative experiment with paired eyes, one treated with vehicle and the other with 100 μM IWP-953. Displayed are flow traces from both eyes at four sequential pressure steps. Notice that flow at each pressure, except for the first is greater in the drug-treated eye. Data from multiple perfusions such as this example are used to generate pressure/flow plots and calculate outflow facility.
Figure 4.
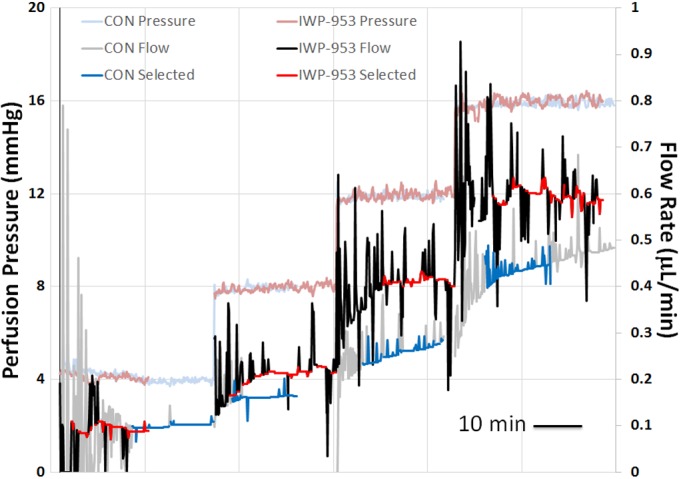
Representative perfusion traces showing flow data over time from paired eyes at sequential pressure steps. The gray trace shows the raw flow data obtained from the vehicle treated eye and the black trace shows flow data from the contralateral eye perfused with 100 μM IWP-953. Red and blue highlighted regions of traces represent data used to calculate average flow rate at each corresponding pressure step for control and IWP-953-treated eyes, respectively. Calculated outflow facilities for this pair are 0.027 and 0.041 μL/min/mm Hg for the control and IWP-953-treated eyes, respectively. Trace spikes indicate rapid increases in flow rate (that subside over time) from syringe pump that is attempting to maintain user-defined perfusion pressure.
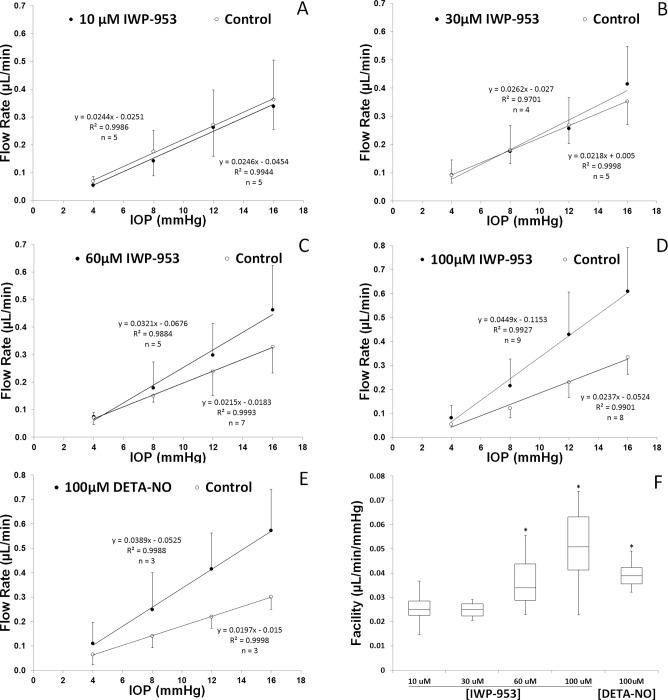
Shown in Figures 5A through 5E are pressure/flow plots for each treatment. Linear regression analysis of the slope of pressure–flow curves estimates outflow facility (pressure-dependent outflow). Results show that increasing concentrations of IWP-953 improved outflow facility, with a maximum and significant (P = 0.002) effect at the highest concentration (0.045 vs. 0.024 μL/min/mm Hg); approximately doubling outflow facility (89.5% increase). We also observed that the 60-μM concentration was significantly different than paired controls (0.032 vs. 0.022 μL/min/mm Hg), increasing outflow facility by 49.3%. In contrast, the two lowest concentrations were without detectable effect (0.024 vs. 0.025 and 0.026 vs. 0.022 μL/min/mm Hg), As a positive control, a cohort of eyes was treated with 100 μM DETA-NO, which significantly (P = 0.030) increased outflow facility by 97.5%. A side-by-side comparison of treatment effects on outflow facility are shown in panel F of Figure 5.
Figure 5.
Pressure versus flow plots showing impact of escalating concentrations of IWP-953 on conventional outflow function of enucleated mouse eyes. Ex vivo perfusions were conducted using (A) 10 μM, (B) 30 μM, (C) 60 μM, and (D) 100 μM IWP-953, or vehicle as control for comparisons. Eyes were subjected to four pressure steps (4, 8, 12, and 16 mm Hg), and flow was continuously monitored. Linear regression was performed on mean flow data at each pressure step (±SD) to estimate outflow facility. As a positive control (E), 100 μM DETA-NO was perfused into a separate cohort of eyes, with contralateral eyes perfused with vehicle. A summary of outflow facility calculations at each concentration is shown in panel F as a box-and-whisker plot (*P < 0.05).
Discussion
We provide evidence that IWP-953 stimulation of the sGC–cGMP pathway in primary cultures of HTM cells significantly increased cGMP production in myofibroblast-like HTM120 and HTM123 and endothelial-like HTM130 and HTM141 cultures, but the magnitude of intracellular cGMP production is less pronounced in endothelial-like HTM130 and HTM141 cells. Importantly, pharmacologic effects of IWP-953 observed in vitro translated in an ex vivo model of IOP control, eliciting a substantial increase in outflow facility. These data suggest a novel, previously unrecognized mechanism functionally linking pharmacologic effects of sGC stimulation and modulation of the sGC–cGMP pathway to therapeutic benefit in an established model of glaucoma.
Soluble guanylate cyclase stimulators act synergistically with NO to increase the activity of sGC and thus may be therapeutically useful to enhance NO signaling, particularly in conditions or diseases in which NO signaling may be impaired.26 Recent FDA approval of the sGC stimulator riociguat (Adempas; Bayer Pharma AG, Leverkusen, Germany) for treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension highlights the therapeutic potential of this class of drugs and importantly, provides clinical validation for sGC as a therapeutic target.27,28 IWP-953 is a novel sGC stimulator that elicits potent in vitro pharmacologic effects, concentration-dependently stimulating cGMP production in HEK-293 cells (EC50: 17 nM) in the presence of DETA-NO. This stimulation is sGC-dependent as it can be inhibited by the presence of the sGC inhibitor, ODQ. Similar to findings in HEK-293 cells, in vitro exposure of primary cultures of HTM cells to IWP-953 in the presence of DETA-NO resulted in significant and concentration-dependent increases in intracellular cGMP levels. Interestingly, the magnitude of this response clearly differentiates myofibroblast-like HTM120 and HTM123 cells from endothelial-like HTM130 and HTM141 cells, with markedly higher intracellular cGMP levels detected in HTM120 and HTM123 cultures. Due to the nature of our TM cell isolation technique, all cultures are mixed. However, cultures are often dominated by endothelial or myofibroblastic cell isolates, depending upon the condition of the donor tissue and age of the donor, explaining the spectrum of responses of these four cell strains. Taken together, our functional data with primary cultures of human cells from the TM are consistent with previous reports showing select populations of cells in the TM tissue and in cell culture with contractile characteristics.17–21
Cells that occupy the beams and plates of the inner TM are endothelial-like, while cells that reside in the juxtacanalicular connective tissue (JCT) are myofibroblast-like and highly contractile.29 Stimulation with IWP-953 in the absence of DETA-NO also increased cGMP levels in all HTM cultures, but the potency of this effect was generally one to three orders of magnitude below that observed in the presence of DETA-NO, providing further evidence for strong synergy between sGC stimulators and NO and the likely importance of this synergy for the observed pharmacologic activities. These findings suggest that the highly contractile myofibroblast cell population within the TM/JCT network is a primary target of IWP-953, thereby modulating sGC–cGMP pathway downstream events by a mechanism(s) potentially involving cytoskeletal actin fiber assembly/disassembly. Changes in the dimensions of the trabecular outflow pathway, through IWP-953 induced alterations in cell morphology and contractile tone of these myofibroblasts could then lead to decreased outflow resistance in the inner wall region and lower IOP.
The activity of cGMP phosphodiesterases is generally recognized as the major elimination pathway controlling intracellular cGMP levels. However, findings from recent studies in different model systems have identified an important role for the ATP-binding cassette transporter multidrug resistance–associated protein-4 (MRP4) in intracellular cGMP homeostasis, including IOP by controlling the relaxation characteristics of cells in the conventional AH outflow pathway.30–32 Multidrug resistance–associated protein-4, via a mechanism of directing the unidirectional efflux of cGMP, has been described as an overflow pump supporting the decrease of intracellular cGMP levels under conditions of strongly induced cGMP production and importantly, cGMP for potential paracrine actions.33,34 Consistent with this postulated role of MRP4, we found significantly increased levels of extracellular cGMP in culture supernatants of primary culture of HTM cells treated with IWP-953 and DETA-NO, in a concentration-dependent manner. Interestingly, clinical studies found significantly lower cGMP concentrations in AH from POAG patients, compared to cGMP levels in the AH of normal pressure glaucoma patients, but no physiological role of extracellular cGMP in the TM outflow pathway is currently known.35,36 However, extracellular cGMP following activation of the intestinal guanylate cyclase-C/cGMP pathway has recently been shown to elicit potent analgesic effects in models of visceral pain via modulation of the activity of colonic sensory afferents. Furthermore, direct inhibitory effects of extracellular cGMP on central nervous system neurons results in reduced excitability and inhibition of neurotransmitters, suggesting that studies aiming to elucidate a potential role of extracellular cGMP in the modulation of AH outflow pathways are warranted.37–41
Next we examined pressure-dependent outflow in perfused enucleated mouse eyes ex vivo. Pressure-controlled perfusion directly measures conventional outflow in the absence of confounding factors such as AH inflow, uveoscleral outflow, and episcleral venous pressure. Intracamerally perfused IWP-953 stimulated a concentration-dependent increase in outflow, and the effect of IWP-953 observed at the highest concentration (100 μM) was significant and comparable to that of DETA-NO. Notably, this potent effect on mouse outflow facility was not dependent on costimulation with an exogenous NO donor, suggesting the presence of a basal NO tone in conventional outflow tissues. Since the bulk of conventional outflow resistance is generated proximal to or at the endothelium of the Schlemm's canal or downstream vessels and Schlemm's canal cells produce NO, it is likely that NO released from Schlemm's canal cells is responsible for the basal NO tone and thus, the regulation of conventional outflow.12 Our results with IWP-953 in mouse eyes provide further evidence supporting the concept of pharmacologic targeting of the sGC–cGMP pathway for the treatment of POAG.42–46 Moreover, direct targeting the sGC–cGMP pathway with a sGC stimulator possesses advantages over NO donor strategies. NO donors, in addition to stimulating the sGC–cGMP pathway, may also elicit nonspecific biological responses via a cGMP-independent, noncanonical pathway, through covalent posttranslational S-nitrosylation of target proteins.47,48
Finally, both the sGC activator BAY-58-2667 and the sGC stimulator YC-1 increase conventional outflow mechanistically linked to changes in TM cell volume, mediated through activation of the sGC–cGMP–cGK1 pathway in a manner dependent on the large-conductance calcium-activated potassium channel (BKCa), a channel with dense distribution in the TM that integrates multiple signals leading to cell relaxation.6 However, both BAY-58-2667 and YC-1 elicit biphasic effects on TM cell volume, characterized by sGC–cGMP dependent decreases at lower concentrations, but cGMP independent increases at higher concentrations.6 While we have not investigated the effects of IWP-953 on TM cell volume in this study, our data support a concentration-dependent effect on increased outflow facility.
To date, the only proven treatment strategy for glaucoma is to lower IOP, either by increasing outflow or decreasing the production of AH. Pharmacologic inhibitors of ROCK are a novel class of drugs currently in late-stage clinical development that selectively target the conventional outflow pathway downstream of NO–sGC–cGMP signaling.5 Mechanistically, these drugs act independent of cytosolic calcium levels via a singular mode of action, diminishing the contractile tone of conventional outflow pathway tissues and disrupting components of the actin–myosin cytoskeleton, thereby increasing outflow facility and lowering IOP.49–51 In contrast, the effects of IWP-953 relaxing the contractile tone of the TM are likely mediated through multiple mechanisms. Activation of the cGMP downstream effector cGK1 directly counteracts the effects of the Rho-ROCK pathway by phosphorylation and activation of myosin light chain phosphatase, resulting in myosin light chain dephosphorylation and relaxation of the TM contractile tone. Simultaneously, via regulation of BKCa channels, cGKI decreases calcium influx into the cytosol, thus inactivating calcium/calmodulin-dependent myosin light chain kinase and preventing increased TM contractility.5,52
In summary, these studies provide compelling evidence that IWP-953 acts on sGC to enhance NO–cGMP signaling predominantly in highly contractile myofibroblast-like TM cells, an effect that translated in an established model of glaucoma, increasing outflow facility. These findings suggest sGC stimulators have the potential to emerge as a new therapeutic class of ocular hypotensive drugs selectively targeting the conventional outflow pathway.
Supplementary Material
Acknowledgments
The authors thank G. Todd Milne for critical reading and constructive comments on this manuscript and W. Michael Dismuke for assisting with the construction of Figure 4.
The project was supported by Ironwood Pharmaceuticals, EY022359, EY005722, and Research to Prevent Blindness Foundation.
Disclosure: P. Ge, Ironwood (E); I.D. Navarro, None; M.M. Kessler, Ironwood (E); S.G. Bernier, Ironwood (E); N.R. Perl, Ironwood (E); R. Sarno, Ironwood (E); J. Masferrer, Ironwood (E); G. Hannig, Ironwood (E); W.D. Stamer, Ironwood (F)
References
- 1. Chowdhury UR,, Hann CR,, Stamer WD,, Fautsch MP. Aqueous humor outflow: dynamics and disease. Invest Ophthalmol Vis Sci. 2015; 56: 2993–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kass MA,, Heuer DK,, Higginbotham EJ,, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701–713. [DOI] [PubMed] [Google Scholar]
- 3. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963; 69: 783–801. [DOI] [PubMed] [Google Scholar]
- 4. Johnson M. What controls aqueous humor outflow resistance? Exp Eye Res. 2006; 82: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Challa P,, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Invest Drugs. 2014; 23: 81–95. [DOI] [PubMed] [Google Scholar]
- 6. Dismuke WM,, Sharif NA,, Ellis DZ. Human trabecular meshwork cell volume decrease by NO-independent soluble guanylate cyclase activators YC-1 and BAY-58-2667 involves the BKCa ion channel. Invest Ophthalmol Vis Sci. 2009; 50: 3353–3359. [DOI] [PubMed] [Google Scholar]
- 7. Kee C,, Kaufman PL,, Gabelt BT. Effect of 8-Br-cGMP on aqueous humor dynamics in monkeys. Invest Ophthalmol Vis Sci. 1994; 35: 119–123. [PubMed] [Google Scholar]
- 8. Ellis DZ,, Dismuke WM,, Chokshi BM. Characterization of soluble guanylate in NO- induced increases in aqueous humor outflow facility and in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009; 50: 1808–1813. [DOI] [PubMed] [Google Scholar]
- 9. Kotikoski H,, Alajuuma P,, Moilanen E,, et al. Comparison of nitric oxide donors in lowering intraocular pressure in rabbits: role of cyclic GMP. J Ocul Pharmacol Ther. 2002; 18: 11–23. [DOI] [PubMed] [Google Scholar]
- 10. Kotikoski H,, Vapaatalo H,, Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res. 2003; 26: 119–123. [DOI] [PubMed] [Google Scholar]
- 11. Buys ES,, Ko Y-C,, Alt C,, et al. Soluble guanylate cyclase α1-deficient mice: a novel murine model for primary open angle glaucoma. PLoS One. 2013; 8: e60156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang JYH,, Stamer WD,, Bertrand J,, et al. The role of nitric oxide in murine conventional outflow. Am J Physiol Cell Physiol. 2015; 309: C205 –C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lei Y,, Zhang X,, Song M,, Wu J,, Sun X. Aqueous humor outflow physiology in NOS3 knockout mice. Invest Ophthalmol Vis Sci. 2015; 56: 4891–4898. [DOI] [PubMed] [Google Scholar]
- 14. Stasch J-P,, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol. 2009; 191: 277–308. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt HH,, Schmidt PM,, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol. 2009; 191: 309–339. [DOI] [PubMed] [Google Scholar]
- 16. Nakai T,, Perl NR,, Iyengar RR,, et al. Discovery of IWP-051, a novel orally bioavailable soluble guanylate cyclase stimulator with sustained and dose-dependent hemodynamic effects. BMC Pharm Toxicol. 2015; 16 (suppl 1): A59. [Google Scholar]
- 17. Coroneo MT,, Korbmacher C,, Fluegel C,, Stiemer B,, Luetjen-Drecoll E,, Wiederholt M. Electrical and morphological evidence for heterogenous populations of cultured bovine trabecular meshwork cells. Exp Eye Res. 1991; 52: 375–388. [DOI] [PubMed] [Google Scholar]
- 18. Fluegel C,, Tamm E,, Luetjen-Drecoll E. Different cell populations in bovine trabecular meshwork: an ultrastructural and immunocytochemical study. Exp Eye Res. 1991; 52: 681–690. [DOI] [PubMed] [Google Scholar]
- 19. Stamer WD,, Seftor RE,, Williams SK,, Samaha HA,, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995; 14: 611–617. [DOI] [PubMed] [Google Scholar]
- 20. Stamer WD,, Roberts BC,, Howell DN,, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm's canal. Invest Ophthalmol Vis Sci. 1998; 10: 1804–1812. [PubMed] [Google Scholar]
- 21. O'Brien ET,, Wang Y,, Ying H,, Yue BY. Differential expression of genes in cultured cells from juxtacanalicular trabecular meshwork and Schlemm's canal. J Ocul Pharmacol Ther. 2014; 30: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stamer WD,, Roberts BC,, Epstein DL,, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000; 20: 347–350. [PubMed] [Google Scholar]
- 23. Lei Y,, Overby DR,, Boussommier-Calleja A,, Stamer WD,, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011; 52: 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rogers ME,, Navarro ID,, Perkumas KM,, et al. Pigment epithelium-derived factor decreases outflow facility. Invest Ophthalmol Vis Sci. 2013; 54: 6655–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boussommier-Calleja A,, Li G,, Wilson A,, et al. Physical factors affecting outflow facility measurements in mice. Invest Ophthalmol Vis Sci. 2015; 56: 8331–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Follmann M,, Griebenow N,, Hahn MG,, et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed. 2013; 52: 2–23. [DOI] [PubMed] [Google Scholar]
- 27. Rubin LJ,, Galie N,, Grimminger F,, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study (PATENT-2). Eur Respir J. 2015; 45: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 28. Simonneau G,, D'Armini AM,, Ghofrani HA,, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J. 2015; 45: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 29. Dismuke WM,, Liang J,, Overby DR,, Stamer WD. Concentration-related results of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp Eye Res. 2014; 120: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pattabiraman PP,, Pecen PE,, Rao PV. MRP4-mediated regulation of intracellular cAMP and cGMP levels in trabecular meshwork cells and homeostasis of intraocular pressure. Invest Ophthalmol Vis Sci. 2013; 54: 1636–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krawutschke C,, Koesling D,, Russwurm M. Cyclic GMP in vascular relaxation: export is of similar importance as degradation. Arterioscler Thromb Vasc Biol. 2015; 35: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 32. Tchernychev B,, Ge P,, Kessler MM,, et al. MRP4 modulation of the guanylate cyclase- C/cGMP pathway: effects on linaclotide-induced electrolyte secretion and cGMP efflux. J Pharmacol Exp Ther. 2015; 335: 1–9. [DOI] [PubMed] [Google Scholar]
- 33. Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H,, Grube M, Koeck K,, Kroemer HK,. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab Rev. 2005; 37: 253–278. [DOI] [PubMed] [Google Scholar]
- 34. Sager G. Cyclic GMP transporters. Neurochem Int. 2004; 45: 865–873. [DOI] [PubMed] [Google Scholar]
- 35. Galassi F,, Sodi A,, Ucci F,, Renieri G,, Pieri B,, Masini E. Ocular hemodynamics and nitric oxide in normal pressure glaucoma. Acta Ophthalmol Scand Suppl. 2000; 232: 37–38. [DOI] [PubMed] [Google Scholar]
- 36. Galassi F,, Renieri G,, Sodi A,, Ucci F,, Vannozzi L,, Masini E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004; 88: 757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cervetto C,, Maura G,, Marcoli M. Inhibition of presynaptic release-facilitatory kainite autoreceptors by extracellular cyclic cGMP. J Pharmacol Exp Ther. 2010; 332: 210–219. [DOI] [PubMed] [Google Scholar]
- 38. Linden DJ,, Dawson TM,, Dawson VL. An evaluation of the nitric oxide/cGMP/cGMP- dependent protein kinase cascade in the induction of cerebellar long-term depression in culture. J Neurosci. 1995; 15: 5098–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poupoloupou C,, Nowak LM. Extracellular 3′,5′ cyclic guanosine monophosphate inhibits kainite-activated responses in cultured mouse cerebellar neurons. J Pharmacol Exp Ther. 1998; 286: 99–109. [PubMed] [Google Scholar]
- 40. Castro J,, Harrington AM,, Hughes PA,, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine-3′,5′- monophosphate. Gastroenterology. 2013; 145: 1334–1346. [DOI] [PubMed] [Google Scholar]
- 41. Silos-Santiago I,, Hannig G,, Eutamene H,, et al. Gastrointestinal pain: unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain. 2013; 154: 1820–1830. [DOI] [PubMed] [Google Scholar]
- 42. Sumida GM,, Stamer WD. S1P receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am J Physiol Cell Physiol. 2011; 300: C1164 –C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Millard LH,, Woodward DF,, Stamer WD. The role of prostaglandin EP4 receptor in the regulation of human outflow facility. Invest Ophthalmol Vis Sci. 2011; 52: 3506–3513. [DOI] [PubMed] [Google Scholar]
- 44. Boussommier-Calleja A,, Bertrand J,, Woodward DF,, Ethier CR,, Stamer WD,, Overby DR. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012; 53: 5838–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li G,, Farsiu S,, Chiu SJ,, et al. Pilocarpine-induced dilation of Schlemm's canal and prevention of lumen collapse at elevated intraocular pressures in living mice visualized by OCT. Invest Ophthalmol Vis Sci. 2014; 55: 3737–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Overby DR,, Bertrand J,, Schicht M,, Paulsen F,, Stamer WD,, Luetjen-Drecoll E. The structure of the trabecular meshwork its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci. 2014; 55: 3727–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gould N,, Doulias P-T,, Tenopoulou M,, Raju K,, Ischiropoulos H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J Biol Chem. 2013; 288: 26473–26479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hess DT,, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012; 287: 4411–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rao PV,, Deng PF,, Kumar J,, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y27632. Invest Ophthalmol Vis Sci. 2001; 42: 1029–1037. [PubMed] [Google Scholar]
- 50. Honjo M,, Tanihara H,, Inatani M,, et al. Effects of rho-associated kinase inhibitor Y- 27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001; 42: 137–144. [PubMed] [Google Scholar]
- 51. Honjo M,, Inatani M,, Kido N,, et al. Effects of protein kinase inhibitor, HA 1077, on intraocular pressure and outflow facility in rabbit eyes. Arch Ophthalmol. 2001; 119: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 52. Hofmann F,, Feil R,, Kleppisch T,, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006; 86: 1–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.