Abstract
A3 adenosine receptor (A3AR) agonists have been reported to influence cell death and survival. The effects of an A3AR agonist, 1-[2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-D-ribofuranonamide (Cl-IB-MECA), on apoptosis in two human leukemia cell lines, HL-60 and MOLT-4, were investigated. Cl-IB-MECA (≥30 μM) increased the apoptotic fractions, as determined using fluorescence-activated cell sorting (FACS) analysis, and activated caspase 3 and poly-ADP-ribose-polymerase. Known messengers coupled to A3AR (phospholipase C and intracellular calcium) did not seem to play a role in the induction of apoptosis. Neither dantrolene nor BAPTA-AM affected the Cl-IB-MECA-induced apoptosis. Cl-IB-MECA failed to activate phospholipase C in HL-60 cells, while UTP activated it through endogenous P2Y2 receptors. Induction of apoptosis during a 48 hr exposure to Cl-IB-MECA was not prevented by the A3AR antagonists [5-propyl-2-ethyl-4-propyl-3-(ethylsulfanylcarbonyl)-6-phenylpyridine-5-carboxylate] (MRS 1220) or N-[9-chloro-2-(2-furanyl)[l,2,4]triazolo[l,5-c]quinazolin-5-yl]benzeneacetamide (MRS 1523). Furthermore, higher concentrations of MRS 1220, which would also antagonize A1 and A2A receptors, were ineffective in preventing the apoptosis. Although Cl-IB-MECA has been shown in other systems to cause apoptosis through an A3AR-mediated mechanism, in these cells it appeared to be an adenosine receptor-independent effect, which required prolonged incubation. In both HL-60 and MOLT-4 cells, Cl-IB-MECA induced the expression of Fas, a death receptor. This induction of Fas was not dependent upon p53, because p53 is not expressed in an active form in either HL-60 or MOLT-4 cells. Cl-IB-MECA-induced apoptosis in HL-60 cells was augmented by an agonistic Fas antibody, CH-11, and this increase was suppressed by the antagonistic anti-Fas antibody ZB-4. Therefore, Cl-IB-MECA induced apoptosis via a novel, p53-independent up-regulation of Fas. Published by Elsevier Science Inc.
Keywords: Adenosine receptor, Antagonist, Poly-ADP-ribose polymerase, CD95, APO-1, Phospholipase C, HL-60, MOLT-4
1. Introduction
Following the introduction of A3AR-selective ligands [1–3], the A3AR has been demonstrated to have diverse physiological functions, including its effects on inflammation [4], hypotension [5], mast cell degradation [6], protection of brain and heart [7–9], and apoptosis [10–15]. Specifically, potent A3AR agonists showed dual effects leading to either cellular protection or death. In rat astroglial and human astrocytoma cells, micromolar concentrations of A3AR agonists reduced the cell number, while nanomolar concentrations promoted cytoskeletal changes that were associated with cytoprotection [13,16]. In chick ventricular myocyte culture [14] and in the isolated rabbit heart [17], the activation of the A3AR showed a preconditioning-like effect that improved the outcome following an ischemic injury. Similar effects were observed in vivo in a gerbil model of global ischemia [8]. In promyelocytic human leukemia HL-60 cells [11], the A3AR agonists IB-MECA (l-[6-[[(3-iodophenyl)-methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-D-ribofuranuronamide) and Cl-IB-MECA induced apoptosis at high concentrations. At lower concentrations, they protected the cells from apoptosis induced by A3AR antagonists. In CHO cells transfected with the human A3AR, effects on the cell cycle were induced with high concentrations of Cl-IB-MECA in those cells expressing the A3AR, but not in control cells [18].
This study sought to probe the mechanism of the apoptotic cell death induced by Cl-IB-MECA in the HL-60 and MOLT-4 leukemic cell lines.
2. Materials and methods
2.1. Materials
HL-60 and MOLT-4 cells were obtained from the ATCC. RPMI 1640 medium, fetal bovine serum, penicillin, streptomycin, and glutamine were purchased from Gibco BRL. Cl-IB-MECA and MRS 1220 (N-[9-chloro-2-(2-furanyl)-[l,2,4]triazolo[l,5-c]quinazolin-5-yl]benzeneacetamide) were obtained from RBI-Sigma. MRS 1523 [5-propyl-2-ethyl-4-propyl-3-(ethylsulfanylcarbonyl)-6-phenylpyri-dine-5-carboxylate] was synthesized as described previously [19]. A TACS™ 2 TdT-DAB in situ apoptosis detection kit was obtained from Trevigen. Dowex AG 1-X8 resin was purchased from Bio-Rad. Anti-Fas (C20), anti-Bcl-2 (100), anti-Bax (B-9), and anti-TRAIL (C-19) were purchased from Santa Cruz Biotechnology. Anti-Fas antibodies (CH-11 and ZB-4) were purchased from Upstate Biotechnology. Anti-Cpp32 and anti-PARP (C2–10) were purchased from Pharmingen. All other reagents were purchased from Sigma.
2.2. Cell culture and preparation
HL-60 or MOLT-4 cells were grown at 37° in a humidified incubator with 5% CO2/95% air in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/mL of penicillin, 100 mg/mL of streptomycin, and 2 mM L-glutamine. The culture was maintained by splitting every third day. For each experiment, the cells undergoing log phase growth were collected and resuspended in growth medium to 0.5 × 106 cells/mL, and 3 mL aliquots were placed into the individual wells of several 6-well culture plates. Test compounds dissolved in DMSO at the appropriate concentration (or only DMSO as control) were added to each well. The final concentration of DMSO in each sample did not exceed 0.2%.
2.3. Cell viability analysis
Cell viability was measured using the Trypan blue exclusion test. Trypan blue was mixed with the cell suspension (final concentration of dye was 0.2%), and the numbers of unstained (live) and stained (dead) cells were counted between 5 and 7 min after the dye was added.
2.4. Apoptosis analysis by flow cytometry
After treating HL-60 or MOLT-4 cells with various reagents, the cells were washed twice with cold PBS by centrifugation (500 g for 5 min at room temperature), washed again with cold citrate buffer (250 mM sucrose, 5% DMSO, 40 mM trisodium citrate, pH 7.5), and fixed with 2% paraformaldehyde at 4°. The cells were washed, resuspended in 100 μL of cold citrate buffer, and treated with 900 μL of trypsin solution (500 units/mL of trypsin, 3.4 mM trisodium citrate, 0.1% Tergitol (type NP-40, Sigma), 1.5 mM spermine, 0.5 mM Tris (pH 7.5)) for 10 min at room temperature. Then 750 μL of RNase solution (500 μg/mL of trypsin inhibitor, 100 μg/mL of RNase A, 3.4 mM trisodium citrate, 0.1% NP-40, 1.5 mM spermine, 0.5 mM Tris (pH 7.5)) was added. After a 10 min incubation at room temperature, cells were stained by the addition of 750 μL of propidium iodide solution (0.6 mM propidium iodide, 3 mM spermine, 3.4 mM trisodium citrate, 0.1% NP-40, 0.5 mM Tris (pH 7.5)). The apoptotic fraction was quantified for 104 cells by analyzing the sub-G1 (sub-diploid) population by measuring the fluorescence activity of propidium iodide-stained DNA of fixed cells on a FacsCalibur (Becton Dickinson).
2.5. Immunoblotting analysis
Proteins from the treated HL-60 or MOLT-4 cells were extracted using a lysis buffer (0.5% NP-40,120 mM NaCl, 40 mM Tris (pH 8.0)). After SDS-PAGE, the protein bands were transferred to nitrocellulose paper, and were blocked with 5% powdered non-fat milk. They were incubated overnight with the primary antibodies and for 1 hr with the horseradish peroxidase linked secondary antibodies. Immunoblots were developed with enhanced chemiluminescence (ECL) reagents (Pierce).
2.6. Phospholipase C assay
The amount of inositol phosphates was measured by a modification of the method of Baek et al. [20]. After labeling with myo-[3H]-inositol (1 μCi/106 cells) for 24 hr at 37°, LiCl was added (final concentration was 20 mM). Then the cells were resuspended to a density of 2 × 107 cells/mL in RPMI 1640 medium containing 0.5% fetal bovine serum, 20 mM HEPES (pH 7.2), 20 mM LiCl, and 1 mg/mL of bovine serum albumin. An aliquot (150 μL) of cell suspension was transferred to each well of a 96-well plate that contained either Cl-IB-MECA or UTP The plates were incubated for 30 min at 37°. The reaction was terminated by the addition of 100 μL of ice-cold 180 mM formic acid. After centrifugation (2000 g for 10 min at 4–6°), the supernatants were neutralized with 300 μL of 60 mM NH4OH and applied to Bio-Rad Dowex AG 1-X8 anion exchange columns. The columns were washed with water followed by a 60 mM sodium formate solution containing 5 mM sodium tetraborate. Total inositol phosphates were eluted with 1 M ammonium formate containing 0.1 M formic acid, and the amounts of radioactivity were measured using a liquid scintillation counter (Beckman).
2.7. HPLC analysis of MRS 1220 stability
To the culture medium used in the experiments, 2 vol. of acetone was added and the mixture was centrifuged (500 g for 10 min at room temperature). The supernatant was removed and concentrated under a stream of N2. This method was shown to recover the MRS 1220 efficiently from the growth medium. The amount of MRS 1220 in the concentrate was analyzed with a Hewlett-Packard 1090 HPLC apparatus equipped with a Phenomenex® RP-C18 analytical column (250 mm × 4.6 mm, linear gradient solvent system: 0.1 M triethylammonium acetate/CH3CN from 0/100 to 60/40 in 20 min, flow rate 1 mL/min). UV detection at 260 nm was used. Under these conditions, MRS 1220 had a retention time of 4.8 min.
3. Results
3.1. Induction of apoptosis by Cl-IB-MECA
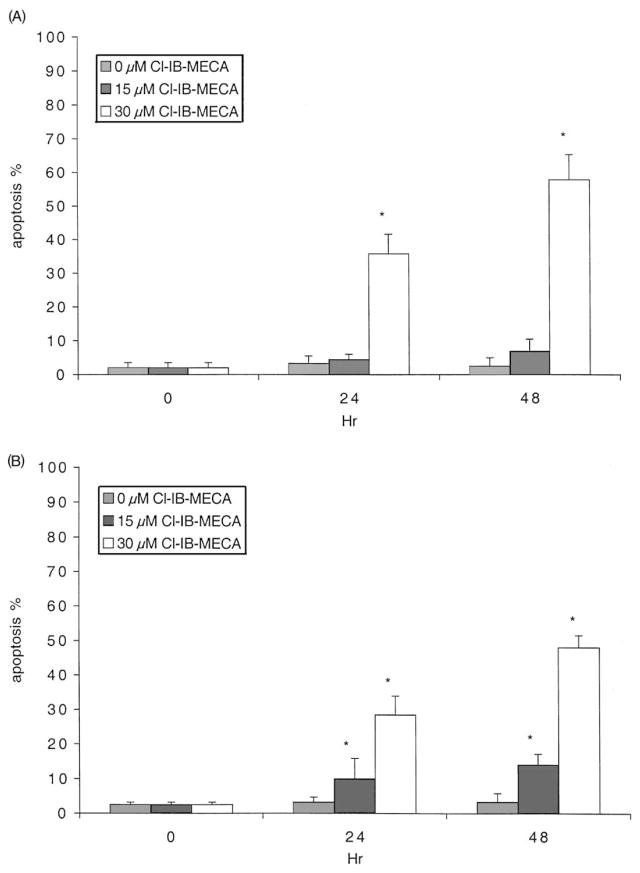
HL-60 or MOLT-4 cells were treated with 0–30 μM Cl-IB-MECA for 24 or 48 hr, and the fraction of cells undergoing apoptosis was determined using FACS. Significant apoptosis was induced by 30 μM Cl-IB-MECA in both cell lines (36 and 58% in HL-60 cells, and 29 and 48% in MOLT-4 cells, at 24 and 48 hr, respectively), as indicated in Fig. 1. This apoptosis rate was confirmed using the terminal deoxynucleotide transferase-mediated dUTP-bio-tin nick-end labeling (TUNEL) method (TACS™ 2 TdT-DAB in situ apoptosis detection kit; data not shown). The degree of cell death determined using the dye exclusion technique was also consistent with the degree of apoptosis. In HL-60 cells, no significant apoptosis was observed at a concentration of Cl-IB-MECA of ≤10 μM or at exposure times shorter than 24 hr. Therefore, a 30 μM concentration was used to study the mechanism of apoptosis induced by Cl-IB-MECA in subsequent experiments. Apoptosis was not observed at 48 hr when the cells were treated for only 4 or 8 hr with Cl-IB-MECA.
Fig. 1.
Induction of apoptosis by Cl-IB-MECA in HL-60 (A) and MOLT-4 (B) cells. After treatment with 0, 15, or 30 μM Cl-IB-MECA for 24 or 48 hr, the cells were collected and then analyzed by flow cytometry as described in Section 2. Data shown are means ± SD of at least three independent experiments. Key: significantly different with respect to the 0 hr value, *P < 0.01 (Student’s t-test).
3.2. Effects of calcium modifiers on apoptosis induced by Cl-IB-MECA
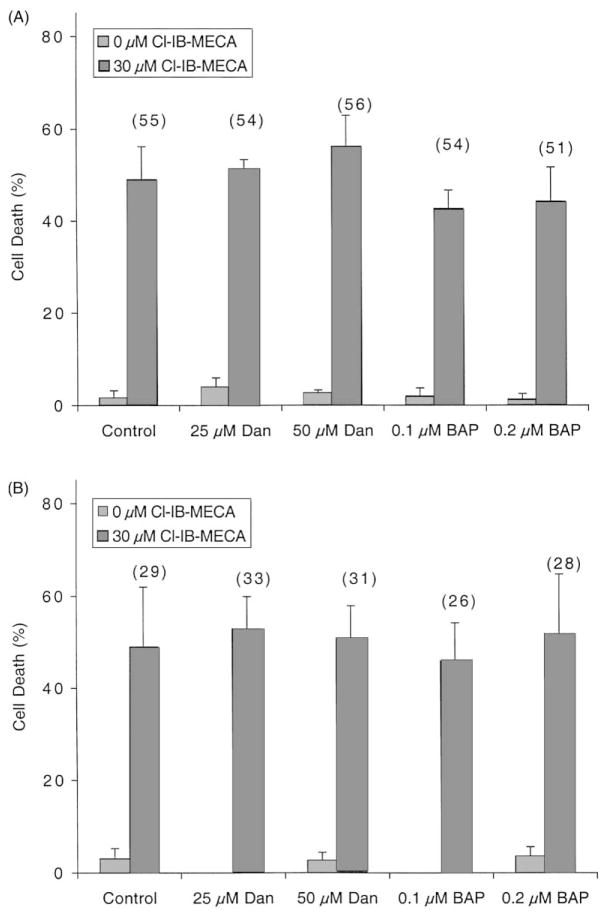
The role of intracellular calcium in Cl-IB-MECA-induced apoptosis was examined, since it has been reported that Cl-IB-MECA increases intracellular calcium levels [10,15]. Fig. 2 shows that the Cl-IB-MECA-induced apoptosis and cell death were not affected when the release of calcium from intracellular sources was blocked by dantrolene (25 or 50 μM), or when the intracellular calcium was chelated following preincubation with BAPTA-AM (0.1 or 0.2 μM). Since the apoptosis rates were not affected by the addition of calcium modifiers, any potential change in intracellular calcium levels by Cl-IB-MECA would not likely be involved in apoptosis induced by Cl-IB-MECA.
Fig. 2.
Effects of calcium modifiers on Cl-IB-MECA-induced cell death and apoptosis. HL-60 (A) or MOLT-4 (B) cells were incubated for 48 hr in culture medium containing calcium modulators (Dan: dantrolene, BAP: BAPTA-AM) with or without 30 μM Cl-IB-MECA. The degree of cell death was determined by the Trypan blue exclusion test. Results are means ± SD from the combined data of two independent experiments performed in triplicate. Percent apoptosis, as determined by flow cytometry, is shown in parentheses.
3.3. Effects ofA3AR antagonists on apoptosis induced by Cl-IB-MECA
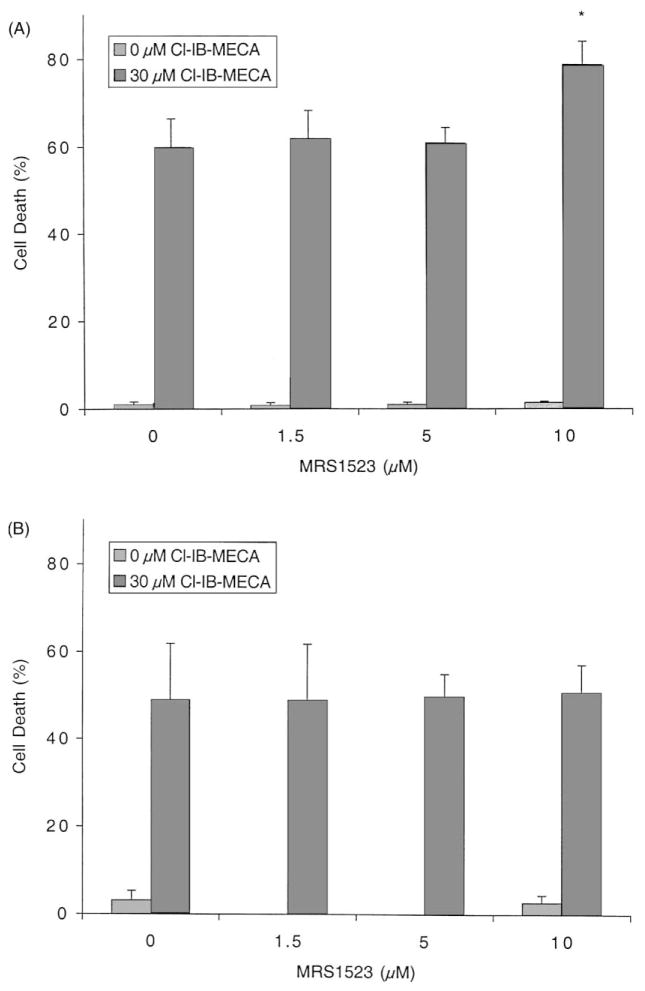
Two human A3AR-selective antagonists, MRS 1523 (also selective in rat) and MRS 1220, were examined for their ability to block Cl-IB-MECA-induced apoptosis in HL-60 and MOLT-4 cells. As shown in Fig. 3, a 48 hr incubation with MRS 1523 (0–10 μM) did not improve cell viability, which was reduced by 30 μM Cl-IB-MECA. At the highest concentration (10 μM), MRS 1523 slightly augmented the effect of 30 μM Cl-IB-MECA in HL-60 cells. MRS 1220 (0–5 μM) also did not affect the apoptotic rate induced by 30 μM Cl-IB-MECA (data not shown). At 5 μM, MRS 1220 would be expected to block A1 and A2A receptors [1]. Thus, the action of 30 μM Cl-IB-MECA at 48 hr appeared to be adenosine receptor-independent.
Fig. 3.
Effects of the A3AR antagonist MRS 1523 on Cl-IB-MECA-induced cell death. HL-60 (A) or MOLT-4 (B) cells were incubated for 48 hr in culture medium containing 0–10 μM MRS 1523 with or without 30 μM Cl-IB-MECA. Cell viability was determined by the Trypan blue exclusion test as described in Section 2. Data are means ± SD of three independent experiments. Key: significantly different with respect to 0 μM MRS 1523, *P < 0.01 (Student’s t-test).
3.4. Phospholipase C activity
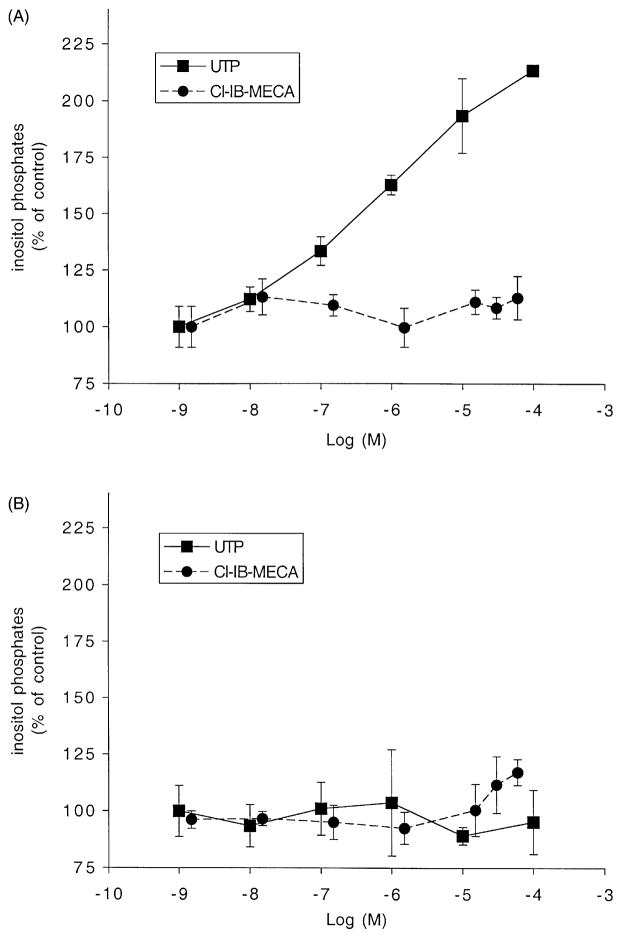
It has been reported that the A3AR is coupled to phospholipase C (PLC) [21–23], considered one of the main second messenger systems coupled to the A3AR. As shown in Fig. 4, however, the production of inositol phosphates was not increased by up to 60 μM Cl-IB-MECA in either HL-60 or MOLT-4 cells. To ascertain that a functional PLC system was present, we examined the effect of UTP, which activates endogenous P2Y2 nucleotide receptors in HL-60 cells. Treatment of HL-60 cells with UTP-activated PLC with an approximately 2-fold increase at 10 μM. Therefore, HL-60 cells seemed to either lack the A3AR or at least its ability to couple to PLC. UTP had no effect in MOLT-4 cells.
Fig. 4.
Production of inositol phosphates by Cl-IB-MECA. HL-60 (A) or MOLT-4 (B) cells were labeled with myo-[3H]inositol (1 μCi/106 cells) for 24 hr. Then the cells were treated for 30 min at 37° with Cl-IB-MECA or UTP in RPMI 1640 medium containing 0.5% fetal bovine serum, 20 mM HEPES (pH 7.2), 20 mM LiCl, and 1 mg/mL of bovine serum albumin. [3H]-Inositol phosphates were extracted and separated by Dowex AG 1-X8 as described in Section 2. Results are means ± SD from the combined data of two independent experiments performed in triplicate.
3.5. Western blot analysis
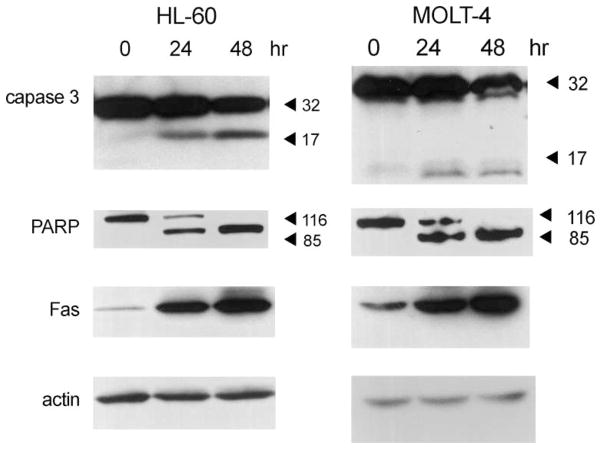
To understand the mechanism of apoptosis induced by Cl-IB-MECA (30 μM), several apoptotic biomarkers were investigated by means of immunoblotting (Fig. 5). Caspase 3 and poly-ADP-ribose-polymerase (PARP) were activated in both leukemic cell lines in a time-dependent manner, showing that Cl-IB-MECA-induced apoptosis through the caspase pathway. We also observed a significant increase of Fas following 24 and 48 hr exposures to Cl-IB-MECA. There were no changes in the expression of TRAIL, Bcl-2, or Bax (data not shown).
Fig. 5.
Activation of caspase 3 and PARP and increase of Fas expression in HL-60 and MOLT-4 cells. After treating cells with 30 μM Cl-IB-MECA for 24 and 48 hr, proteins were extracted, separated, and analyzed by Western blotting as described in Section 2. Antibody detection of actin bands demonstrated that the same amount of protein was present in each lane. Blots shown are representative of at least three independent experiments.
3.6. Effects of agonistic (CH-11) and antagonistic (ZB-4) anti-Fas antibodies on apoptosis induced by Cl-IB-MECA
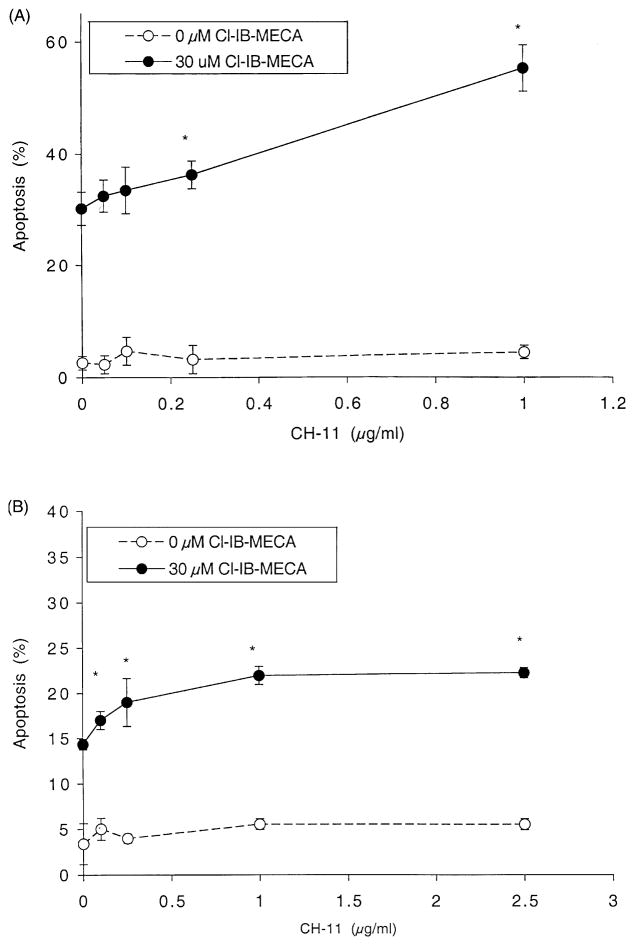
Due to its pronounced up-regulation in these cell lines, the involvement of Fas expression in Cl-IB-MECA-induced apoptosis was investigated further. HL-60 (Fig. 6A) and MOLT-4 (Fig. 6B) cells were treated for 24 hr with Cl-IB-MECA and/or CH-11, an agonistic Fas antibody [24], and apoptosis was determined using FACS. The apoptosis rate in both cell lines was not changed significantly by CH-11 alone during 24 hr, although there were slight increases of cell death at 1 (μg/mL, using the Trypan blue exclusion test (data not shown). Apoptosis induced by Cl-IB-MECA (30 μM) in HL-60 cells (Fig. 6A) was augmented to 130 and 180% of control with 0.25 and 1.0 μg/mL of CH-11, respectively. Augmentation effects by Cl-IB-MECA and subthreshold doses of CH-11 were observed in MOLT-4 cells (Fig. 6B). A Fas antagonist was also used to demonstrate that this increase in Cl-IB-MECA-induced apoptosis elicited by CH-11 was dependent upon Fas. The pretreatment of HL-60 cell cultures with the Fas antagonistic antibody ZB-4 (4 or 8 μg/mL) for 1 hr, and its continued presence during the subsequent incubation, reduced the degree of apoptosis induced by prolonged (24 hr) exposure to Cl-IB-MECA (30 μM) and CH-11 (1 μg/mL) to the control level of approximately 30% (data not shown). These results implied that increased Fas expression mediated the apoptotic effect of Cl-IB-MECA.
Fig. 6.
Effect of CH-11 on Cl-IB-MECA-induced apoptosis. HL-60 (A) or MOLT-4 (B) cells were incubated for 24 hr in culture medium containing various concentrations of CH-11 with or without 30 μM Cl-IB-MECA. The apoptotic rate was determined by the Trypan blue exclusion test and by flow cytometry as described in Section 2. Data are means ± SD of three independent experiments. Key: significantly different with respect to 0 μg/mL of CH-11, *P < 0.01 (Student’s t-test).
4. Discussion
It has been reported that A3AR agonists induce cell death in leukemic and other cells [10–15], while the mechanisms remain unknown. The present study was undertaken to determine how Cl-IB-MECA, an A3AR agonist, induced apoptosis in leukemic cells.
Intracellular calcium has been known to modulate or transduce many intracellular signals including those of programmed cell death or apoptosis. The increase in intracellular free calcium following the activation of the A3AR has been reported in HL-60 cells and in cardiac myocytes [10,15]. We have investigated whether Cl-IB-MECA caused apoptotic cell death through an increase of intracellular calcium levels. As shown in Fig. 2, the induction of cell death by Cl-IB-MECA was not affected when the calcium release from sarcoplasmic reticulum was inhibited by dantrolene, or when the free intracellular calcium was removed by chelation. Therefore, the change of intracellular calcium did not seem to play a role in the Cl-IB-MECA-induced apoptosis.
The A3AR increases intracellular calcium levels through the coupling to PLC [1]. Therefore, we tested the activity of PLC in these leukemic cells upon treatment with Cl-IB-MECA (Fig. 4). Inositol phosphates were not increased by Cl-IB-MECA in either HL-60 or MOLT-4 cells. However, inositol phosphates were increased by UTP used as a positive control that acts through P2Y2 receptors known to be expressed in HL-60 cells (Fig. 4). When UTP was added to HL-60 cells, it increased the production of inositol phosphates in a concentration-dependent manner, demonstrating the existence of purinergic receptors coupled to PLC. However, MOLT-4 cells appear to lack P2Y2 or P2Y4 receptors, which are normally activated by UTP, although the presence of P2Y6 receptors in MOLT-4 cells has been reported [25]. Therefore, Cl-IB-MECA did not act through a PLC-coupled A3AR.
Additional evidence that the apoptotic effect of Cl-IB-MECA was not exerted through A3AR activation was obtained using the selective A3AR antagonist MRS 1523. The presence of this antagonist in the medium throughout the incubation did not block or diminish the Cl-IB-MECA-induced cell death (Fig. 3). Another antagonist of adenosine receptors, MRS 1220 (at 1 and 5 μM), also had no effect (data not shown). Since no antagonism was observed, the stability of MRS 1220 in the experimental conditions was investigated. It was demonstrated by HPLC analysis that MRS 1220 remained intact during a 48 hr incubation in the presence of HL-60 cells (data not shown). Therefore, a mechanism other than activation of adenosine receptors seems to be responsible. In rat cardiac myocytes [26], a lower concentration of antagonist (MRS 1523, 1 μM) was reported to antagonize apoptosis induced by 20 μM IB-MECA, but only when the agonist was present for a short period, i.e. 2 hr. In the leukemic cells, however, we observed that the short periods of treatment with antagonists (2, 4.5, 9, 18 hr) also did not prevent the apoptosis induced by Cl-IB-MECA (24 hr). Under conditions similar to those of Fig. 1, a newly reported A3 agonist [27], MRS 1898 (30 μM), did not induce apoptosis significantly (5% at 48 hr compared with 60% for Cl-IB-MECA), which is additional evidence that the Cl-IB-MECA-induced apoptosis in HL-60 cells does not occur through the A3AR (data not shown). Nevertheless, the presence of A3AR mRNA in HL-60 cells has been shown [10] by reverse transcription-polymerase chain reaction (RT-PCR). It was not possible, using 125I-AB-MECA, to detect radioligand binding to the A3AR in HL-60 cell membranes [10]. There may be an insufficient amount of A3AR on the cell membrane to transduce the signal. Cl-IB-MECA may induce apoptosis through a pathway different from A3AR activation. However, there is also a possibility that the A3AR may contribute to apoptosis by activating a pathway different from the second messenger signals tested above or that prolonged agonist exposure may have desensitized the A3AR. The G proteins that function in coupling to the A3AR may be involved in the apoptotic process. For example, the activation of Akt, which leads to an anti-apoptotic cascade, by the m1 or m2 muscarinic receptors, both of which are G protein-coupled receptors, seems to be signaled through Gαq, Gαi; and βγ without activation of protein kinase C (PKC) [28].
When apoptosis was induced in HL-60 and MOLT-4 cells by Cl-IB-MECA, activation of caspase 3 and PARP was observed (Fig. 5). There was also an increase of Fas expression, which may mediate the apoptotic cell death (Fig. 5). Fas induction was not observed when apoptosis was induced by 10 μM camptothecin, a topoisomerase I inhibitor, for 5 hr (data not shown). Thus, Cl-IB-MECA-induced apoptosis occurred through a pathway different from camptothecin, and the activation of the Fas signal is an important step in the pathway.
Fas, a member of the tumor necrosis factor (TNF) receptor family, has been considered to have an important role in the regulation of death in many cell types, especially in mediating signals to induce lymphocytic apoptosis and to prevent autoimmune disease [29,30]. Liver cells infected with hepatitis C virus showed up-regulation of Fas, which is thought to be activated by the Fas ligand (FasL) on the T cells in an inflamed lesion of the liver [31]. It was also suggested that the induction of Fas expression may be one of the mechanisms of action of chemotoxic drugs and thus might enhance the cell susceptibility to Fas-mediated apoptosis [32].
The importance of p53 in Fas expression has been reported recently. In human vascular smooth muscle cells, p53 activation transiently increased surface Fas expression [33]. Fas was up-regulated in p53+/+, but not in p53−/− human leukemic cells when apoptosis was induced by irradiation [34,35]. The transfection of wild-type p53 into a p53-null adenocarcinoma induced the marked up-regulation of Fas [36]. In human colon cancer cell lines, the induction of Fas by 5-fluorouracil and leucovorin was also found to be p53-dependent [37]. It was suggested that Fas enhanced p53-mediated apoptosis [38], and that p53 sensitized cells to Fas-induced apoptosis [33]. According to the data that we have shown, the increased expression of Fas by Cl-IB-MECA seems to be a novel mechanism for the induction of apoptosis in human leukemia cells, which do not have functional p53. HL-60 cells are known to be p53-null [39], and MOLT-4 cells have a mutated p53 that cannot be expressed [40]. Therefore, our results suggest a deviation from previously characterized pathways of apoptosis. It would be intriguing to understand how Cl-IB-MECA induced the expression of Fas and if the pathway is related to that of p53 signals. Although it is uncertain how Cl-IB-MECA induced the expression of Fas in these cells, we have shown that Fas can be up-regulated independently of p53.
Since Fas-mediating signaling plays an important part in the regulation of lymphocyte populations, the intervention by bisindolmaleimide VIII, which facilitates Fas-induced apoptosis signaling processes, was suggested as a possible strategy for the treatment of autoimmune disease, by enhancing apoptosis to eliminate self-reactive T cells [41]. Widely used anticancer drugs, such as methotrexate and doxorubicin, have been known to act through the up-regulation of FasL expression, leading to apoptosis of Fas-sensitive tumor cells [42]. Here we suggest that the up-regulated Fas expression by Cl-IB-MECA may also be a good candidate strategy for autoimmune disease or for cancer treatment in combinations with those applications.
Abbreviations
- A3AR
A3 adenosine receptor
- FACS
fluorescence-activated cell sorting
- PLC
phospholipase C
- PARP
poly-ADP-ribose-polymerase
References
- 1.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998;19:184–91. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HO, Ji XD, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem. 1994;37:3614–21. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knutsen LJS, Sheardown MJ, Roberts SM, Mogensen JP, Olsen UB, Thomsen C, Bowler AN. Adenosine A1- and A3-selective N-alkoxypurines as novel cytokine modulators and neuroprotectants. Drug Dev Res. 1998;45:214–21. [Google Scholar]
- 4.Linden J. Cloned adenosine A3 receptors—pharmacological properties, species-differences and receptor functions. Trends Pharmacol Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 5.Hannon JP, Pfannkuche HJ, Fozard JR. A role for mast-cells in adenosine A3 receptor-mediated hypotension in the rat. Br J Pharmacol. 1995;115:945–52. doi: 10.1111/j.1476-5381.1995.tb15902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fozard JR, Pfannkuche HJ, Schuurman HJ. Mast cell degranulation following adenosine A3 receptor activation in rats. Eur J Pharmacol. 1996;298:293–7. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- 7.Auchampach JA, Rizvi A, Qiu Y, Tang X-L, Maldonado C, Teschner S, Bolli R. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res. 1997;80:800–9. doi: 10.1161/01.res.80.6.800. [DOI] [PubMed] [Google Scholar]
- 8.Von Lubitz DKJE, Lin RCS, Boyd M, Bischofberger N, Jacobson KA. Chronic administration of adenosine A3 receptor agonist and cerebral ischemia: neuronal and glial effects. Eur J Pharmacol. 1999;367:157–63. doi: 10.1016/s0014-2999(98)00977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Lubitz DKJE, Carter MF, Deutsch SI, Lin RCS, Mastropaolo J, Meshulam Y, Jacobson KA. The effects of adenosine A3 receptor stimulation on seizures in mice. Eur J Pharmacol. 1995;275:23–9. doi: 10.1016/0014-2999(94)00734-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohno Y, Sei Y, Koshiba M, Kim HO, Jacobson KA. Induction of apoptosis in HL-60 human promyelocytic leukemia cells by adenosine A3 receptor agonists. Biochem Biophys Res Commun. 1996;219:904–10. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y, Sei Y, Abbracchio MP, Jiang JL, Kim YC, Jacobson KA. Adenosine A3 receptor agonists protect HL-60 and U-937 cells from apoptosis induced by A3 antagonists. Biochem Biophys Res Commun. 1997;232:317–22. doi: 10.1006/bbrc.1997.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbieri D, Abbracchio MP, Salvioli S, Monti D, Cossarizza A, Ceruti S, Brambilla R, Cattabeni F, Jacobson KA, Franceschi C. Apoptosis by 2-chloro-2′-deoxy-adenosine and 2-chloro-adenosine in human peripheral blood mononuclear cells. Neurochem Int. 1998;32:493–504. doi: 10.1016/s0197-0186(97)00129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbracchio MP, Ceruti S, Brambilla R, Barbieri D, Camurri A, Franceschi C, Giammarioli AM, Jacobson KA, Cattabeni F, Malorni W. Adenosine A3 receptors and viability of astrocytes. Drug Dev Res. 1998;45:379–86. doi: 10.1002/(sici)1098-2299(199811/12)45:3/4<379::aid-ddr38>3.0.co;2-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stickler J, Jacobson KA, Liang BT. Direct preconditioning of cultured chick ventricular myocytes. Novel functions of cardiac adenosine A2a and A3 receptors. J Clin Invest. 1996;98:1773–9. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shneyvays V, Nawrath H, Jacobson KA, Shainberg A. Induction of apoptosis in cardiac myocytes by an A3 adenosine receptor agonist. Exp Cell Res. 1998;243:383–97. doi: 10.1006/excr.1998.4134. [DOI] [PubMed] [Google Scholar]
- 16.Abbracchio MP, Rainaldi G, Giammarioli AM, Ceruti S, Brambilla R, Cattabeni F, Barbieri D, Franceschi C, Jacobson KA, Malorni W. The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-xL: studies in human astroglioma cells. Biochem Biophys Res Commun. 1997;241:297–304. doi: 10.1006/bbrc.1997.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill RJ, Oleynek JJ, Magee W, Knight DR, Tracey WR. Relative importance of adenosine A1 and A3 receptors in mediating physiological or pharmacological protection from ischemic myocardial injury in the rabbit heart. J Mol Cell Cardiol. 1998;30:579–85. doi: 10.1006/jmcc.1997.0621. [DOI] [PubMed] [Google Scholar]
- 18.Brambilla R, Cattabeni F, Ceruti S, Barbieri D, Franceschi C, Kim YC, Jacobson KA, Klotz KN, Lohse MJ, Abbracchio MP. Activation of the A3 adenosine receptor affects cell cycle progression and cell growth. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:225–34. doi: 10.1007/s002109900186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li AH, Moro S, Melman N, Ji XD, Jacobson KA. Structure-activity relationships and molecular modeling of 3,5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J Med Chem. 1998;41:3186–201. doi: 10.1021/jm980093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baek SH, Seo JK, Chae C-B, Suh P-G, Ryu SH. Identification of the peptides that stimulate the phosphoinositide hydrolysis in lymphocyte cell lines from peptide libraries. J Biol Chem. 1996;271:8170–5. doi: 10.1074/jbc.271.14.8170. [DOI] [PubMed] [Google Scholar]
- 21.Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DKJE, Jacobson KA, Cattabeni F. G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038–45. [PubMed] [Google Scholar]
- 22.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–90. [PubMed] [Google Scholar]
- 23.Ali H, Cunha-Melo JR, Saul WF, Beavan MF. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion of antigen-stimulated RBL-2H3 cells. J Biol Chem. 1990;265:745–53. [PubMed] [Google Scholar]
- 24.Quirk SM, Cowan RG, Joshi SG, Henrikson KP. Fas antigen-mediated apoptosis in human granulosa-luteal cells. Biol Reprod. 1995;52:279–87. doi: 10.1095/biolreprod52.2.279. [DOI] [PubMed] [Google Scholar]
- 25.Communi D, Robaye B, Janssens R, Parmentier M, Boeynaems J-M. Receptors responsive to extracellular uracil nucleotides. Drug Dev Res. 1998;45:130–4. [Google Scholar]
- 26.Shneyvays V, Jacobson KA, Li A-H, Nawrath H, Zinman T, Issac A, Shainberg A. Induction of apoptosis in rat cardiocytes by A3 adenosine receptor activation and its suppression by isoproterenol. Exp Cell Res. 2000;257:111–26. doi: 10.1006/excr.2000.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K, Ravi G, Ji X-d, Marquez VE, Jacobson KA. Ring-constrained (N)-methanocarba-nucleosides as adenosine receptor agonists: independent 5′-uronamide and 2′-deoxy modifications. Biorg Med Chem Lett. 2001;11:1333–7. doi: 10.1016/s0960-894x(01)00213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for α and βγ subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinase γ. J Biol Chem. 1998;273:19080–5. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–76. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 31.Nasir A, Arora HS, Kaiser HE. Apoptosis and pathogenesis of viral hepatitis C—an update. In Vivo. 2000;14:297–300. [PubMed] [Google Scholar]
- 32.Labroille G, Dumain P, Lacombe F, Belloc F. Flow cytometric evaluation of Fas expression in relation to response and resistance to anthracyclines in leukemic cells. Cytometry. 2000;39:195–202. [PubMed] [Google Scholar]
- 33.Bennett M, Macdonald K, Chan S-W, Luzio JP, Simari R. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis P. Science. 1998;282:290–3. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y-R, Tan T-H. Lack of correlation in JNK activation and p53-dependent Fas expression induced by apoptotic stimuli. Biochem Biophys Res Commun. 1999;256:595–9. doi: 10.1006/bbrc.1999.0383. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Ruan S, Jabbur JR, Consoli U, Clodi K, Shiku H, Owen-Schaub LB, Andreeff M, Reed JC, Zhang W. Differential p53 phosphorylation and activation of apoptosis-promoting genes Bax and Fas/APO-1 by irradiation and ara-C treatment. Cell Death Differ. 1998;5:584–91. doi: 10.1038/sj.cdd.4400382. [DOI] [PubMed] [Google Scholar]
- 36.Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, Roth JA, Deisseroth AB, Zhang WW, Kruzel E, Radinsky R. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol. 1995;15:3032–40. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petak I, Tillman DM, Houghton JA. p53 dependence of Fas induction and acute apoptosis in response to 5-fluorouracil-leucovorin in human colon carcinoma cell lines. Clin Cancer Res. 2000;6:4432–41. [PubMed] [Google Scholar]
- 38.Rakkar AN, Katayose Y, Kim M, Craig C, Ohri E, Li Z, Cowan KH, Seth PA. Novel adenoviral vector expressing human Fas/CD95/APO-1 enhances p53-mediated apoptosis. Cell Death Differ. 1999;6:326–33. doi: 10.1038/sj.cdd.4400498. [DOI] [PubMed] [Google Scholar]
- 39.Wolf D, Rotter V. Major deletions in the gene encoding the p53 tumor-antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci USA. 1985;82:790–4. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigues NR, Rowan A, Smith MEF, Kerr IB, Bodmer WF, Gannon JV, Lane DP. p53 mutations in colorectal-cancer. Proc Natl Acad Sci USA. 1990;87:7555–9. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou T, Song L, Yang P, Wang Z, Lui D, Jope RS. Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T cell-mediated autoimmune diseases. Nat Med. 1999;5:42–8. doi: 10.1038/4723. [DOI] [PubMed] [Google Scholar]
- 42.Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia. Nat Med. 1996;2:574–7. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]