Abstract
Background
In the field of cellular therapy potential cell entrapment in the lungs following intravenous administration in a compromised or injured pulmonary system is an important concern that requires further investigation. We developed a rat model of inflammatory and fibrotic lung disease to mimic the human clinical condition of obliterative bronchiolitis (OB) and evaluate the safety of intravenous infusion of mesenchymal stromal cells (MSCs). This model was used to obtain appropriate safety information and functional characterization to support the translation of an ex vivo generated cellular product into human clinical trials. To overcome spontaneous recovery and size limitations associated with current animal models we used a novel multiple dose bleomycin strategy to induce lasting lung injury in rats.
Methods
Intratracheal instillation of bleomycin was administered to rats on multiple days. MSCs were intravenously infused 7 days apart. Detailed pulmonary function tests including forced expiratory volume, total lung capacity, and invasive hemodynamic measurements were conducted to define the representative disease model and monitor cardiopulmonary hemodynamic consequences of the cell infusion. Post euthanasia assessments included a thorough evaluation of lung morphology and histopathology.
Results
The double dose bleomycin instillation regimen resulted in severe and irreversible lung injury and fibrosis. Cardiopulmonary physiological monitoring reveled that no adverse events could be attributed to the cell infusion process.
Discussion
Although our study did not show the infusion of MSCs to result in an improvement in lung function or rescue of damaged tissue this study does confirm the safety of MSC infusion into damaged lungs.
Keywords: mesenchymal stromal cells, MSC, stem cells, lung injury, pulmonary function, cardiac function, cardiopulmonary characterization, safety, cellular therapy
Introduction
The safety and clinical feasibility of administration of ex vivo expanded bone marrow (BM) derived mesenchymal stromal cells (MSCs) was originally shown more than 15 years ago (1) and has since been investigated in hundreds of clinical trials for a wide variety of clinical indications different routes of administration, and cell doses (2–7). However, there are still theoretical safety concerns regarding the intravenous injection of MSCs, especially for lung disorders, since the lung is the first tissue with capillaries that these cells encounter. For example, it has been shown that a large proportion of cells are retained in the lung after intravenous injection of MSCs in a mouse model of brain injury (8). When human MSCs were directly injected into the myocardium in a mouse myocardial infarction model, 83% of the injected cells were identified in the lungs 15 minutes post injection with an estimated halflife of 24 hours (9). In another study, intravenous infusion of MSCs resulted in multiple pulmonary artery embolisms and infarct of the right lung (10). These results underscore the importance of evaluating the safety of MSC injections in animal models of lung injury prior to the initiation of human clinical studies, especially for pulmonary disorders. It has been shown that MSCs can protect the lungs from acute lung injury induced by bleomycin and reduce lung transplant rejection in rodent models (11–16). However, detailed and clinically relevant pulmonary function tests have not been assessed in detail in any animal model (17).
To overcome previous reports of spontaneous recovery using a single bleomycin instillation, we developed a double dose instillation strategy (18). Intratracheal instillation of bleomycin is associated with an increase in pulmonary artery and right ventricular (RV) pressure (19). In this study, we therefore investigated the potential effects of cellular infusion on cardiopulmonary pressure changes in this model. Without additional doses of bleomycin, animals often return to their baseline state, where fibrosis and inflammation resolve (20). This study was designed to demonstrate safety by infusing MSCs at two methodical points representative of the most vulnerable stages of lung injury; one during the pro-inflammatory phase and then again during the pro-fibrotic phase of the injury.
This study was designed to support an Investigational New Drug (IND) application to U.S. Food and Drug Administration to demonstrate safety of infused MSC and justify their safe use in future human clinical studies. In this study we test the safety of intravenous infusion of MSCs into a lung injury model, perform detailed pulmonary function tests, and assess right ventricular hemodynamic changes during the MSC infusion. A single intratracheal or intranasal delivery of bleomycin, a chemotherapeutic agent which induces tissue damage through direct DNA strand breakage and the generation of free radicals, to mouse lungs has been the most common animal model of pulmonary fibrosis (21, 22). While bleomycin administration results in dose dependent damage to the lung resulting in pathology similar to bronchiolitis obliterans (BO), mice often spontaneously recover from the insult after 28 days limiting the utility of this transient model. Just as important, due to their small size, mouse models are less suited for evaluating clinically relevant cell delivery methods and representative dosing, and do not allow detailed physiologic monitoring. To develop a more representative pre-clinical model we designed a 2-dose bleomycin rat lung injury model to represent chronic lung injury which lacks any signs of spontaneous recovery and is amenable to the clinically relevant cardiopulmonary physiological monitoring.
Materials and Methods
Animals
All animal procedures were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee and followed guidelines of the NIH Guide for the Care and Use of Laboratory Animals. Male Sprague Dawley rats (Harlan, Indianapolis, IN) were housed singly to minimize stress and facilitate animal observations. All animals were maintained in environmentally controlled rooms at 22 °C and 50% to 55% humidity with a 12/12-hour light/dark cycle. All animals were provided a standard laboratory diet (NIH-07 Rat and Mouse Chow) and water ad libitum. Daily monitoring included assessments of food and water intake, animal weight, behavior, body condition and mortality. Twenty-four (24) animals were divided into three groups for this study. All animals were between 7 and 8 weeks old and weighed between 215 and 264 g.
Animals were allowed to acclimate for up to 5 days before being placed on study. Animals were assessed for pulmonary function on study day 0. Immediately following pulmonary function testing (PFT), bleomycin (Sigma-Aldrich, St. Louis, MO) was instilled intratracheally at 3.0 U/kg in 300 µl of sterile isotonic saline using a high pressure aerosolizing syringe (PennCentury, Wyndmoor, PA). Four days later, a second dose of bleomycin was instilled in the same fashion. Repeat PFT testing was subsequently performed on days 7 and 14 (Figure 1). Intravenous injection of MSC or vehicle was performed on days 8 and 15. Saturation of peripheral oxygen (SpO2) data was collected during the intravenous infusion. Group 1- lung injury animals received intravenous vehicle control solution (Freezing medium diluted with Plasmalyte A), Group 2- lung injury animals received low dose MSC, and Group 3- lung injury animals received a high dose MSC treatment. On day 15 a second intravenous infusion of vehicle, high dose, or low dose MSC was administered to each of the respective groups. Right ventricular pressures were measured during the final intravenous infusion on day 15. Immediately following this last intravenous infusion animals were euthanized with an overdose of pentobarbital (120 mg/kg). At the time of euthanasia gross pathological analysis was performed on all major tissues and organ systems. A certified veterinary pathologist then harvested tissues and organs to allow for histological evaluation.
Figure 1. Overall Study Design and Time Points.
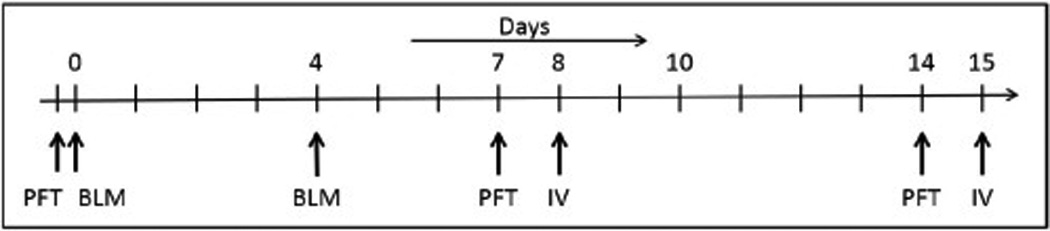
Study schema depicting study procedures and assessment time points. PFT = Pulmonary function test; BLM = Bleomycin instillation; IV = intravenous infusion
Pulmonary function testing
Pulmonary function measurements were captured on lightly anesthetized (ketamine/xylazine) rats instrumented with an orotracheal tube, using a total body plethysmograph (Buxco Research Systems, Wilmington, NC) equipped with software-controlled valves to measure thoracic gas volume and forced expiratory volume (FEV). This pulmonary function testing was controlled and data were collected using a PowerLab system (ADIntstruments, Colorado Springs, CO).
Blood oxygen levels
Animals were anesthetized with isoflurane at a constant concentration of 2% in 100% oxygen. SpO2 was measured with a clinical pulse oximeter (GE Datex Ohmeda 3900) with a probe attached to a foot pad during lung function measurements providing values during tidal and ventilator breathing.
Respiratory and heart Rate
Respiratory rate was captured and recorded for 1 minute durations at 5 minute intervals during the infusion period. A pulse oximeter was used to obtain the heart rate which was captured and recorded every 5 minutes during the infusion period. The pulse oximeter sensor was methodically placed on the front foot of each animal to ensure adequate conductivity to obtain a valid result.
Right ventricle pressure during MSC infusion
Measurement of pulmonary arterial pressure was performed in accordance with standard practice in cases where there is no pulmonic valve stenosis. More specifically, pulmonary artery systolic pressure is equal to right ventricular systolic pressure under these normal conditions. While animals were anaesthetized with isoflurane a fluid-filled custom-made catheter was inserted into the jugular vein and directed to the right ventricle. Placement of the catheter was determined by the displayed pressure wave on an Ivy Biomedical monitor (Ivy Biomedical, Branford, CT). Right ventricular pressure was measured and captured throughout the second intravenous infusion which occurred on day 15.
Blood gases
Two hundred microliters of arterial blood was collected on day 15 from a main peripheral artery and analyzed for pH, pO2 and pCO2. These arterial blood samples were analyzed using a clinical blood gas machine (pHOx Basic, Nova Biomedical, Waltham, MA).
MSC intravenous infusion
The MSCs used in these studies were produced by University of Wisconsin-Madison Waisman Biomanufacturing, a cGMP compliant contract manufacturing facility that supports early-stage human clinical studies. The MSCs were characterized as previously described (23). Briefly, MSCs were tested for sterility (no contamination), endotoxin (<0.5 EU/mL), viability (>83% post thaw), identity/purity (standard flow cytometry for MSC markers), and potency (immunopotency assay). MSCs were cryopreserved in a solution consisting of Plasmalyte A with 2.5% DMSO, 10% Human Serum Albumin and stored in the vapor phase of liquid nitrogen until used for these studies. Frozen MSCs were thawed and immediately diluted 5-fold in Plasmalyte A prior to infusion. Rats were intravenously infused with Plasmalyte A alone, low MSC dose of 2.8 × 106 /kg or high MSC dose of 5.6 × 106 cells/kg. The infusion rate was kept constant at 2 mL/hour using a syringe infusion pump (Harvard Apparatus, Holliston, MA) with the cannula inserted directly into the jugular vein. This infusion was followed by a 0.5 mL Plasmalyte A “flush” at a rate of 4 mL/hour. The total infusion volume ranged between 1.0 to 1.5 mL. Throughout the duration of the infusion animals were monitored for heart rate, respiration rate, and SpO2.
Cell thaw and viability
A single vial of MSC (6×106 cells/mL) was thawed at 37 °C until ice crystals dissolved. Immediately following the thaw, 1.6 mL of Plasmalyte A (1:5 dilution) was slowly added to the freshly thawed cell product to minimize osmotic shock to the cell suspension. The infusion parameters used in this study were selected to mimic those to be utilized in the human clinical study; viability was determined using Trypan Blue (0.4%) exclusion on three different fractions of the infused product.
Animal necropsy and pathology
Necropsy on each animal was performed by a Board-certified veterinary pathologist blinded to treatment groups. Animal weights were documented prior to the necropsy. External and internal gross features of the animals were examined and documented. Necropsy procedures included the removal and examination of the lungs, heart, liver, spleen, kidney, brain, and gonads; each was weighed immediately after harvest. The lung was fixed in 10% formalin at 25 cm water pressure. Tissues were formalin-fixed, trimmed, paraffin-embedded, sectioned at 5 µm, and stained with hematoxylin and eosin. Sections of lung were also stained with Trichrome. The veterinary pathologist was unblinded prior to histopathological analysis.
Blood coagulation
At the study endpoint at 15 days, blood was collected from at least five representative animals from each group and analyzed for prothrombin time (PT) in seconds, activated partial thromboplastin time (APTT) in seconds, and fibrinogen concentration in mg/dL. It should be noted that any changes in these values may provide an early indication of liver disease, development of thromboembolism, or anti-coagulation abnormalities.
Statistical analysis
The statistical evaluation of animal body weights, organ weights, and blood coagulation utilized multiple t-test with the Holm-Sidak method. Each group was analyzed individually, without assuming a consistent standard deviation. Statistical analysis of SpO2 included the use of one-way ANOVA and corrected for multiple comparison using Tukey’s multiple comparison test. Statistical evaluation of diastolic and systolic pressure employed the use of multiple t-test and corrected for multiple comparison using the Holm-Sidak method. To account for the baseline offset for systolic pressure, the value was subtracted before analysis in all groups. Pulmonary function test FEV0.1 and TLC data were analyzed using one-way ANOVA and corrected for multiple comparison using Tukey’s multiple comparison test.
Results
Survival and animal weights
All animals survived both bleomycin instillations. The subsequent 16.7% (4 of 24 rats) animal attrition rate was the result of procedural complications. One animal died during the pulmonary function test at day 7 which was determined to be the result of equipment failure leading to hyperinflation and lung rupture. A second animal died during the measure of functional residual capacity – the heart stopped beating with no obvious cause (assumed to be an anesthetic-related death). Two additional animals died during the cell infusion due to excessive bleeding caused by the catheter implantation procedure on day 15. No differences were noted in the number of deaths between any of the three treatment groups.
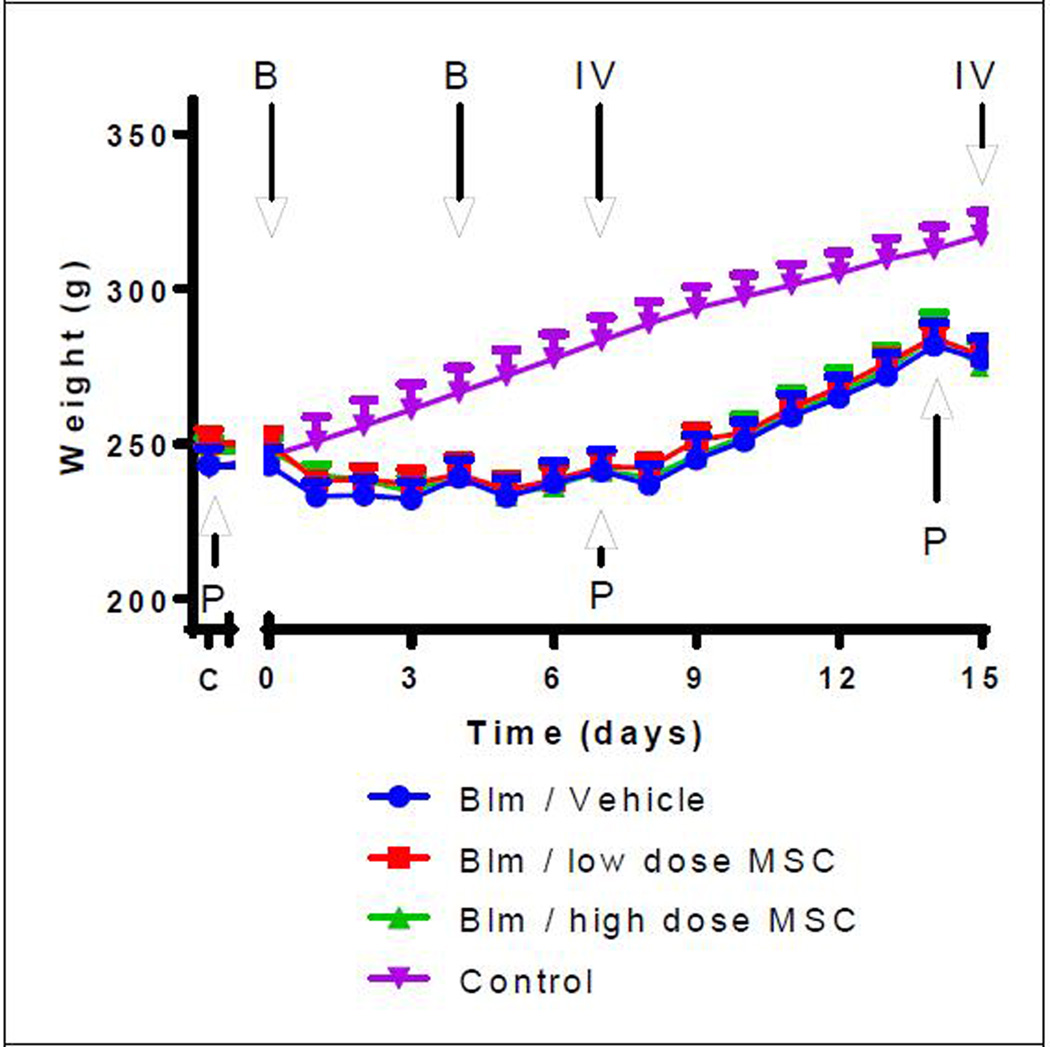
Initial animal weights before being placed on study averaged 247 ± 2.7 g for all three study groups. On average animals lost 17.8 ± 9.8 g after the bleomycin treatment (Figure 2) but at the time of study termination animals had gained an average of 2.29 ± 1.05 g/day resulting in an average weight of 277 ± 18 g (Table 1). Body weights of the representative control animals, not receiving bleomycin exposure, were significantly higher than all bleomycin treatment groups starting at day 4 (p<0.05). There was no significant difference in final body weights between animals of any bleomycin treatment group. The initial observed weight loss was attributed to a reduction in daily food and water intake a result of compromised health from the bleomycin instillation or stress from the pulmonary function test. No difference in food or water intake was observed between the three treatment groups.
Figure 2. Change in animal weights over the course of the study.
All bleomycin treated animals initially lost weight as a result of the bleomycin. Weight gain steadily increased in all groups after day 8 and continued until the PFT on day 14. C = pre-bleomycin instillation; IV = intravenous injection; B = Bleomycin instillation; IV = intravenous infusion; P = Pulmonary function test; Group 1- Blm/Vehicle Control; Group 2- Blm/low dose MSC; Group 3- Blm/high dose MSC, Group 4- Control (representative normal healthy animals with no bleomycin exposure).
Table 1.
Animal body and organ weights
| Group (n) |
Body Weight |
Lung | Liver | Spleen | Kidney | Gonads | Heart | Brain | |
|---|---|---|---|---|---|---|---|---|---|
| B/V (7) | Mean(SD) | 278 (±21) | 2.56 (±0.38) | 11.52 (±1.81) | 0.63 (±0.10) | 1.07 (±0.11) | 1.84 (±0.10) | 1.01 (±0.11) | 1.62 (±0.12) |
| B/L (7) | Mean(SD) | 279 (±10) | 2.57 (±0.37) | 11.08 (±1.17) | 0.64 (±0.13) | 1.03 (±0.05) | 1.75 (±0.07) | 0.97 (±0.05) | 1.67 (±0.11) |
| B/H (8) | Mean(SD) | 275 (±22) | 2.68 (±0.40) | 11.50 (±0.98) | 0.66 (±0.10) | 1.07 (±0.14) | 1.78 (±0.09) | 1.18 (±0.32) | 1.59 (±0.21) |
No significant differences were noted between any groups for any organs. Weight is presented in grams. SD = standard deviation. Group 1- Bleomycin/Vehicle Control (B/V); Group 2- Bleomycin Low dose MSC (B/L); Group 3- Bleomycin High dose MSC (B/H). n= total number of samples/includes all animals that survived to day 15.
Pulmonary function test results
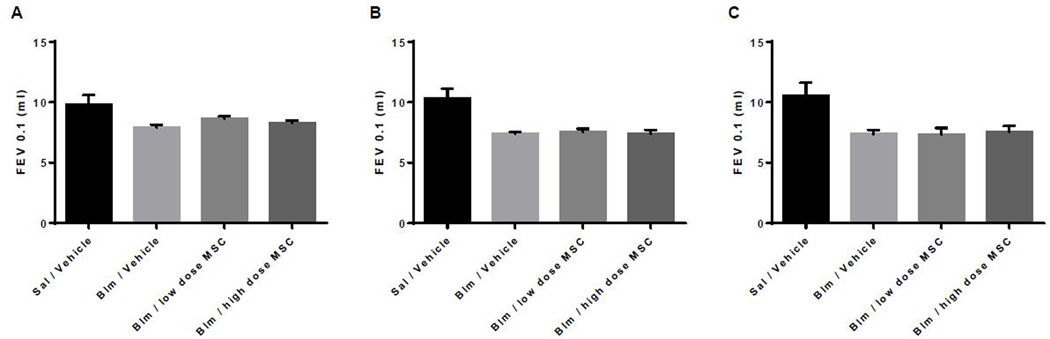
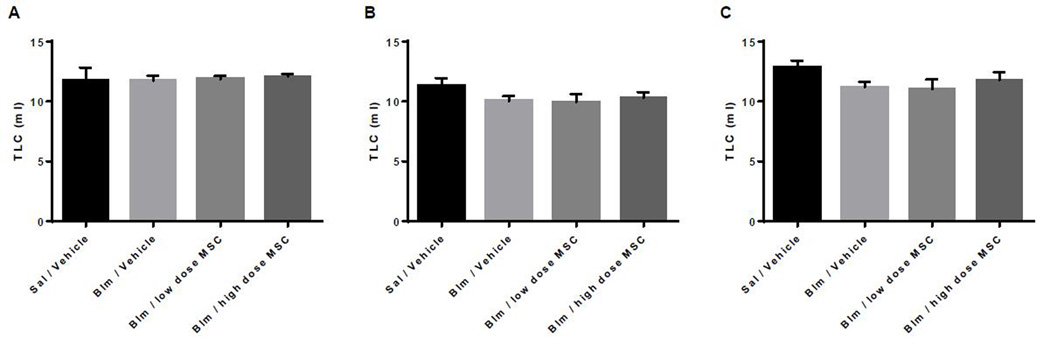
Respiratory rate did not change during the intravenous infusions nor was it different between the three groups during either of the two infusions. Pulmonary Function Tests (PFT) were performed at baseline on day 0, day 7 (3 days post second bleomycin dose) and on day 14. As shown in Figures 3 and 4, lung injury caused by the bleomycin-instillation was characterized by the loss of forced expiratory volume (FEV0.1) and total lung capacity (TLC). No difference was detected between the three bleomycin treated groups.
Figure 3. Graphic depiction of forced expiratory volume (FEV0.1; mL/0.1seconds) at days 0, 7, and 14.
Group 1- Sal/Vehicle (representative normal healthy animals with no bleomycin exposure), Group 2- Blm/Vehicle, Group 3- Blm/low dose MSC, Group 4- Blm/high dose MSC. Data presented at Baseline or day 0 (A), day 7 (B), and day 14 (C).
Figure 4. Total lung capacity (TLC) in mL at days 0, 7, and 14.
Group 1- Sal/Vehicle (representative normal healthy animals with no bleomycin exposure), Group 2- Blm/Vehicle, Group 3- Blm/low dose MSC, Group 4- Blm/high dose MSC. Data presented at Baseline or day 0 (A), day 7 (B), and day 14 (C).
Blood oxygen levels
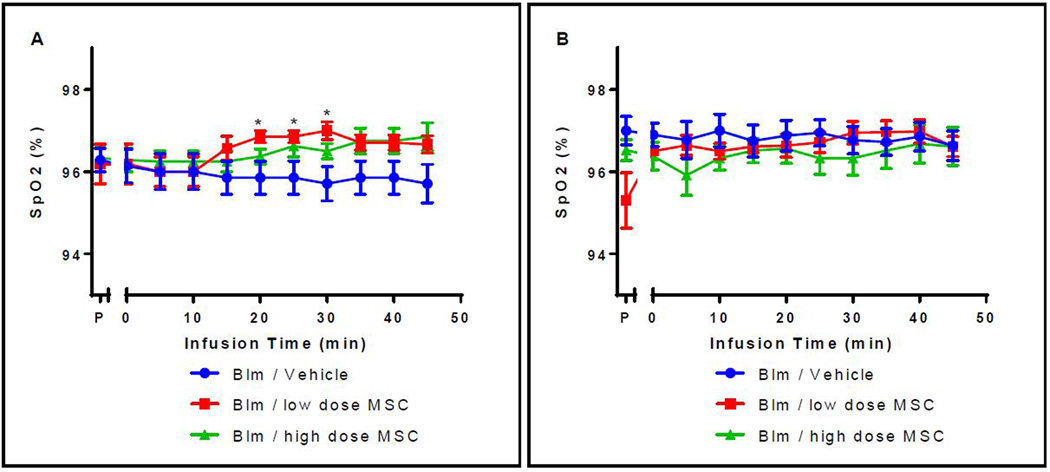
Saturation of peripheral oxygen (SpO2) was not different between groups at the start of the first intravenous infusion averaging 96.2 ± 0.9% and did not vary more than one percent throughout the course of first infusion (Figure 5A). Importantly, the SpO2 values were not significantly different between animals or groups during the second intravenous infusion starting at 96.3 ± 2.7% prior to infusion and ending at 96.6 ± 2.5% (Figure 5B).
Figure 5. Change in SpO2 values during the first (A) and second (B) intravenous infusion.
(A) After 20, 25, and 30 minutes there was a significant difference in saturated blood oxygen levels between the Blm/low MSC dose and the Blm/vehicle control groups (p=0.038, 0.038, 0.019 respectively). No other significant differences were detected between other time points or groups. P = pre-bleomycin instillation; Group 1- Blm/Vehicle Control; Group 2- Blm/low dose MSC; Group 3- Blm/high dose MSC. (B) After the second intravenous infusion, there were no significant differences in saturated blood oxygen levels either within groups or between groups at any of the time points evaluated. P = pre-bleomycin instillation; Group 1- Blm/Vehicle Control; Group 2- Blm/low dose MSC; Group 3- Blm/high dose MSC.
Heart rate and Right ventricular pressures
Heart rate remained steady throughout the 45 minute infusion and was not different between the three bleomycin treatment groups at either the first (255 ± 6.8 bpm) or the second intravenous infusion (304 ± 3.3 bpm).
Right ventricular (RV) systolic pressures were slightly higher in cell infused rats on Day 15 compared to the vehicle-infused rats at baseline but were not significantly different (p=0.331 and 0.215) (Figure 6A). RV systolic pressures did not change throughout the intravenous infusion. Diastolic pressures were also not different at baseline prior to intravenous infusion between groups (p=0.517 and 0.458) and remained steady during the infusions for all three groups and were not different between groups at any time (p>0.27) (Figure 6B).
Figure 6. Systolic and diastolic right ventricular pressure during second intravenous infusion.
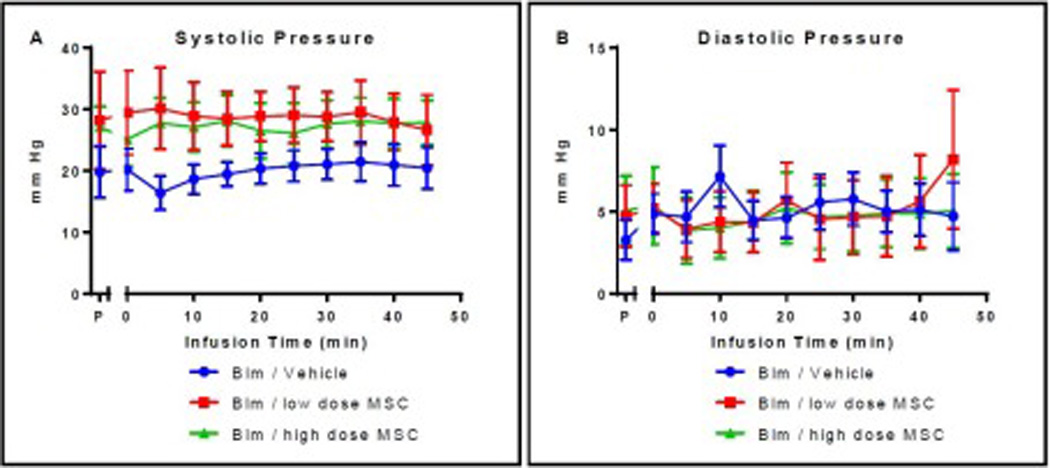
There were no significant differences in systolic (A) or diastolic (B) blood pressure at the time of the second intravenous infusion either within groups or between groups at any of the time points evaluated. P = pre-bleomycin instillation; Group 1- Bleomycin Vehicle; Group 2- Bleomycin Low dose MSC; Group 3- Bleomycin/High dose MSC.
Organ weights and histopathology
There were no significant differences in brain, heart, kidney, liver, lung, spleen, or gonad weights between bleomycin treated groups (Table 1). The mean lung weight of the bleomycin/vehicle control group was 2.56 ± 0.38 g, the low dose group was 2.57 ± 0.37 g, and high dose group was 2.68 ± 0.40 g; there is no statistical difference. Gross examination revealed hemorrhagic areas in the lungs all animals exposed to bleomycin.
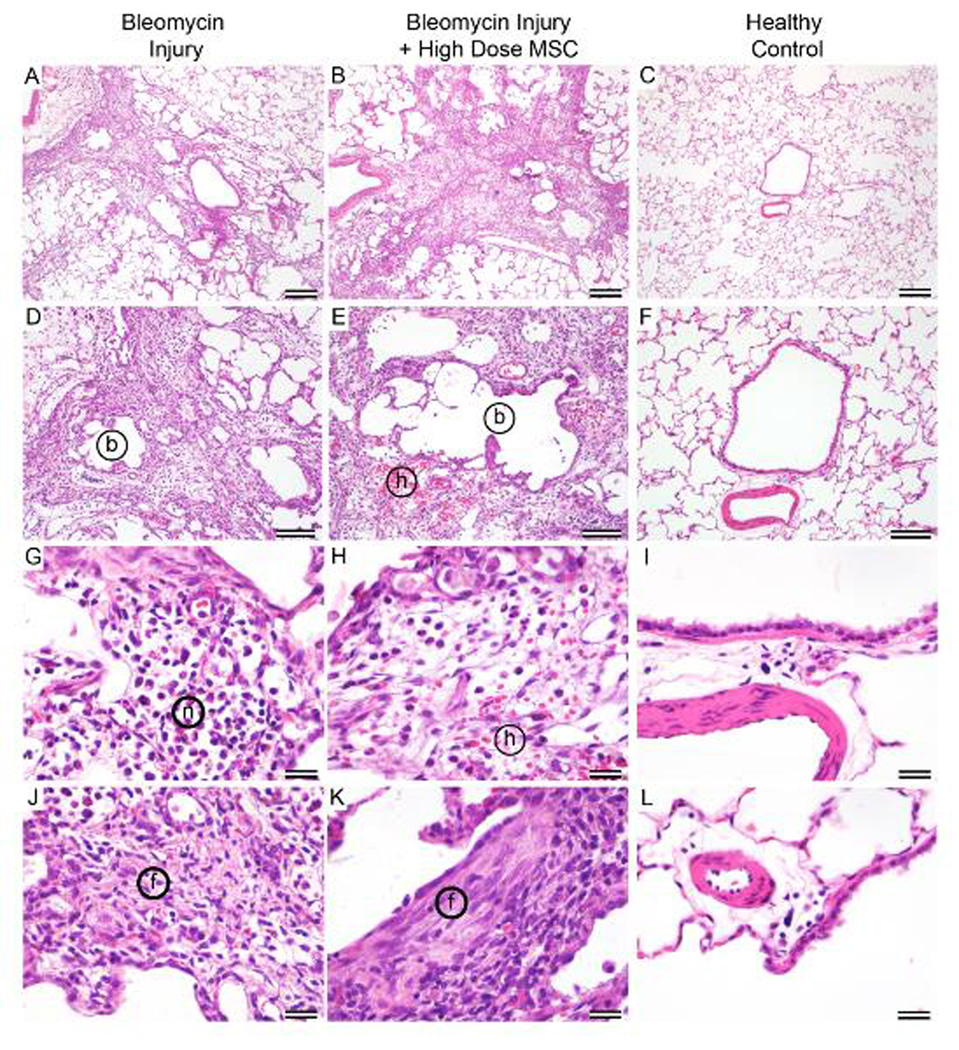
Histopathologic examination identified marked multifocal to coalescing lymphohistiocytic and neutrophilic interstitial inflammation with varying degrees of local edema and hemorrhage, bronchiectasis, alveolar dilation, type II cell hyperplasia, alveolar histiocytosis, and fibrosis. There were no differences in the incidence of any lesion between the three treatment groups. Microscopic examination of the left lung sections of bleomycin treatment groups identified foci of tissue consolidation with inflammatory cells and collagen deposition next to nearly normal alveolar areas (Figure 7). Bronchiectasis and dilated alveoli were common in all study rats (Figure 7A, 7B, 7D, 7E). Inflammatory cells were identified as lymphocytes, macrophages, and neutrophils (Figure 7G, 7H). Collagen deposition was noticeable in all areas of inflammation recognized by H&E staining (Figure 7A, 7B, 7J, 7K). Peribronchial areas of fibrosis often extended along terminal bronchioles and into alveolar spaces. Fibrosis was confirmed by examination of Trichrome-stained slides. All pulmonary lesions seen and described are consistent with the well-described reproducible lung injury model initiated by bleomycin (21, 24).
Figure 7. Pulmonary histology of injured and healthy lungs.
Histological comparison of lung tissue either exposed to bleomycin only (left column), bleomycin plus high cell dose (middle column), or representative healthy animal control (right column). Patchy areas of interstitial inflammation, edema, and fibrosis are similar in rat lungs treated with bleomycin (A) and bleomycin plus cells (B). Areas of airway dilation (bronchiectasis, b) are similarly rimmed with inflammation in rat lungs treated with bleomycin (D) and bleomycin plus cells (E). A small area of acute alveolar hemorrhage (h) is present in E. The inflammation consists of lymphocytes, histiocytes, and neutrophils (n) in G within an edematous (clear) interstitium in both lungs treated with bleomycin (G) and bleomycin plus cells (H). A small amount of acute hemorrhage (h) is present in E and H. Fibrosis (poorly cellular, eosinophilic, extracellular material, (f) is common and similar in inflamed areas of rat lungs treated with bleomycin (J) and bleomycin plus cells (K). Panels C, F, I, and L are form a healthy unmanipulated, non-study rat and are provided for comparison to normal rat lung.
Blood coagulation parameters
At the end of the study blood was analyzed from representative animals of each group for prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen concentration. Changes in these values may serve as early indicators of liver disease, development of thromboembolism, or anti-coagulation deficiency. No differences in PT or APTT times and fibrinogen concentration were observed between animals in any of the three represented bleomycin treated groups, suggesting no adverse effect of the cell infusion on the coagulation parameters in these animals (Table 2).
Table 2.
Blood coagulation parameters
| Group (n) | PT | APTT | Fibrinogen | |
|---|---|---|---|---|
| 1- Bleomycin/Vehicle (5) | Mean(SD) | 17.6 (±2.0) | 44.7 (±42.4) | 226.0 (±48.7) |
| 2- Bleomycin/Low MSC (5) | Mean(SD) | 19.8 (±2.9) | 44.2 (±36.8) | 251.4 (±115.9) |
| 3- Bleomycin/High MSC (6) | Mean(SD) | 19.3 (±3.0) | 46.5 (±36.6) | 257.5 (±167.5) |
No statistical differences between blood coagulation parameters were detected between samples of representative groups. Blood coagulation PT = Prothrombin Time in seconds; APTT = Activated Partial Thromboplastin Time in seconds. Fibrinogen concentration is provided in mg/dL. Group 1- Bleomycin Vehicle Control (B/V); Group 2- Bleomycin Low dose MSC (B/L); Group 3- Bleomycin High dose MSC (B/H). n= total number of samples. SD = standard deviation
Post-thaw cell viability
No significant differences were detected between the starting viability of the freshly thawed cell product compared to the three cell fractions that were collected over a 40 minute period. The starting viability was determined to be approximately 85% and no cell clumping was observed.
Discussion
The primary goal of this study was to develop and characterize a reliable and representative preclinical animal model of lung injury for use in demonstrating the safety of intravenous MSC administration to support an IND application. Repeated bleomycin instillations successfully induced a lung injury model that reasonably mimics human disease (BO) on both gross and microscopic levels. A common limitation of lung injury models is spontaneous recovery; our rat model demonstrates persistent changes in pulmonary function, thereby improving its utility. Finally, we evaluated the safety of intravenous administration of MSCs in a compromised lung model and did not observe any acute adverse effects of cell administration on cardiopulmonary function.
This study was specifically designed to demonstrate safety by infusing MSCs at two methodical points representative of the most vulnerable stages of lung injury; one during the pro-inflammatory stage and then again during the pro-fibrotic stage of the injury. Our repeated dose bleomycin instillation method resulted in persistent lung injury which included the lymphohistiocytic and neutrophilic interstitial pneumonia with bronchiectasis, alveolar dilation, and mild obvious fibrosis. These characteristics are consistent with those reported in the bleomycin two-dose mouse and three-dose rat models (15, 18, 25). Intratracheal instillation of bleomycin causes inflammatory and fibrotic reactions within a few days. The first seven days post-bleomycin instillation is marked by increases in pro-inflammatory cytokines, which is then followed by pro-fibrotic markers that peak around day 14 (26). The fibrotic stage may persist for an additional 1–2 weeks beyond that. During this stage there is increased deposition of extracellular matrix (collagen) leading to areas or patches of fibrosis. We successfully demonstrated recapitulation of persistent lung injury and used this model to confirm the safety of infusing MSCs at successive time points during the characteristic pro-inflammatory and pre-fibrotic stages of lung injury.
Full physiological characterization of pulmonary disease in animal models of stem cell therapy often lacks appropriate clinically relevant endpoints, specifically pulmonary function testing (27). Therefore, we characterized our double dose bleomycin rat lung injury model utilizing clinically relevant pulmonary function tests, which has not been previously reported. In this study, we found a significant decrease in total lung capacity (TLC) and detected a significant reduction in FEV 0.1 which is an expected result in lungs with decreased compliance due to inflammation and fibrosis. Bleomycin is known to cause a significant reduction in forced vital capacity (FVC) in some single dose mouse models (28) but other studies report no change in FVC or FEV (27). These differences could be animal model-dependent or the result of timing of evaluation after bleomycin instillation. We did not detect noticeable or statistical differences in cardiopulmonary function between the bleomycin low and bleomycin high cell dose treatment groups tested. We also did not detect a drop in SpO2 with bleomycin treatment, which is contrary to other studies (29); however, our SpO2 evaluation was performed on animals ventilated with 100% oxygen rather than room air, which likely explains this difference.
Using this rat lung injury model we tested the safety of multiple intravenous infusions of human MSCs. As anticipated, all animals tolerated multiple intravenous infusions of low and high dose MSCs with no reportable adverse effects on body weight, food or water consumption, and body appearance. As you might expect pulmonary function was severely reduced by the bleomycin treatment, but MSC infusions did not similarly negatively impact pulmonary function. Although our study could not show that administration of human cells in this lung injury model improved pulmonary function it did demonstrate that infusion of MSCs in animals with injured or compromised lungs is safe. Importantly, blood oxygenation, breathing, and heart rate remained unchanged during the cell infusions. Critically, the expected and observed pulmonary hypertension induced by bleomycin was not further exacerbated by the MSC infusion.
The safety findings of this study are consistent with a large volume of published animal studies indicating that systemic infusion of MSC is a safe procedure (30–33). This study was designed to include a generous margin of error; the high cell dose of 5.6 × 106 cells/kg used in this preclinical safety study is higher than a typical dose used in human clinical studies but did not result in adverse events attributed to the cellular product dose, route of administration, or administration schedule. Importantly, we did not find an increase in the right ventricular pressure or any decrease in SpO2 following cellular product administration. It should be noted that this severe lung injury model was developed to evaluate safety of human cell infusions rather than the ability for the cells to reverse the effects of lung injury. However, we continue to hypothesize that our cellular product may minimize or prevent the progression of lung injury which will be one of the endpoints in a human clinical trial. The short-term follow up of our study animals may be considered a limitation of this study if a long-term effect of MSC infusion is of interest.
In summary, exposure of bleomycin-instilled rats to two infusions of human MSCs seven days apart using a maximum cell dose equivalent of 5.6 × 106 cells/kg was not associated with any unexpected adverse cardiopulmonary hemodynamic findings. There were also no abnormalities in general appearance, health, or appearance of any organ system evaluated in any test animal different from the expected effects of bleomycin as demonstrated in the control group. Also, there were no histological pathology findings that were attributed to the infusion of MSCs in this study. We therefore conclude that intravenous infusion of MSC into rats with damaged lungs is safe under these experimental conditions tested.
Acknowledgments
This work was supported by Production Assistance for Cellular Therapies (PACT) program from NIH/NHLBI at the University of Wisconsin- Madison Waisman Biomanufacturing (PACT Contract # HHSN268201000010C).
Abbreviations
- MSC
Mesenchymal Stromal Cells
- BO
Bronchiolitis Obliterans
- PFT
Pulmonary Function Test
- FEV
Forced Expiratory Volume
- Blm
Bleomycin
- TLC
Total Lung Capacity
- FVC
Forced Vital Capacity
- BALT
Bronchial Associated Lymphoid Tissue
- PT
Prothrombin Time
- APTT
Activated Partial Thromboplastin Time
- RV
Right Ventricular
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995 Oct;16(4):557–564. [PubMed] [Google Scholar]
- 2.Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11(5):503–515. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindblad RW, Ibenana L, Wagner JE, McKenna DH, Jr, Hei DJ, Hematti P, et al. Cell therapy product administration and safety: data capture and analysis from the Production Assistance for Cellular Therapies (PACT) program. Transfusion. 2015 Mar;55(3):674–679. doi: 10.1111/trf.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014 May;54(5):1418–1437. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galipeau J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013 Jan;15(1):2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stabler CT, Lecht S, Lazarovici P, Lelkes PI. Mesenchymal stem cells for therapeutic applications in pulmonary medicine. British medical bulletin. 2015 Sep;115(1):45–56. doi: 10.1093/bmb/ldv026. [DOI] [PubMed] [Google Scholar]
- 8.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009 Jun;18(5):683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009 Jul 2;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung JW, Kwon M, Choi JC, Shin JW, Park IW, Choi BW, et al. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei medical journal. 2013 Sep 1;54(5):1293–1296. doi: 10.3349/ymj.2013.54.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G, et al. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PloS one. 2009;4(11):e8013. doi: 10.1371/journal.pone.0008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grove DA, Xu J, Joodi R, Torres-Gonzales E, Neujahr D, Mora AL, et al. Attenuation of early airway obstruction by mesenchymal stem cells in a murine model of heterotopic tracheal transplantation. J Heart Lung Transplant. 2011 Mar;30(3):341–350. doi: 10.1016/j.healun.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manning E, Pham S, Li S, Vazquez-Padron RI, Mathew J, Ruiz P, et al. Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum Gene Ther. 2010 Jun;21(6):713–727. doi: 10.1089/hum.2009.147. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jun 26;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America. 2003 Jul 8;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplantation proceedings. 2008 Jun;40(5):1700–1705. doi: 10.1016/j.transproceed.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 17.Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009 May;77(3):370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Pinart M, Serrano-Mollar A, Llatjos R, Rocco PR, Romero PV. Single and repeated bleomycin intratracheal instillations lead to different biomechanical changes in lung tissue. Respir Physiol Neurobiol. 2009 Mar 31;166(1):41–46. doi: 10.1016/j.resp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Williams JH, Jr, Bodell P, Hosseini S, Tran H, Baldwin KM. Haemodynamic sequelae of pulmonary fibrosis following intratracheal bleomycin in rats. Cardiovascular research. 1992 Apr;26(4):401–408. doi: 10.1093/cvr/26.4.401. [DOI] [PubMed] [Google Scholar]
- 20.Chung MP, Monick MM, Hamzeh NY, Butler NS, Powers LS, Hunninghake GW. Role of repeated lung injury and genetic background in bleomycin-induced fibrosis. Am J Respir Cell Mol Biol. 2003 Sep;29(3 Pt 1):375–380. doi: 10.1165/rcmb.2003-0029OC. [DOI] [PubMed] [Google Scholar]
- 21.Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 2011 Sep;17(5):355–361. doi: 10.1097/MCP.0b013e328349ac2b. [DOI] [PubMed] [Google Scholar]
- 22.Williamson JD, Sadofsky LR, Hart SP. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp Lung Res. 2015 Mar;41(2):57–73. doi: 10.3109/01902148.2014.979516. [DOI] [PubMed] [Google Scholar]
- 23.Bloom DD, Centanni JM, Bhatia N, Emler CA, Drier D, Leverson GE, et al. A reproducible immunopotency assay to measure mesenchymal stromal cell-mediated T-cell suppression. Cytotherapy. 2015 Feb;17(2):140–151. doi: 10.1016/j.jcyt.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones AW. Bleomycin lung damage: the pathology and nature of the lesion. British Journal of Diseases of the Chest. 1978;72(4):321–326. [PubMed] [Google Scholar]
- 25.Huang K, Kang X, Wang X, Wu S, Xiao J, Li Z, et al. Conversion of bone marrow mesenchymal stem cells into type II alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats. Molecular medicine reports. 2015 Mar;11(3):1685–1692. doi: 10.3892/mmr.2014.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med. 2006 Apr 1;173(7):769–776. doi: 10.1164/rccm.200505-717OC. [DOI] [PubMed] [Google Scholar]
- 27.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010 Jan;42(1):96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 28.Kurotsu S, Tanaka K, Niino T, Asano T, Sugizaki T, Azuma A, et al. Ameliorative effect of mepenzolate bromide against pulmonary fibrosis. J Pharmacol Exp Ther. 2014 Jul;350(1):79–88. doi: 10.1124/jpet.114.213009. [DOI] [PubMed] [Google Scholar]
- 29.Andrianifahanana M, Wilkes MC, Gupta SK, Rahimi RA, Repellin CE, Edens M, et al. Profibrotic TGFbeta responses require the cooperative action of PDGF and ErbB receptor tyrosine kinases. FASEB J. 2013 Nov;27(11):4444–4454. doi: 10.1096/fj.12-224907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzouvelekis A, Paspaliaris V, Koliakos G, Ntolios P, Bouros E, Oikonomou A, et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med. 2013;11:171. doi: 10.1186/1479-5876-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013 Jun;143(6):1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011 Aug;20(8):1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 33.Quimby JM, Webb TL, Habenicht LM, Dow SW. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther. 2013;4(2):48. doi: 10.1186/scrt198. [DOI] [PMC free article] [PubMed] [Google Scholar]