Abstract
Background
Chemotherapy administration and supportive management for solid tumors is intended to take place in the ambulatory setting, but little is known about why some patients experience treatment-related, adverse events so severe as to require acute inpatient care.
Objective
Identify predictors of initial and repeated unplanned hospitalizations and potential financial impact among Medicare patients with early-stage (stages I–III) colorectal cancer receiving outpatient chemotherapy.
Methods
Advanced statistical modeling was used to analyze a cohort of patients (N = 1485) from the Surveillance, Epidemiology and End Results (SEER)–Medicare database diagnosed from 2003–2007 with colorectal cancer as their first primary malignancy. Patients were age 66 and older at diagnosis, had uninterrupted Medicare Parts A and B coverage with no health maintenance organization (HMO) component, and received chemotherapy at least one time.
Results
Female sex, younger age, multiple comorbidities, rural geography, higher high school completion rates, and lower median income per census tract were significant predictors of the likelihood of initial unplanned hospitalizations. Non-White race, receipt of radiation therapy, rural geography and higher weighted comorbidity scores were factors associated with the number of hospitalizations experienced. The total Medicare charges calculated for these admissions was $38,976,171, with the median charge per admission at $20,412.
Discussion
Demographic and clinical factors were identified that form the foundation of work towards development of a risk factor profile for unplanned hospitalization. Further work is needed to incorporate additional clinical data to create a clinically applicable model.
Keywords: colorectal neoplasms, comorbidity, chemotherapy, hospitalization, outpatients, SEER-Medicare
Quality and value are critical to processes and outcomes in effective and efficient health systems. Unplanned and potentially avoidable hospitalization events may signal lapses in quality and contribute to diminished value. Factors associated with unplanned and potentially avoidable hospitalization are not well understood.
In oncology, chemotherapy for solid tumors (such as colorectal cancers) is intended to be administered and managed primarily in the outpatient setting once initial surgery to resect disease is completed; however, a proportion of patients will experience treatment-related symptoms so severe as to disrupt therapy and require inpatient care to resolve. The therapeutic goal in early-stage (stages I–III) disease is to cure or achieve long-term disease remission, and therefore every attempt is made for this population to provide full doses of drug on the prescribed schedule. The average age at colorectal cancer diagnosis is 69 (Howlader et al., 2015), and though older adults typically experience the highest proportion of cancer diagnoses annually, they tend to be underrepresented in clinical trials, limiting knowledge about how adverse events are precipitated and experienced by this population. This study uses the National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results (SEER)–Medicare database to develop a cohort of older adult patients with early-stage colorectal cancer to identify factors associated with both an initial unplanned hospitalization as well as repeated readmissions over the course of chemotherapy administration. The use of a large dataset such as SEER–Medicare allows exploration of “real world” data outside of the clinical trial setting, and a longitudinal view of care across settings ranging from prior to cancer diagnosis and throughout the treatment trajectory.
Related Literature
Unplanned Hospitalization
Factors associated with the initial unplanned hospitalization in a population intended to receive care exclusively in the outpatient setting may be distinct from readmission, where a patient is discharged from an inpatient stay, then must return to the hospital for further planned or unplanned inpatient care (Fessele & Atkins, 2012; Jencks, Williams, & Coleman, 2009; Mulder, Tzeng, & Vecchioni, 2012). Unplanned hospitalization is defined as “an unexpected admission for management of a severe disease or treatment-related event that cannot be controlled in the outpatient setting” (Fessele & Atkins, 2012), and for this study, it was limited to those occurring during the period during which the patient is receiving chemotherapy. Patients with metastatic or stage IV disease were excluded from this analysis to minimize the inclusion of hospitalizations related to cancer progression.
As data regarding these events are limited for this population, larger scale studies are needed to identify factors related to the incidence of initial, unplanned hospitalization and multiple readmissions in the patients intended to be treated exclusively in the outpatient setting. Any unplanned hospitalization may present a significant clinical disruption in regards to the ability to maintain potentially curative chemotherapy dose and schedule, exposure to possible nosocomial and iatrogenic complications, financial burdens associated with an inpatient stay, and the impact on the patient and family’s quality of life (Aparicio et al., 2013; Calfee, 2012).
Colorectal cancer was selected as the focus of this study as this tumor type has been described among the most frequently admitted in several reviews (Grant, Ferrell, Rivera, & Lee, 1995; Hassett et al., 2011; Weaver et al., 2006). Reports from single institution-based studies in patients with colorectal cancer indicate that higher (stages III and IV) disease stage, receipt of chemotherapy or radiation therapy, multiple (three or more) comorbidities, and inadequate support in the home are associated with unplanned hospitalizations (González et al., 2005; Hassett et al., 2011; Weaver et al., 2006).
Prior large dataset studies exploring predictors of unplanned hospitalizations among patients with several cancers also found that the presence of multiple comorbidities, higher disease stage (Nurgalieva, Liu, & Du, 2009), unmarried status, race, and geographic location (Du, Osborne, & Goodwin, 2002) were significant predictors of unplanned hospitalization. In addition, higher rates of toxicity among older adults compared to those reported in clinical trials associated with the administered regimens were noted (Du et al., 2002; Hassett, O’Malley, Pakes, Newhouse, & Earle, 2006).
Though current geriatric oncology guidance recommends assessment of a patient’s physiologic rather than chronologic age to determine appropriate use of chemotherapy for older adults, it is acknowledged that some major organ function changes do occur with time, such as decreased renal and bone marrow function (Extermann et al., 2002), possibly impacting the incidence and severity of adverse events that might interrupt treatment. Underrepresentation of older adults in clinical trials limits the data available to interpret risks to this group (Hurria et al., 2015).
There is evidence that sex may play a role in determining the degree of toxicity experienced. Females receiving chemotherapy for colorectal cancers have been noted to experience higher rates of myelosuppression, diarrhea and mucositis in several studies (González et al., 2005; Nottage et al., 2003; Pal & Hurria, 2010; Sloan et al., 2002; Zalcberg, Kerr, Seymour, & Palmer, 1998). This may be related to biologic differences in clearance of 5-fluorouracil—the most commonly administered drug—though the exact mechanism underlying this observation is unclear.
Purpose
This study focuses on patients receiving chemotherapy for early-stage disease for colorectal cancer intended to be delivered exclusively in the outpatient setting, and the identification of predictors of both initial hospitalization and multiple readmissions. This work fills an important gap in literature, as no data were found addressing these problems in this population. The first aim examined which demographic and clinical factors were associated with an initial, unplanned hospitalization in patients with early-stage colorectal cancer in the SEER-Medicare linked database. The second aim studied which factors were associated with the number of readmissions for treatment-related, serious adverse events in these patients. An exploratory aim was to identify the potential financial impact of these unplanned hospitalizations.
Methods
Data Source
Data for this study originate from the SEER–Medicare linked database, which combines information from two sources: the National Cancer Institute’s SEER program and the Center for Medicare and Medicaid Services (CMS) claims data through a linking process to allow researchers to view clinical and administrative data for a single patient across time and settings of care (Warren, Klabunde, Schrag, Bach, & Riley, 2002). The SEER program has collected data on incident cancer cases diagnosed within 17 cancer registries across the United States since 1973, capturing approximately 28% of all national cases (NCI, 2012). SEER data includes patient demographics, cancer type, stage, initial surgical and radiation treatments (but not specific chemotherapy regimens), and survival status, and the quality of data is considered highly valid according to the North American Association of Central Cancer Registries (Bray & Parkin, 2009).
Medicare, a federally administered health insurance program, covers approximately 93% of Americans over age 65 (U.S. Department of Health and Human Services, 2014), which automatically includes Part A benefits that provide for hospital and skilled-nursing facility costs, as well as hospice and some other home health services. About 96% of covered beneficiaries choose to obtain Part B benefits, which cover physician and outpatient services. Parts C and D optional benefits cover Health Maintenance Organization (HMO) plans for which CMS is payor, and prescription drug coverage, respectively (CMS, 2012).
The SEER–Medicare database comprised several file types, each generated from a separate source. Table 1 illustrates the main file types in this study, including the unit of measurement in each file. The Patient Entitlement and Diagnosis Summary File (PEDSF) originates from the SEER registry information, and is formatted as one observation per patient case. The availability of PEDSF data at the start of this study included cases diagnosed with colorectal cancer through 2007, with claims-derived data for each case through 2009. For example, the record of a patient diagnosed with colorectal cancer in 2005 was found in the PEDSF file for that tumor type and year, and included all of the SEER registry information. A researcher then explored the patient’s associated Medicare-derived files from 2004 (prior to the cancer diagnosis to identify pre-existing comorbid conditions) through the end of available data in 2009.
TABLE 1.
SEER-Medicare File Types Utilized
| File type | Content | Data | Source | Coding comment |
|---|---|---|---|---|
| PEDSF | Demographics | Age | SEER Registry | Continuous |
| Sex | SEER Registry | Categorical | ||
| Race | SEER Registry | Categorical | ||
| Marital status | SEER Registry | Categorical | ||
| Cancer diagnosis | ICD-O-3a | SEER Registry | Categorical | |
| Cancer stage | SEER Registry | Categorical | ||
| Local SEER Registry | SEER Registry | Categorical | ||
| Diagnosis date | SEER Registry | Transformed: SAS datesb | ||
| Initial treatment | Radiation therapy | SEER Registry | Categorical | |
| Census tract | Education | Census 2000 | Non-HS graduates (%) | |
| Income | Census 2000 | Mdn | ||
| Medicare coverage | Parts A, B, or C (months) | Medicare | Categorical | |
| Urbanization | Population density | 2004 ARF | Categoricalc | |
| NCH | Outpatient claimsd | Dates of caree | Medicare | Transformed: SAS datesb |
| Chemotherapy | Medicare | Categoricalf | ||
| ICD-9-CMg | Medicare | Continuous | ||
| OUTPT | Outpatient claimsh | Dates of caree | Medicare | Transformed: SAS datesb |
| Chemotherapy | Medicare | Categoricalf | ||
| ICD-9-CMg | Medicare | Continuous | ||
| MEDPAR | Inpatient servicesi | Dates of carej | Medicare | Transformed: SAS datesb |
| ICD-9-CMg | Medicare | Continuous |
Note. ARF = Area Resource File; Mdn = median; MEDPAR = Medicare Provider Analysis and Review; NCH = National Carrier History ; OUTPT = Outpatient; PEDSF = Patient Entitlement and Diagnosis Summary File; SEER = Surveillance, Epidemiology, and End Results.
ICD-O-3 = International Classification of Diseases for Oncology, 3rd Edition.
Calendar dates were transformed to SAS date values (number of days since January 1, 1960).
Big Metro = Counties of metro areas of 1 million population or more; Metro/Urban = Urban population of 20,000 through Counties in metro areas of up to 1 million population; Less Urban/Rural = Completely rural, or less than 2,500 urban population through Urban population up to 19, 999.
Provider and Medicare Part B claims from nonhospital, outpatient settings.
Date outpatient care service rendered.
Healthcare Common Procedure Coding System (HCPCS) J codes.
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Provider and Medicare Part B claims from hospital-based outpatient settings.
Medicare Part A claims for inpatient services.
Admission and discharge dates.
The Medicare Provider Analysis and Review (MEDPAR) File is derived from the Medicare Part A claims data generated during a hospitalization. Each observation within MEDPAR represents a single hospital stay for a SEER–Medicare patient. The National Carrier History (NCH) and Outpatient (OUTPT) files describe services such as provider visits and treatments administered in the ambulatory setting. NCH data represents provider claims from physicians, nurse practitioners, and physician assistants empaneled as independent Medicare providers across settings and specialties, as well as claims from laboratory and freestanding ambulatory care centers. OUTPT data represents similar claims from the outpatient departments within hospitals, and are separate in nature and structure from the MEDPAR claims.
Each observation in the NCH and OUTPT files represents a single-billed line item. Using a hypothetical scenario to illustrate a one-day chemotherapy administration in a private oncologist’s office; the researcher might note several separate observations for the same date in the NCH file, including the provider exam, a complete blood count to assure patient eligibility prior to treatment, each individual drug charge, and an administration charge (Lamont et al., 2005). The initial variable in the PEDSF, MEDPAR, NCH, and OUTPT files is the patient identification (ID) assigned by SEER-Medicare, allowing desired data to be obtained for a particular patient as he or she receives care and generates claims to Medicare at various locations over time.
Population and Cohort Construction
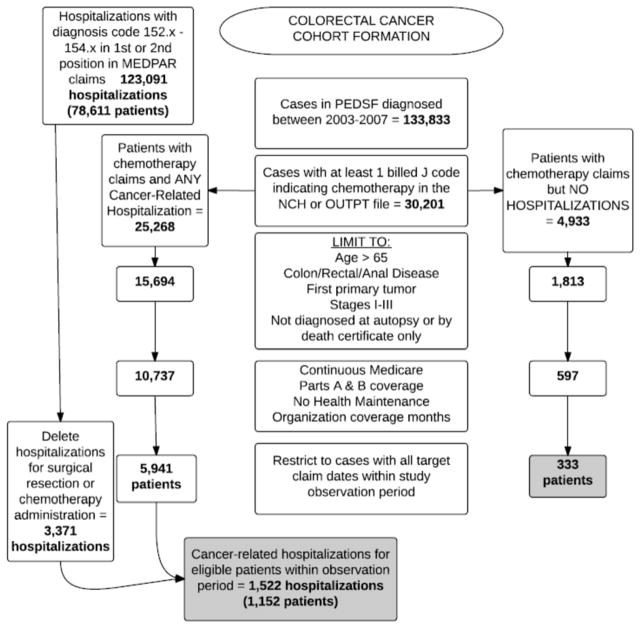
The Rutgers University Institutional Review Board deemed use of the SEER–Medicare database as nonhuman subject research, and the study was authorized to proceed. Files were initially obtained inclusive of the entire population of Medicare-eligible patients diagnosed in contributing SEER registry areas with the desired cancer type for the years requested. Cases were retained initially if the patient had stage I–III colorectal cancer diagnosed in the years 2003 through 2007, was 66 years of age or greater at the time of cancer diagnosis, and had continuous Parts A and B Medicare coverage during the period of observation—but no participation in Part C (HMO). Cases diagnosed on autopsy or by death certificate only were excluded (see Figure 1 for cohort construction numbers). No sampling occurred so as to capture the entire possible population eligible for inclusion.
FIGURE 1.
Formation of the colorectal cohort. SEER-Medicare files initially contain the entire population of all patients with the requested tumor type diagnosed in contributing SEER sites across the nation. Eligibility and other study criteria quickly reduce the remaining population available for analysis.
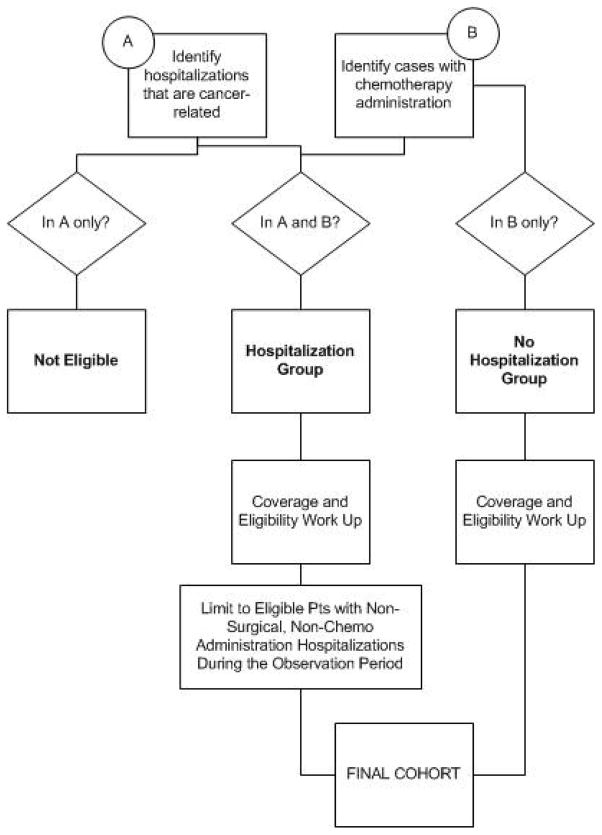
Cohort construction was designed to identify all eligible patient cases with early-stage colorectal cancer who received chemotherapy in the ambulatory setting, including those who experienced a subsequent hospitalization within 30 days of treatment administration. Patients who received chemotherapy were identified by searching the multiyear ambulatory claims files (NCH and outpatient) for observations with a billing code containing a J9 value, which designates chemotherapy agents (Lamont et al., 2005). Cancer-related hospitalizations (as opposed to hospitalizations for noncancer conditions in patients with a history of cancer) were identified by searching the multiyear hospital claims file (MEDPAR) for the tumor type of interest in either the first or second position of 10 possible International Classification of Diseases (ICD)-9-CM diagnostic codes assigned to the admission (Mayer, Travers, Wyss, Leak, & Waller, 2011). When a patient case was located in both “received chemotherapy” and “had a cancer-related hospitalization” file searches, that case was assigned to the “hospitalized” group (Figure 2). When codes for chemotherapy administration or a surgical procedure were found in a MEDPAR file observation, the hospitalization was removed from final analysis to restrict the events of interest to unplanned hospitalizations likely to be related to treatment-related, adverse events rather disease progression within the cohort or staging surgeries. Where a case was found in the “received chemotherapy” file only, that patient was assigned to the “no hospitalization” group.
FIGURE 2.
Logic model illustrating flow of cases into Hospitalized or Nonhospitalized groups within the colorectal cohort.
Medicare Coverage
To optimize the capture of billed claims and the associated diagnostic coding information, SEER–Medicare researchers typically apply what is referred to as “most likely to have (consistent) claims” criteria (Warren et al., 2002). By ensuring uninterrupted Medicare Parts A and B coverage, with no transfer into a Medicare HMO product over the course of the study, the researcher is most able to detect all diagnostic codes and claims for a patient as they receive services from providers and institutions that accept this insurance. The key observation time periods for this study included a one-year period prior to cancer diagnosis to identify pre-existing comorbidities, and a hospitalization observation period, ranging from the day after the first chemotherapy administration (to avoid missed data resulting from the method Medicare uses when a patient receives both outpatient and inpatient claims on the same date) through 30 days after the last chemotherapy administration on record (Figure 3).
FIGURE 3.
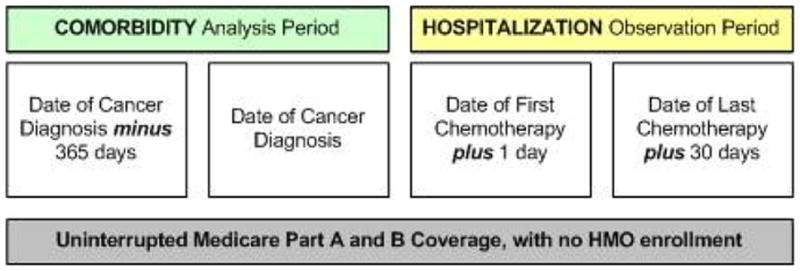
Key dates defining the study observation periods.
Cases remaining with “no hospitalizations” at this point formed that final group segment for comparative analysis (n = 333). The “all cancer-related hospitalizations” file was then restricted to include only admissions associated with dates within the hospitalization observation period for the eligible patient cases. The remaining observations formed the final hospitalization group for analysis (Figure 1). Table 2 describes the characteristics of nonhospitalized and hospitalized groups that composed this cohort.
TABLE 2.
Cohort Characteristics
| Hospitalized (n = 1152)
|
Nonhospitalized (n = 333)
|
|||||
|---|---|---|---|---|---|---|
| Characteristic | M | (SD) | Range | M | (SD) | Range |
| Age at diagnosis (yrs) | 77.3 | (5.0) | 66–97 | 78.9 | (5.7) | 66–97 |
| Educationa | 19.9 | (13.4) | 0–68.8 | 19.0 | (13.0) | 0.71–71.7 |
| n | %b | n | %b | |||
| Sex (female) | 647 | (56.2) | 132 | (39.6) | ||
| Comorbidity scorec | ||||||
| 0 | 477 | (41.4) | 198 | (59.5) | ||
| 1 | 300 | (26.0) | 83 | (24.9) | ||
| 2 | 197 | (17.1) | 33 | (9.9) | ||
| ≥3 | 178 | (15.4) | 19 | (5.70) | ||
| Range (pre-recode) | 0–9 | 0–5 | ||||
| Married (yes) | 630 | (54.7) | 176 | (52.9) | ||
| Urbanization | ||||||
| Big metro | 618 | (53.7) | 181 | (54.4) | ||
| Metro/urban | 386 | (33.5) | 126 | (37.8) | ||
| Less urban/rural | 147 | (12.8) | 26 | (7.8) | ||
| SEER Registry Region | ||||||
| New Jersey | 249 | (21.6) | 79 | (23.7) | ||
| Westd | 317 | (27.5) | 114 | (34.2) | ||
| Southe | 262 | (22.7) | 60 | (18.0) | ||
| Mid/Northeastf | 324 | (28.1) | 80 | (24.0) | ||
| Race (White) | 1025 | (89.0) | 289 | (87.0) | ||
| Incomeg | 45333 | 7–200008 | 45857 | 7887–200008 | ||
| Disease stage | ||||||
| 0 | 4 | (0.4) | 27 | (8.1) | ||
| I | 101 | (8.8) | 102 | (30.6) | ||
| II | 294 | (25.5) | 43 | (12.9) | ||
| III | 658 | (57.1) | 67 | (20.1) | ||
| Unknownh | 95 | (8.3) | 94 | (28.2) | ||
| Radiation treatment | ||||||
| External beam | 280 | (24.8) | 100 | (30.6) | ||
| Nonei | 849 | (75.2) | 227 | (69.4) | ||
| Chemotherapyj | ||||||
| 5-Fluorouracil | 1010 | (87.7) | 166 | (49.9) | ||
| Oxaliplatin | 586 | (50.9) | 61 | (18.3) | ||
| MAbs | 439 | (38.1) | 62 | (18.6) | ||
| Irinotecan | 295 | (25.6) | 23 | (6.9) | ||
Note. MAbs = monoclonal antibodies.
Census tract percentage of non-high school graduates.
Percentages based on column totals.
NCI Combined Index.
California, Hawaii, Seattle.
Kentucky, Louisiana, Georgia.
Michigan, Connecticut, Iowa, New Mexico, Utah.
Census tract median income.
Not missing.
Includes refused.
Patients generally received more than one drug class during the study period.
Comorbidity Analysis
Both hospitalized and nonhospitalized cases underwent weighted comorbidity analysis, utilizing the NCI Combined Index (Klabunde, Legler, Warren, Baldwin, & Schrag, 2007) to provide a weighted comorbidity score for each patient case. The index extends the classic Charlson Comorbidity Index (CCI; Charlson, Pompei, Ales, & MacKenzie, 1987) to study designs that utilize administrative data generated from both the inpatient and outpatient areas. The presence (initially assigned a score of 1) or absence (assigned a score of 0) of 14 noncancer conditions is detected from claims data. Each condition score is then multiplied by a coefficient estimate for two-year, noncancer mortality through use of a Cox proportional hazards model derived during method development (Klabunde, Potosky, Legler, & Warren, 2000). The weighted scores are then summed to provide a single value.
Analytic Methods
Data were available from 16 NCI-SEER registries. Based on geographical considerations, these data were grouped into four SEER registry regions. In order to properly account for geographical differences, and the resulting within region correlations that may occur with cases from the same region, population averaged statistical models were estimated using generalized estimating equations (GEE). Missing data were minimal, affecting less than 30 cases where information on receipt of radiation was not documented. These cases were coded as if they did not receive or refused radiation in order to retain them in the overall analysis.
GEE is a statistical modeling technique that builds on the classical generalized linear model to allow for within region correlated data (Liang & Zeger, 1986). For the first study aim, factors associated with the initial admission, the method was used with a binomial distribution and logit link to predict the probability of a “case/event” (i.e., hospitalization) as a linear function of predictors, in a similar manner to logistic regression. However, the variance of the binary response was adjusted for the likelihood that cases from the same region are more similar. Results are interpreted in terms of odds ratios, giving the likelihood of hospitalization versus nonhospitalization for each independent variable. For the second study aim, the GEE model with a Poisson distribution and log link was used to predict the number of hospitalizations, conditional on at least one hospitalization occurrence. Results are interpreted using an incidence rate (Rothman, 2002). Data step programming in SAS version 9.3 was used to perform data management, integration, and manipulation. Statistical modeling was completed with the PROC GENMOD SAS procedure.
After assessing the characteristics and frequency distributions of the independent variables, bivariate models were fit to assess the association between each independent variable with the dependent variable. A nominal level of significance of .05 was used. In the model-building steps, a level of significance of .15 was used, and independent variables meeting this criterion in bivariate analyses were retained in further modeling stages. Advanced statistical modeling was then performed. After considering independent variables that were known to be associated with hospitalization, and including in each model by default regardless of statistical significance, we proceeded through an extensive model selection procedure. Two statistical criteria were considered in model building and selection. A two-fold approach using statistical (p-values) and the quasi-likelihood under the independence model criterion (QIC) goodness of fit statistic (Pan, 2001) were used at each step of modeling to aid selection of the best final models (see Tables 3 and 4). We present results here of the unadjusted bivariate modeling and final multivariable modeling results. (Additional detailed results are available from the corresponding author upon request.)
TABLE 3.
Initial Unplanned Hospitalization in Colorectal Cancer Treatment: GEE Prediction Models
| Predictor | Bivariatea
|
Multivariateb,c
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Sex (female) | 1.95 | [1.48, 2.57] | <.0001 | 2.27 | [1.83, 2.81] | <.0001 |
| Age (yrs) | 0.94 | [0.92, 0.96] | <.0001 | 0.94 | [0.92, 0.96] | <.0001 |
| Race (non-White) | 0.83 | [0.59, 1.18] | .30 | 0.77 | [0.48, 1.25] | .29 |
| NonHS graduates (%)d | 1.02 | [1.01, 1.03] | <.0001 | 0.94 | [0.88, 0.99] | .03 |
| Income (Mdn)d | 0.95 | [0.94, 0.96] | <.0001 | 0.94 | [0.90, 0.99] | .03 |
| Urbanizatione | ||||||
| Big Metro | 0.60 | [0.52, 0.69] | <.0001 | 0.68 | [0.58, 0.80] | <.0001 |
| Metro/urban | 0.54 | [0.52, 0.57] | <.0001 | 0.58 | [0.56, 0.60] | <.0001 |
| Comorbidityf | ||||||
| 0 | 0.26 | [0.14, 0.48] | <.0001 | 0.22 | [0.10, 0.46] | <.0001 |
| 1 | 0.38 | [0.26, 0.58] | <.0001 | 0.36 | [0.21, 0.61] | .0002 |
| 2 | 0.64 | [0.35, 1.17] | .15 | 0.58 | [0.27, 1.12] | .10 |
| Radiation therapy (yes) | 0.75 | [0.54, 1.04] | .08 | |||
| Marital status (married) | 0.93 | [0.65, 1.33] | .69 | |||
Note. N = 1485. CI = confidence interval; GEE = generalized estimating equations; HS = high school; Mdn = median; NCI = National Cancer Institute; OR = odds ratio; QIC = quasi-likelihood under independence model.
Unadjusted models.
Adjusted model.
QIC = 1464.6376.
Census tract data.
Reference group is less urban/rural.
NCI Combined Index. Reference group is 3+.
TABLE 4.
Number of Unplanned Hospitalizations in Colorectal Cancer: GEE Prediction Models
| Predictor | Unadjusted (bivariate) models
|
Adjusted (multivariate) modela
|
||||
|---|---|---|---|---|---|---|
| Multiplier | 95% CI | p | Multiplier | 95% CI | p | |
| Sex (female) | 1.07 | [0.98, 1.17] | .13 | |||
| Age (yrs) | 0.99 | [0.99, 1.00] | .55 | |||
| Race (non-White) | 1.13 | [1.04, 1.23] | .003 | 1.19 | [1.03, 1.37] | .02 |
| NonHS graduates (%)b | 1.02 | [0.98, 1.07] | .20 | |||
| Income (Mdn)b | 0.96 | [0.92, 1.00] | .09 | |||
| Urbanizationc | ||||||
| Big metro | 0.81 | [0.69, 0.96] | .02 | 0.79 | [0.68, 0.93] | .004 |
| Metro/urban | 0.79 | [0.70, 0.89] | .0002 | 0.78 | [0.71, 0.87] | <.0001 |
| Comorbidityd | ||||||
| 0 | 0.96 | [0.86, 1.08] | .55 | 0.96 | [0.85, 1.07] | .45 |
| 1 | 0.99 | [0.90, 1.10] | .97 | 1.01 | [0.90, 1.13] | .89 |
| 2 | 1.12 | [1.07, 1.16] | <.0001 | 1.13 | [1.09, 1.17] | <.0001 |
| Radiation therapy (yes) | 1.04 | [0.98, 1.10] | .15 | 1.06 | [1.00, 1.12] | .03 |
| Marital status (married) | 1.06 | [1.02, 1.10] | .004 | |||
Note. N = 333. Outcome was a count variable. CI = confidence interval; GEE = generalized estimating equations; HS = high school; Mdn = median; NCI = National Cancer Institute; OR = odds ratio; QIC = quasi-likelihood under independence model.
QIC = 1889.7619.
Census tract data.
Reference group is less urban/rural.
NCI Combined Index. Reference group is 3+.
The total amount of all charges for all services and the amount of payment made by Medicare to institutions associated with each identified unplanned hospitalization was computed through use of the MEDPAR file variables TOTCHRGS (total charges) and REIMBAMT (reimbursement amount), respectively. No adjustments, including cost-to-charge ratio calculations, were made to these variables in this study, as the intent was to obtain a general estimate of the financial impact of potentially avoidable events.
Results
Cohort
The cohort consisted of 1,485 patients, 52.5% female, 11.4% of non-White race, with a mean age of 77.6 years. Of these, 77.5% (n = 1, 152) experienced at least one unplanned hospitalization. There were a total of 1, 522 hospitalizations, and the mean number per patient was 1.8 (SD = 1.3, range of 1 to17), with a median length of stay of five days. The total Medicare charges calculated for these admissions was $38,976,171, with the median charge per admission at $20,412. Median Medicare reimbursement per hospitalization equaled $6,734.
Initial Unplanned Hospitalization
Factors examined for the relationship to initial unplanned hospitalization included age, comorbidity, sex, marital status, receipt of radiation therapy, race, education, income, urbanization, and SEER registry. After controlling for other variables in the adjusted model, the following factors were found to be significant: for each year of increasing age, the likelihood of hospitalization decreased by 6.1% (OR = 0.94, 95% CI: [0.92, 0.96], p < .0001). Comorbidity was supported as a predictor, whereas compared with a weighted NCI Combined Index score of 3+ (indicating multiple comorbid conditions) patients with no comorbidities had a decreased likelihood of hospitalization of 78.1% (OR = 0.22, 95% CI: [0.10, 0.46], p < .0001). Those with a comorbidity score of 1 had a decreased likelihood of 63.9% (OR = 0.36, 95% CI: [0.21, 0.62], p = .0002) and in those with a score of 2, the likelihood of hospitalization decreased by 44.2% (OR = 0.56, 95% CI: [0.28, 1.12], p = .10).
Female patients were more than twice as likely to be hospitalized as males (OR = 2.27, 95% CI: [1.84, 2.81], p < .0001). For each 10% increment decrease in census tract level rate of high school completion, the likelihood of hospitalization decreased by 6.02% (OR = 0.94, 95% CI: [0.89, 0.99], p = .03), and for each $10,000 increment increase in census tract level median income, the likelihood of hospitalization decreased by 5.34% (OR = 0.95, 95% CI: [0.90, 0.99], p = .03). As compared to patients living in an area designated as completely rural (urban population less than 20,000), those living in counties with metro areas of one million or more (big metro) had a decreased likelihood of hospitalization of 31.4% (OR = 0.69, 95% CI: [0.59, 0.80], p < .0001), and those in areas with an urban population of between 20,000 and one million (metro/urban) had a decreased likelihood of 41.6% (OR = 0.58, 95% CI: [0.57, 0.60], p < .0001).
Number of Unplanned Hospitalizations
The same factors were examined related to the number of unplanned hospitalizations experienced. Controlling for other variables in the model, cases designated in the SEER record with a non-White race had 1.19 times the number of unplanned hospitalization as compared to Whites (95% CI: [1.03, 1.37], p < .02). Patients who received radiation therapy as part of their initial treatment plan had an increased number of hospitalizations, multiplied by 1.06 as compared to those who did not undergo that treatment (95% CI: [1.0, 1.12], p = .03), and patients with a comorbidity score of 2 had 1.14 times the number of hospitalizations as compared to those with a score of 3 or more [95% CI: [1.10, 1.18], p < .0001). Degree of urbanization again influenced unplanned hospitalization. After controlling for other variables, as compared to those patients living in a completely rural area, those in a Big Metro area or Metro/Urban area had a decreased likelihood of unplanned hospitalization, multiplied by 0.80 (95% CI: [0.68, 0.93], p = .004) or 0.79 (95% CI: [0.71, 0.87], p < .0001), respectively.
Discussion
This study examined predictors of unplanned hospitalization over the course of chemotherapy administration and management intended to be provided exclusively in the outpatient setting among patients with early-stage colorectal cancer. Consistent with the literature described earlier, we observed that sex was a significant predictor, as females in the colorectal cohort experienced twice the likelihood of initial unplanned hospitalization than males. Sex was not a statistically significant predictor of the number of hospitalizations experienced.
Age was a significant predictor, but inverse to what was expected. Each year of additional age was associated with a 6% decrease in the likelihood of initial unplanned hospitalization. Though this may appear a counterintuitive result, a bias towards offering lower doses of anticancer treatments to patients based upon their chronologic age is evident in the literature (Hurria et al., 2008; Sargent et al., 2001; Sundararajan et al., 2002) and could contribute towards the appearance of fewer severe toxicities leading to hospitalization. Though the specific drugs administered could be precisely identified through billing data, the exact dose could not (as the unit of measurement is at the billed vial size), rather than indicative of true milligram per meter squared dosing.
A number of socioeconomic variables predicted unplanned hospitalizations in this study, including non-White race, living in a less densely populated area, a census tract with higher rates of high school graduation or a lower median income. Further study is needed to explore the true impact of these variables to individual patients—especially given the unexpected difference in directionality of the education and income findings—and a claims-based data source may not be the most informative setting to explore these factors.
As expected, the influence of pre-existing comorbid conditions predicted unplanned hospitalizations. Compared to patients with an NCI Combined Index weighted score of 3 or greater, those with 0 (no comorbidities) or 1 were significantly less likely to experience an initial hospitalization. Patients with a score of 2 experienced an increased number of hospitalizations than those with scores of 0, 1, or 3 or more. This study utilized the total weighted index score for analysis, but this finding highlights the need for future inquiry regarding the impact of how specific comorbid conditions may impact chemotherapy toxicity and unplanned hospitalizations. It may be that certain conditions predispose patients more heavily than others, and the use of a single weighted score may not be as informative to predict risk.
Receipt of radiation therapy was a statistically significant predictor of the number of hospitalizations in the colorectal cohort, though it was not significant as a predictor of the initial admission. Radiation therapy is a localized intervention as compared with the systemic effects of chemotherapy, but may cause intense and lasting effects in the areas treated, such as nausea, vomiting and diarrhea, as well as dermatitis and bone marrow suppression (Baglan et al., 2002; Cai et al., 2013), possibly augmenting the systemic effects of chemotherapy.
Nursing implications
Though the results presented here require additional study before comprising an evidence-based, clinically applicable risk factor profile, eventual development of such prospective tools for early identification of those patients most likely to experience unplanned hospitalizations will guide nurses to provide targeted, proactive interventions early in the course of care where the effect will be most pronounced. Care protocols utilized by nurses in generalist and advanced practice roles may be targeted by risk level, including patient education with special instruction on important clinical signs and symptoms that should trigger urgent contact with the oncology service to obtain early outpatient management.
Future work
To build on these results, we are next working to apply the cohort development strategies to a regional electronic data warehouse (EDW) data source that will provide access to more extensive medical record-derived clinical data and a broader age range of patients. This type of inquiry will also facilitate clarification of specific pre-existing, noncancer comorbidities experienced, as well as race/ethnicity and the socioeconomic status variables that were significant in this study. Access to the EDW will provide more robust clinical data, such as chemotherapy dosing, patient functional status, symptom incidence, intensity, timing, and attempted outpatient management strategies implemented prior to an unplanned hospitalization, as well as more detail about the reason for the admission.
Additionally, subgroup analyses exploring the relationships between patients with specific organ system comorbidities and risk of unplanned hospitalizations for the most frequently observed toxicities are warranted. For example, a logical subanalysis would be to explore the toxicity patterns and risk of hospitalization in patients identified to have a diagnosis of diabetes prior to starting colorectal cancer treatments, as the high incidence of nausea and vomiting is likely to heavily impact this population. Identification of such clear-cut predictors will aid nurses to stratify education, supportive therapies, and patient monitoring to those patients at highest risk of avoidable complications.
Limitations
Though there are many advantages to utilization of a large dataset such as SEER-Medicare for nursing inquiry, it is important to note that a number of limitations exist. While claims data may provide clinical information (such as specific diagnostic and drug codes), reporting on key additional findings (such as symptom incidence and intensity), actual drug dosing, as well as functional status assessments, are not recorded in this way. Another challenge relates to data age. SEER-Medicare data typically lags two years behind availability, and is updated approximately every two years. For this study, which began in 2011, claims data were available through the end of 2009, and an update was not released until 2013, after which the majority of data analysis was completed.
It was hoped to include disease stage as a variable in the study models, but the proportion of cases where the diagnostic stage was coded as “unknown” (not missing) by the contributing cancer registry was significantly higher in the non-hospitalized group (X2 = 25.82, 1, p < .0001), which was not compatible with GEE analysis. Most cancer registries reside within a hospital setting where medical record access is directly available, providing a possible explanation for the higher rate of SEER diagnostic classification among those patients in the hospitalized group.
It is also important to note that the intent of this study was to identify predictive factors associated with the event of unplanned hospitalization among patients receiving chemotherapy—not to determine the specific causes of these admissions. The level of detail available in a claims-based dataset does not provide sufficient clinical information to accurately assess the etiology of unplanned hospitalizations.
Conclusion
This study represents a first step to identify patients prior to initiation of chemotherapy who are at high risk of severe treatment-related adverse events that may result in unplanned hospitalizations. Further work is needed to refine understanding of the impact of these factors and to add additional clinical variables, such as symptom experience, functional status, and chemotherapy dosing.
Acknowledgments
The authors acknowledge that this study was conducted as Fessele’s dissertation work in partial fulfillment of the requirements for the PhD degree at Rutgers, and was written as part of a postdoctoral fellowship at the University of Utah. This publication was supported in part by the National Institute of Nursing Research of the National Institutes of Health under Award Number T32NR013456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The authors wish to acknowledge Cynthia Ayres, PhD, RN, Associate Professor, Rutgers, The State University of New Jersey for her contributions to the dissertation committee that supervised this project.
Footnotes
The authors report no conflicts of interest to report.
Contributor Information
Kristen L. Fessele, Postdoctoral Fellow, University of Utah College of Nursing.
Matthew J. Hayat, Associate Professor, School of Public Health,Georgia State University.
Deborah K. Mayer, Professor, School of Nursing, Director of Cancer Survivorship, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill.
Robert L. Atkins, Associate Professor of Childhood Studies and Nursing, Director, New Jersey Health Initiatives – Robert Wood Johnson Foundation, Rutgers, The State University of New Jersey.
References
- Aparicio T, Jouve JL, Teillet L, Gargot D, Subtil F, Le Brun-Ly V, … Mitry E. Geriatric factors predict chemotherapy feasibility: Ancillary results of FFCD 2001–02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. Journal of Clinical Oncology. 2013;31:1464–1470. doi: 10.1200/JCO.2012.42.9894. [DOI] [PubMed] [Google Scholar]
- Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume realationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. International Journal of Radiation Oncology Biology•Physics. 2002;52:176–183. doi: 10.1016/S0360-3016(01)01820-X. [DOI] [PubMed] [Google Scholar]
- Bray F, Parkin DM. Evaluation of data quality in the cancer registry: Principles and methods. Part I: Comparability, validity and timeliness. European Journal of Cancer. 2009;45:747–755. doi: 10.1016/j.ejca.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Cai X, Wu H, Peng J, Zhu J, Cai S, Cai G, Zhang Z. Tolerability and outcomes of radiotherapy or chemoradiotherapy for rectal cancer in elderly patients aged 70 years and older. Radiation Oncology. 2013;8:86. doi: 10.1186/1748-717X-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee DP. Crisis in hospital-acquired, healthcare-associated infections. Annual Review of Medicine. 2012;63:359–371. doi: 10.1146/annurev-med-081210-144458. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. 2011 edition of the Statistical Supplement. 2012 Retrieved from http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareMedicaidStatSupp/2011.html. [PubMed]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Du XL, Osborne C, Goodwin JS. Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. Journal of Clinical Oncology. 2002;20:4636–4642. doi: 10.1200/JCO.2002.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extermann M, Chen H, Cantor AB, Corcoran MB, Meyer J, Grendys E, … Balducci L. Predictors of tolerance to chemotherapy in older cancer patients: A prospective pilot study. European Journal of Cancer. 2002;38:1466–1473. doi: 10.1016/S0959-8049(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Fessele K, Atkins R. Unplanned hospitalizations for adverse events during outpatient chemotherapy: Conceptual and methodological delineation during analysis of a large, oncology-focused administrative dataset [Abstract] Oncology Nursing Forum. 2012;39:E543. Retrieved from https://onf.ons.org/file/5261/download. [Google Scholar]
- González JR, Fernandez E, Moreno V, Ribes J, Peris M, Navarro M, … Borrás JM. Sex differences in hospital readmission among colorectal cancer patients. Journal of Epidemiology & Community Health. 2005;59:506–511. doi: 10.1136/jech.2004.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M, Ferrell BR, Rivera LM, Lee J. Unscheduled readmissions for uncontrolled symptoms: A health care challenge for nurses. Nursing Clinics of North America. 1995;30:673–682. [PubMed] [Google Scholar]
- Hassett MJ, O’Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. Journal of the National Cancer Institute. 2006;98:1108–1117. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- Hassett MJ, Rao SR, Brozovic S, Stahl JE, Schwartz JH, Maloney B, Jacobson JO. Chemotherapy-related hospitalization among community cancer center patients. The Oncologist. 2011;16:378–387. doi: 10.1634/theoncologist.2010-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, … Cronin KA. SEER cancer statistics review (CSR), 1975–2012. 2015 Retrieved from http://seer.cancer.gov/csr/1975_2012/
- Hurria A, Levit LA, Dale W, Mohile SG, Muss HB, Fehrenbacher L, … Cohen HJ. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. Journal of Clinical Oncology. 2015 doi: 10.1200/JCO.2015.63.0319. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Hurria A, Wong FL, Villaluna D, Bhatia S, Chung CT, Mortimer J, … Naeim A. Role of age and health in treatment recommendations for older adults with breast cancer: The perspective of oncologists and primary care providers. Journal of Clinical Oncology. 2008;26:5386–5392. doi: 10.1200/JCO.2008.17.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. New England Journal of Medicine. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Annals of Epidemiology. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000;53:1258–1267. doi: 10.1016/S0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- Lamont EB, Herndon JE, II, Weeks JC, Henderson IC, Lilenbaum R, Schilsky RL, Christakis NA. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants. Journal of the National Cancer Institute. 2005;97:1080–1083. doi: 10.1093/jnci/dji189. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.2307/2336267. [DOI] [Google Scholar]
- Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. Journal of Clinical Oncology. 2011;29:2683–2688. doi: 10.1200/JCO.2010.34.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder BJ, Tzeng HM, Vecchioni ND. Preventing avoidable rehospitalizations by understanding the characteristics of “frequent fliers. Journal of Nursing Care Quality. 2012;27:77–82. doi: 10.1097/NCQ.0b013e318229fddc. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Overview of the SEER program. 2012 Retrieved from http://seer.cancer.gov/about/overview.html.
- Nottage M, McLachlan SA, Brittain MA, Oza A, Hedley D, Feld R, … Moore MJ. Sucralfate mouthwash for prevention and treatment of 5-fluorouracil-induced mucositis: A randomized, placebo-controlled trial. Supportive Care in Cancer. 2003;11:41–47. doi: 10.1007/s00520-002-0378-8. [DOI] [PubMed] [Google Scholar]
- Nurgalieva Z, Liu CC, Du XL. Risk of hospitalizations associated with adverse effects of chemotherapy in a large community-based cohort of elderly women with ovarian cancer. International Journal of Gynecological Cancer. 2009;19:1314–1321. doi: 10.1111/IGC.0b013e3181b7662d. [DOI] [PubMed] [Google Scholar]
- Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. Journal of Clinical Oncology. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341X.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. Epidemiology: An introduction. 1. New York, NY: Oxford Press; 2002. [Google Scholar]
- Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, … Francini G. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. New England Journal of Medicine. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- Sloan JA, Goldberg RM, Sargent DJ, Vargas-Chanes D, Nair S, Cha SS, … Loprinzi CL. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. Journal of Clinical Oncology. 2002;20:1491–1498. doi: 10.1200/JCO.20.6.1491. [DOI] [PubMed] [Google Scholar]
- Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Annals of Internal Medicine. 2002;136:349–357. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Profile of older Americans. 2014 Retrieved from http://www.aoa.acl.gov/Aging_Statistics/Profile/2014/docs/2014-Profile.pdf.
- Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Medical Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- Weaver C, Schiech L, Held-Warmkessel J, Kedziera P, Haney E, DiLullo G, … Barsevick A. Risk for unplanned hospital readmission of patients with cancer: Results of a retrospective medical record review. Oncology Nursing Forum. 2006;33:E44–E52. doi: 10.1188/06.ONF.E44-E52. [DOI] [PubMed] [Google Scholar]
- Zalcberg J, Kerr D, Seymour L, Palmer M. Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. European Journal of Cancer. 1998;34:1871–1875. doi: 10.1016/S0959-8049(98)00259-7. [DOI] [PubMed] [Google Scholar]