Abstract
IMPORTANCE
Buprenorphine opioid agonist treatment (OAT) has established efficacy for treating opioid dependency among persons seeking addiction treatment. However, effectiveness for out-of-treatment, hospitalized patients is not known.
OBJECTIVE
To determine whether buprenorphine administration during medical hospitalization and linkage to office-based buprenorphine OAT after discharge increase entry into office-based OAT, increase sustained engagement in OAT, and decrease illicit opioid use at 6 months after hospitalization.
DESIGN, SETTING, AND PARTICIPANTS
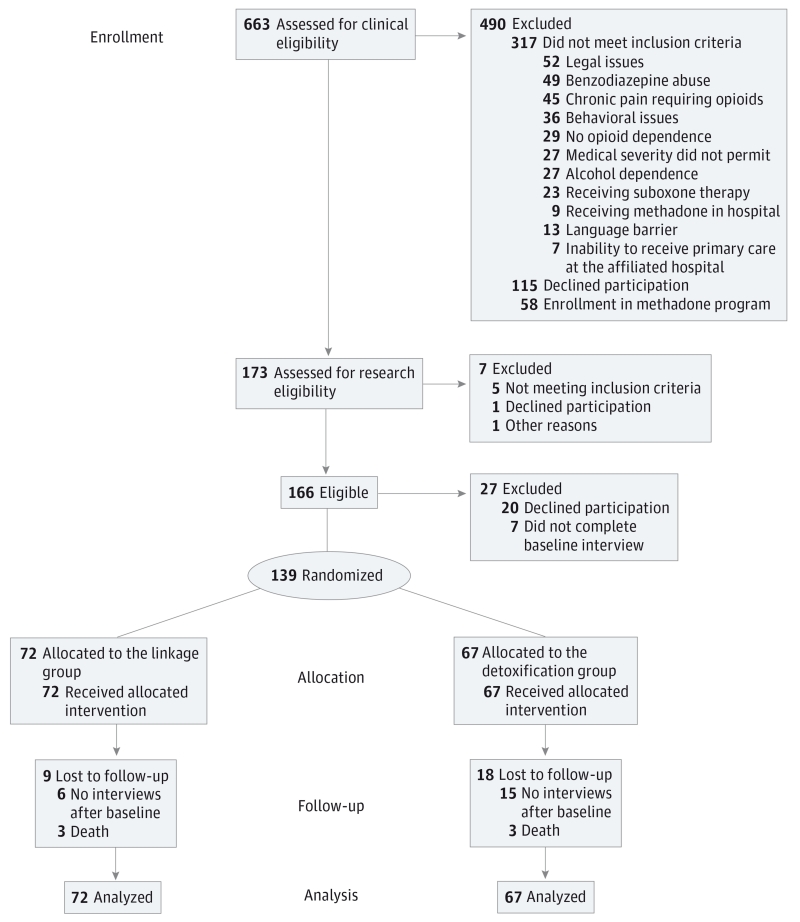
From August 1, 2009, through October 31, 2012, a total of 663 hospitalized, opioid-dependent patients in a general medical hospital were identified. Of these, 369 did not meet eligibility criteria. A total of 145 eligible patients consented to participation in the randomized clinical trial. Of these, 139 completed the baseline interview and were assigned to the detoxification (n = 67) or linkage (n = 72) group.
INTERVENTIONS
Five-day buprenorphine detoxification protocol or buprenorphine induction, intrahospital dose stabilization, and postdischarge transition to maintenance buprenorphine OAT affiliated with the hospital’s primary care clinic (linkage).
MAIN OUTCOMES AND MEASURES
Entry and sustained engagement with buprenorphine OAT at 1, 3, and 6 months (medical record verified) and prior 30-day use of illicit opioids (self-report).
RESULTS
During follow-up, linkage participants were more likely to enter buprenorphine OAT than those in the detoxification group (52 [72.2%] vs 8 [11.9%], P < .001). At 6 months, 12 linkage participants (16.7%) and 2 detoxification participants (3.0%) were receiving buprenorphine OAT (P = .007). Compared with those in the detoxification group, participants randomized to the linkage group reported less illicit opioid use in the 30 days before the 6-month interview (incidence rate ratio, 0.60; 95% CI, 0.46-0.73; P < .01) in an intent-to-treat analysis.
CONCLUSIONS AND RELEVANCE
Compared with an inpatient detoxification protocol, initiation of and linkage to buprenorphine treatment is an effective means for engaging medically hospitalized patients who are not seeking addiction treatment and reduces illicit opioid use 6 months after hospitalization. However, maintaining engagement in treatment remains a challenge.
Hospitalized patients have high rates of substance use—36% smoke cigarettes, 20% drink alcohol hazardously, and 8% use illicit drugs.1-5 In New York, New York, an estimated 4% of hospitalized patients use illicit opioids, and less than one-quarter of opioid users initiate substance treatment in a year.6 Recognizing that hospitalization and acute illness may motivate patients to decrease substance use, researchers have developed brief interventions to decrease tobacco use7 and address risky drinking and alcohol dependence.8-11
Interventions focused on hospitalized opioid users have been rare, yet this population is increasing because of increases in prescription opioid abuse and dependence. Opioid-related emergency department visits increased 183% between 2004 and 2011, and nearly one-quarter of these visits resulted in hospital admission.12 Opioid use (in particular, injection drug use) is associated with myriad medical problems, such as soft tissue infections, endocarditis, human immunodeficiency virus disease, trauma, and overdose,13 that lead to hospitalization where interventions can take place. To treat opioid withdrawal symptoms that may interfere with medical treatment, the standard of care is to manage withdrawal using a tapering schedule of opioid agonist substitution (short-term detoxification) with methadone or buprenorphine.14,15 Referral to substance abuse treatment after discharge is uncommon.16
Longer-term opioid agonist treatment (OAT) with methadone maintenance or office-based buprenorphine administration is reported to decrease substance use and mortality.17-21 Buprenorphine, an opioid partial agonist, has appeal for many opioid-dependent individuals because of the convenience of monitoring and the decreased stigma of receiving it in a general medical office setting. Long-term buprenorphine treatment is typically initiated in community-based settings with treatment-seeking, opioid-dependent individuals.
The single observational study22 of non–treatment-seeking, opioid-dependent patients during hospitalization promoted methadone treatment program referral after discharge and resulted in 82% of participants presenting for outpatient evaluation and 59% starting methadone treatment. Given that buprenorphine can be prescribed for opioid dependence in a primary care setting (unlike methadone), it may offer a more streamlined transition to postdischarge, office-based addiction care. At the time of an acute medical hospitalization, however, many opioid-dependent individuals are not seeking substance abuse treatment, and whether long-term treatment engagement can begin in this setting remains in question.
This study had 2 primary goals. We sought to determine whether offering hospitalized, opioid-dependent patients initiation and linkage to office-based opioid addiction treatment with buprenorphine would facilitate entry into and increase persistence in buprenorphine treatment. We then examined whether this treatment initiation and linkage would decrease illicit opioid use at 6 months.
Methods
Study Design and Recruitment
This study was approved by the Butler Hospital and Boston Medical Center institutional review boards, and all participants provided written informed consent. Hospitalized, opioid-dependent patients were recruited from the inpatient medical service of a safety-net, academic hospital. Research staff, including an addiction nurse specialist, screened the daily hospital record for all new inpatient admissions of persons 18 years or older whose medical history suggested recent opioid use. Potential participants were interviewed by the nurse specialist to determine preliminary study eligibility and interest. Individuals were excluded from the study if they were receiving methadone or buprenorphine maintenance before admission, expressed a desire to harm themselves or others, had alcohol dependence, had benzodiazepine dependence, were not local residents, had surgery or potential jail time pending, required opioids for pain beyond hospitalization, or were pregnant. At this initial screen, all eligible English-speaking patients were offered referral to methadone treatment and informed about this clinical trial. Individuals interested in OAT with buprenorphine after hospital discharge and who were willing to receive it at an affiliated primary care practice were referred to research staff for full eligibility evaluation.
From August 1, 2009, through October 31, 2012, a total of 663 hospitalized, opioid-dependent patients were identified. Of these, 317 did not meet eligibility criteria for the following reasons: legal issues (n = 52), benzodiazepine abuse (n = 49), chronic pain requiring opioid analgesia (n = 45), alcohol dependence (n = 27), medical issues (n = 27), behavioral issues (includes suicidal ideation and leaving against medical advice and cocaine use) (n = 36), no opioid dependence (n = 29), already receiving suboxone (n = 23), language barrier (n = 13), currently in methadone maintenance treatment or receiving methadone while inpatient (n = 9), inability to receive primary care at the affiliated hospital (n = 7) An additional 34 were excluded during the research screen and enrollment process (29 declined, 5 did not meet inclusion criteria, 1 staff unavailable to enroll). Potentially eligible patients refused because they did not want OAT (n = 71), were not interested in study participation (n = 44), or preferred methadone treatment (n = 58).
A total of 145 eligible individuals consented to randomized clinical trial participation. Of these, 139 completed the baseline interview and were assigned to the detoxification (n = 67) or linkage (n = 72) group using permuted block (block sizes of 4 or 6) randomization generated by an off-site statistician (B.A.). Participants in the detoxification group received a buprenorphine induction and 4 days of tapering buprenorphine doses. Participants in the linkage group received buprenorphine induction, received a maintenance dose of buprenorphine during hospitalization, and facilitated linkage into the hospital-affiliated primary care OAT program (Figure 1).
Figure 1.
Screening, Randomization, and Follow-up
Two of 3 participants in the linkage group died before any follow-up interview.
Buprenorphine Treatment
Day 1
For both study groups, the buprenorphine protocol was identical: 2 mg of sublingual buprenorphine and 0.5 mg of nalox-one up to 4 times for a maximum of 8 mg of buprenorphine.
Detoxification Group
Those randomized to the detoxification group received 4 additional days of tapering buprenorphine and naloxone. Daily doses were 8 mg of buprenorphine on day two, 6 mg on day three, 4 mg on day 4, and 2 mg on day 5. This taper plan was administered by hospital nursing staff during hospitalization and self-administered by the participant if discharge occurred before study day 5, in which case participants received a blister pack of the remaining medication and pharmacy instructions. Research staff offered postdischarge treatment referral information.
Linkage Group
Participants in the linkage group received 12 mg of buprenorphine and naloxone on day 2 and 16 mg on day 3 and for the remainder of their hospitalization. Before discharge, research staff facilitated linkage to the hospital-associated primary care buprenorphine OAT. The OAT staff contacted the participant, conducted its own admission process, and scheduled the initial nurse intake visit within 7 days of discharge. A buprenorphine-licensed physician (J.M.L.) performed a clinical assessment before discharge and prescribed buprenorphine, 16 mg/d, to last until the OAT intake appointment. If the participant missed the scheduled OAT intake appointment, he/she did not receive further prescription of buprenorphine and naloxone from the study personnel. However, the participant could reschedule the OAT intake appointment, at which time a new induction would be prescribed by the OAT staff, as clinically appropriate. After intake, the OAT staff determined all ongoing treatment.
Research Assessments
All participants were interviewed at baseline and at 1, 3, and 6 months after enrollment. Follow-up interviews occurred in person or on the telephone. Participants were compensated $15 in gift cards at the baseline interview, $25 at 1 month, $35 at 3 months, and $45 at 6 months. Research interviewers were aware of treatment group assignment at the follow-up assessments.
Primary Outcome Variables
The prespecified primary outcome variables were entry into buprenorphine treatment (in-person intake [anytime between study enrollment and 6 months after enrollment]) at the hospital-associated OAT program, confirmed by OAT electronic medical record review, and length of illicit opioid use (number of days of reported opioid use in the 30 days before the 1-, 3-, and 6-month interviews using a standard 30-day time-line follow-back method).23
Secondary Outcome Variables
Secondary outcome variables included time to entry into the buprenorphine program (days to in-person intake at the hospital-associated OAT program, confirmed by electronic medical record review) and OAT days (number of days of self-reported prescribed OAT [methadone or buprenorphine] in the 30 days before the 1-, 3-, and 6-month interviews using a standard 30-day timeline follow-back method).23
Entry into any substance abuse treatment program was defined as self-reported receipt of any substance abuse treatment, including residential, outpatient counseling, methadone maintenance treatment, or buprenorphine at a facility other than the hospital-associated OAT, during the follow-up period.24 Mortality and presumed cause of death were discovered during tracking of participants for follow-up assessments through medical record review (verified) or report by family members (unverified).
Statistical Analysis
We present descriptive statistics to summarize the characteristics of the cohort. Between-group differences in baseline characteristics were tested using t tests for differences in means and the Pearson χ2 test for differences in categorical distributions and entry into the hospital-associated OAT program. The Cox proportional hazards regression model was used to test for differences in days from hospital discharge to OAT initiation. The study was powered to detect moderate effect sizes. Specifically, the study was powered (1 – β > .8) to detect a 33% between-group difference in the rate of opioid use during follow-up (days of use per 30 follow-up days).
We also evaluated the effect of intervention on rates of illicit opioid use and self-reported OAT (methadone or buprenorphine) during the 6-month follow-up assessment period. All rates were reported as days of use per 30 follow-up days and analyzed as count variables using random-effects Poisson regression. Because the distributions were overdispersed and not well approximated by any exponential family distribution, we used bias-corrected and accelerated bootstrap resampling25 with 5000 replications to estimate 95% and 99% CIs; the CIs that exclude 1 (we report incidence rate ratios [IRRs]) were considered statistically significant at the .05 and .01 levels, respectively. Given multiple outcomes, readers concerned about overall type I error rates may consider P < .01 a conservative standard to evaluate statistical significance. To facilitate interpretation, we converted the predicted rates to mean days of use to provide a more descriptive metric for interpretation. In addition, we used random-effects logistic regression to estimate the effect of intervention on the likelihood of any illicit opioid use during follow-up.
Complete medical record data were available for assessing OAT entry and time to OAT entry. We conducted several analyses to evaluate the degree to which our results might be sensitive to participant attrition when analyzing self-report. We report results based on complete case analysis (n = 116) but also evaluated parallel tests using the worst-case substitution (participants unavailable for follow-up were assumed to not be using OAT and to be using illicit opioids) and last observation carried forward. These alternative methods yielded similar results.
Using a random-effects logistic regression model, we conducted auxiliary analyses to evaluate the consistency of self-reported opioid use with available urine toxicology tests performed during follow-up. For these analyses, we used only the last 4 follow-up days before performing the toxicology tests. We also explored the correlation between days of OAT and illicit opioid use among participants randomized to the linkage group.
Results
The mean (SD) age of the study participants was 40.5 (11.8) years, 99 (71.2%) were men, 60 (43.2%) were non-Hispanic white, 39 (28.1%) were African American, and 30 (21.6%) were Hispanic (Table). The most common hospital discharge diagnoses were cellulitis (52 [37.4%]), drug overdose or withdrawal (20 [14.4%]), human immunodeficiency virus disease (8 [5.8%]), asthma (8 [5.8%]), gastroenterologic illness (7 [5.0%]), chest pain (6 [4.3%]), fever (5 [3.6%]), liver disease (5 [3.6%]), and endocarditis or sepsis (4 [2.9%]). The mean (SD) rate of illicit opioid use at baseline was 20.8 (9.7) days. Fifty-seven (41.0%) reported any prescription OAT in the month before baseline; 55 reported methadone only, 2 reported sub-oxone only, and 1 reported both methadone and suboxone. The intervention groups did not differ significantly with respect to demographic characteristics, baseline frequency of illicit opioid use, or baseline OAT. Overall follow-up rates were 66.2%, 63.3%, and 59.0% at 1, 3, and 6 months, respectively; 116 participants (83.5%) were observed at 1 or more of the follow-up assessments, and 58 (41.7%) were observed at all 3 follow-up assessments. Participants randomized to the linkage group had higher observed follow-up rates at all periods. Between-group differences in follow-up rates were not statistically significant at 1 or 3 months. The linkage group had a significantly higher rate of follow-up at 6 months (χ2 = 6.75, P = .009).
Table.
Background Characteristics by Intervention Groupa
| Characteristic | Total Cohort (N = 139) |
Detoxification (n = 67) |
Linkage (n = 72) |
t or χ2 | P Value |
|---|---|---|---|---|---|
| Age, mean (SD), y | 40.5 (11.8) | 39.6 (11.5) | 41.4 (12.0) | −0.89 | .38 |
| Male sex | 99 (71.2) | 48 (72.6) | 51 (70.8) | 0.01 | .92 |
| Race/ethnicity | 1.26 | ||||
| White | 60 (43.2) | 28 (41.8) | 32 (44.4) | .74 | |
| African American | 39 (28.1) | 17 (25.4) | 22 (30.6) | ||
| Hispanic | 30 (21.6) | 16 (23.9) | 14 (19.4) | ||
| Other | 10 (7.2) | 6 (9.0) | 4 (5.6) | ||
| Illicit opioid use per 30 follow-up days, mean (SD), d |
20.8 (9.7) | 20.9 (9.2) | 20.8 (10.3) | 0.11 | .92 |
| Previous opioid agonist therapy, d | |||||
| Mean (SD) | 57 (41.0) | 25 (37.3) | 32 (44.4) | 0.07 | .39 |
| Observed at 1 month | 92 (66.2) | 40 (59.7) | 52 (72.2) | 2.43 | .12 |
| Observed at 3 months | 88 (63.3) | 39 (58.2) | 49 (68.1) | 1.45 | .23 |
| Observed at 6 months | 82 (59.0) | 32 (47.8) | 50 (69.4) | 6.75 | .009 |
Data are presented as number (percentage) of study participants unless otherwise indicated.
Toxicology test results were consistent with self-reported opioid use on 136 (81.0%) of the 168 urine toxicology tests conducted during follow-up. Self-reported opioid use was not confirmed on 12 (7.1%) testing occasions, and evidence consistent with underreporting was observed for 18 (10.7%) of all tests. The intervention groups did not differ significantly with respect to the likelihood of underreporting opioid use during follow-up (odds ratio [OR], 0.84; 95% CI, 0.24-3.03; z = −0.25; P = .80).
Confirmed Entry and Engagement in OAT
Fifty-two participants (72.2%) randomized to the linkage group entered the hospital’s OAT by 6 months after study entry compared with only 8 participants (11.9%) randomized to the detoxification group (χ21 = 51.41, P < .001). In addition, time-to-event analysis revealed that participants randomized to the linkage group had a significantly shorter time to OAT entry (hazard ratio, 11.81; 95% CI, 5.57-25.03; P < .001). Median time to OAT initiation was 16 days among participants randomized to the linkage group; because fewer than half of the participants randomized to the detoxification group initiated OAT at the clinic, median days could not be calculated.
Twelve participants (16.7%) randomized to the linkage group compared with 2 participants (3.0%) randomized to the detoxification group (χ2 = 7.17, P = .007) were still engaged in OAT at the completion of the 6-month follow-up. Participants randomized to the linkage group received buprenorphine from the clinic for a mean (SD) of 64.4 (61.7) days during the 6-month follow-up. This finding was significantly higher (t137 = −7.06, P < .001) than for those randomized to the detoxification group who received buprenorphine for a mean (SD) of 6.8 (26.2) days.
Self-reported OAT During Follow-up Assessment
On the basis of self-report, the estimated rate of OAT (buprenorphine or methadone) among participants randomized to the linkage group was almost 2.4 times higher throughout the 6-month study period than for those randomized to the detoxification group (IRR, 2.44; 95% CI, 1.99-3.36; P < .01). Expressed as days of OAT use per 30 follow-up days, participants randomized to the linkage group had approximately 16.4 days of OAT compared with approximately 6.4 days in the detoxification group. Statistically consistent results were observed using worst-case substitution (IRR, 2.78; 95% CI, 2.08-3.97; P < .01) and last observation carried forward (IRR, 2.42; 95% CI, 2.19.-3.34; P < .01).
Illicit Opioid Use During Follow-up Assessment
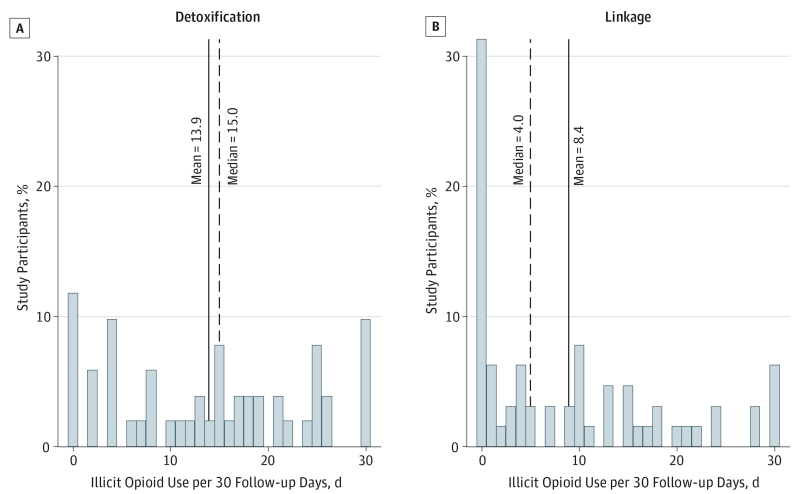
Figure 2 shows the overall rates of illicit opioid use, expressed as days of illicit opioid use per 30 follow-up days. Compared with the detoxification group, participants randomized to the linkage group were more likely to report no illicit opioid use (24 [37.5%] vs 5 [9.0%]). Participants in the linkage group also had lower mean (8.4 vs 13.9) and median (4 vs 15) days of illicit opioid use during follow-up. On the basis of complete case analysis, the estimated rate of illicit opioid use in those in the linkage group was approximately 40% lower (IRR, 0.60; 95% CI, 0.46-0.73; P < .01) than for participants randomized to the detoxification group. The predicted rates of use were 8.6 (linkage group) vs 13.7 (detoxification group) days of illicit opioid use per 30 follow-up days. Parallel random-effects regression models using worst-case substitution (IRR, 0.73; 95% CI, 0.65-0.83; P < .01) and last observation carried forward substitution (IRR, 0.72; 95% CI, 0.64-0.81; P < .01) gave estimated intervention effects substantively and statistically consistent with those observed when using complete case analysis.
Figure 2.
Distribution of Rates of Illicit Opioid Use During Follow-up Assessment by Intervention in 116 Individuals
A, Detoxification group; B, linkage group. To facilitate description, rates were calculated as days of illicit opioid use per 30 follow-up days using all available data, including the mean of all assessments for each study participant with multiple follow-up data or any follow-up time point for participants with one time point.
Relative to those randomized to the detoxification group, the estimated odds of reporting any illicit opioid use were significantly lower among those randomized to the linkage group (OR, 0.13; 95% CI, 0.04-0.77; P = .003). Estimated coefficients for worst-case substitution complete cases (n = 116; OR, 0.22; 95% CI, 0.08-0.60; P = .008) and last observation carried forward (OR, 0.14; 95% CI, 0.05-0.46) were substantively and statistically similar. We conducted auxiliary analysis exploring the association between self-reported OAT and self-reported illicit opioid use during follow-up. Product-moment correlations were −0.57, −0.65, and −0.46 (P < .001) using data from the 1-, 3-, and 6-month interviews, respectively.
Additional Clinical Observations
During follow-up, 15 detoxification participants reported substance abuse treatment other than with the hospital-associated OAT (7 were receiving methadone maintenance, 6 were receiving inpatient detoxification, and 2 were receiving buprenorphine treatment). Three linkage participants reported such treatment (1 was receiving buprenorphine treatment and 2 were receiving inpatient detoxification).
During the study period, 6 participants died of the following causes: congestive heart failure (n = 2), postoperative pulmonary embolism (n = 1), liver failure (n = 1), renal failure (n = 1), and drug overdose (n = 1). No participant who died was engaged in buprenorphine treatment at the time of death.
Discussion
Opioid-dependent participants hospitalized for medical reasons who received induction and linkage to buprenorphine treatment had lower illicit opioid use during a 6-month follow-up period than participants who underwent detoxification during hospitalization. With nearly 75% successfully entering the outpatient buprenorphine treatment provided, the linkage group had greater long-term use of OAT, and more than one-third of participants reported 0 days of illicit opioid use during the study period compared with fewer than 1 of 10 in the detoxification group. This randomized clinical trial confirms what the 1 extant observational study reported: offering treatment to hospitalized, opioid-dependent persons is likely to result in subsequent entry into OAT.22 Furthermore, it is the only study, to our knowledge, to have examined the potential for initiating treatment with buprenorphine in the hospital setting, which can later be dispensed in primary care settings, unlike methadone, which requires referral to federally licensed programs.
With longer retention in OAT (methadone and buprenorphine) associated with better outcomes,15,19 relatively low retention in treatment of participants randomized to the linkage group who began OAT (12 of 52 [23.1%]) is concerning. Indeed, the same hospital-associated, primary care OAT program that treated our study participants reported a retention rate of 51% at 12 months for nonstudy outpatient initiators.26 For several reasons, we expected our treated population to be at higher risk of dropping out of OAT when compared with nonstudy patients who have passed through the bureaucratic and practical barriers necessary to begin outpatient buprenorphine treatment and might be more committed to care. First, participants in our study were not initially seeking treatment; they were offered treatment during a medical hospitalization. Second, medical illness–related needs may take priority over substance use treatment after discharge because many participants were hospitalized with serious medical conditions. Third, getting out-of-treatment, hospitalized patients to maintain buprenorphine treatment after the initial few months appears to require more than treatment as usual in an already effective program.26
Even with the less than ideal retention in OAT programs, the marked decrease in days of opioid use in the linkage group is likely to translate into improved health outcomes. A prior study27 found that days of injection drug use affect health care use, including emergency department visits and hospitalizations. The risk of health complications related to ongoing drug use is high; the survival benefit of OAT is well described.17
Our study had limitations. First, it was conducted at a single institution that had an associated buprenorphine outpatient treatment program. Patients and health care professionals in different clinical contexts may have more logistical barriers to overcome to allow seamless linkage to buprenorphine treatment after a hospitalization. Second, the rates of follow-up assessment were relatively low, with differential study retention by treatment group. These lower rates were in part due to the general difficulty of following up the treatment group, who are often homeless and without telephones. The low assessment rate decreases the strength of the findings. We conducted multiple sensitivity analyses, which, in all cases, confirmed the unadjusted findings, providing greater confidence in the findings. For future studies, frequent and early follow-up for maintaining study contact, drop-in hours for study assessments, cell phones instead of financial compensation, and research assistant visits to homeless shelters and jails may be effective methods to track participants. Third, treatment receipt at sites other than the hospital-associated OAT program was based on self-report; our primary outcome measure (past 30-day illicit opioid use) also depended on self-report. However, an analysis of the available urine drug tests revealed underreporting in only 10.7% of the sample, without differences with respect to the randomization group, confirming that the self-report was likely to be valid and did not affect the study findings. In addition, multiple statistical methods that confirmed our findings helped offset these limitations.
This study indicates the effectiveness of offering induction and linkage to buprenorphine maintenance treatment to hospitalized, opioid-dependent patients. For our protocol to be disseminated, certain policies would need to be implemented. First, hospitals would need a method to identify drug users systematically. This could be accomplished with admission diagnoses, nursing assessments, or physician notes, depending on the electronic health record. Second, discharge planning staff would need to maintain an active referral network of buprenorphine prescribers able to accept new patients on short notice. Third, a dedicated substance use consulting team would need to initiate treatment during hospitalization and provide a bridge subscription to the first outpatient OAT visit. Discharge planning nurses with addiction training could facilitate much of this process. In addition, new methods to retain a higher proportion of patients receiving buprenorphine treatment should be evaluated. Because a high proportion of patients came for the first office-based visit, better retention might require an intensive engagement program at initiation. Candidate methods could include counseling28-30 or patient navigators to help patients engage in medical and social services.31
Conclusions
We present a protocol that successfully initiated and linked hospitalized, non–treatment-seeking, opioid-dependent patients to long-term buprenorphine OAT. Future work should evaluate whether decreased illicit opioid use and increased use of OAT in the 6 months after hospital discharge could have health benefits and prevent subsequent hospitalizations.
Acknowledgments
Funding/Support: This study was supported by grant R01 DA026223 (Dr Stein) and award K24 DA000512 (Dr Stein) from the National Institute on Drug Abuse.
Role of the Sponsors: The National Institute on Drug Abuse had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Anderson and Stein had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Liebschutz, Stein.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Liebschutz, Anderson, Dossabhoy, Stein.
Critical revision of the manuscript for important intellectual content: Liebschutz, Crooks, Herman, Anderson, Tsui, Meshesha, Stein.
Statistical analysis: Anderson.
Obtained funding: Stein.
Administrative, technical, or material support: Liebschutz, Crooks, Herman, Meshesha, Dossabhoy, Stein.
Study supervision: Liebschutz, Herman, Stein.
Conflict of Interest Disclosures: None reported.
Previous Presentations: The results of this study were presented at the Society of General Internal Medicine Annual Meeting; April 24, 2013; Denver, Colorado; and the College on Problems of Drug Dependence Annual Meeting; June 20, 2013; San Diego, California.
Additional Contributions: Reckitt Benckiser Pharmaceuticals provided the study medication and reviewed the manuscript for scientific accuracy. Contributions to the research were made by Joel Hoyte, MD, Meredith Collins, MPH, Sam Masur, BA, Donna Beers, RN, Colleen Labelle, RN, and the physician, nursing, and pharmacy staffs at Boston Medical Center and the office-based opioid treatment program. Dr Hoyte, Ms Collins, Mr Masur, and Ms Beers were compensated for their work.
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00987961
REFERENCES
- 1.Brown RL, Leonard T, Saunders LA, Papasouliotis O. The prevalence and detection of substance use disorders among inpatients ages 18 to 49: an opportunity for prevention. Prev Med. 1998;27(1):101–110. doi: 10.1006/pmed.1997.0250. doi:10.1006/pmed.1997.0250. [DOI] [PubMed] [Google Scholar]
- 2.Cherpitel CJ, Ye Y. Drug use and problem drinking associated with primary care and emergency room utilization in the US general population: data from the 2005 National Alcohol Survey. Drug Alcohol Depend. 2008;97(3):226–230. doi: 10.1016/j.drugalcdep.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore RD, Bone LR, Geller G, Mamon JA, Stokes EJ, Levine DM. Prevalence, detection, and treatment of alcoholism in hospitalized patients. JAMA. 1989;261(3):403–407. [PubMed] [Google Scholar]
- 4.Stein MD, Wilkinson J, Berglas N, O’Sullivan P. Prevalence and detection of illicit drug disorders among hospitalized patients. Am J Drug Alcohol Abuse. 1996;22(3):463–471. doi: 10.3109/00952999609001672. [DOI] [PubMed] [Google Scholar]
- 5.Katz A, Goldberg D, Smith J, Trick WE. Tobacco, alcohol, and drug use among hospital patients: concurrent use and willingness to change. J Hosp Med. 2008;3(5):369–375. doi: 10.1002/jhm.358. [DOI] [PubMed] [Google Scholar]
- 6.McNeely J, Gourevitch MN, Paone D, Shah S, Wright S, Heller D. Estimating the prevalence of illicit opioid use in New York City using multiple data sources. BMC Public Health. 2012;12:443. doi: 10.1186/1471-2458-12-443. doi:10.1186/1471-2458-12-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigotti NA, Munafo MR, Stead LF. Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960. doi: 10.1001/archinte.168.18.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saitz R, Palfai TP, Cheng DM, et al. Brief intervention for medical inpatients with unhealthy alcohol use: a randomized, controlled trial. Ann Intern Med. 2007;146(3):167–176. doi: 10.7326/0003-4819-146-3-200702060-00005. [DOI] [PubMed] [Google Scholar]
- 9.Chick J, Lloyd G, Crombie E. Counselling problem drinkers in medical wards: a controlled study. Br Med J (Clin Res Ed) 1985;290(6473):965–967. doi: 10.1136/bmj.290.6473.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freyer-Adam J, Coder B, Baumeister SE, et al. Brief alcohol intervention for general hospital inpatients: a randomized controlled trial. Drug Alcohol Depend. 2008;93(3):233–243. doi: 10.1016/j.drugalcdep.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Watson HE. A study of minimal interventions for problem drinkers in acute care settings. Int J Nurs Stud. 1999;36(5):425–434. doi: 10.1016/s0020-7489(99)00028-0. [DOI] [PubMed] [Google Scholar]
- 12.Substance Abuse and Mental Health Services Administration [Accessed September 17, 2013];Drug abuse warning network, 2011: national estimates of drug-related emergency department visits. http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm#5.1. [PubMed]
- 13.Stein MD. Medical consequences of substance abuse. Psychiatr Clin North Am. 1999;22(2):351–370. doi: 10.1016/s0193-953x(05)70081-2. [DOI] [PubMed] [Google Scholar]
- 14.Umbricht A, Hoover DR, Tucker MJ, Leslie JM, Chaisson RE, Preston KL. Opioid detoxification with buprenorphine, clonidine, or methadone in hospitalized heroin-dependent patients with HIV infection. Drug Alcohol Depend. 2003;69(3):263–272. doi: 10.1016/s0376-8716(02)00325-3. [DOI] [PubMed] [Google Scholar]
- 15.Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348(18):1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- 16.Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004;164(7):749–756. doi: 10.1001/archinte.164.7.749. [DOI] [PubMed] [Google Scholar]
- 17.Zanis DA, Woody GE. One-year mortality rates following methadone treatment discharge. Drug Alcohol Depend. 1998;52(3):257–260. doi: 10.1016/s0376-8716(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 18.Yancovitz SR, Des Jarlais DC, Peyser NP, et al. A randomized trial of an interim methadone maintenance clinic. Am J Public Health. 1991;81(9):1185–1191. doi: 10.2105/ajph.81.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stancliff S, Joseph H, Fong C, Furst T, Comer SD, Roux P. Opioid maintenance treatment as a harm reduction tool for opioid-dependent individuals in New York City: the need to expand access to buprenorphine/naloxone in marginalized populations. J Addict Dis. 2012;31(3):278–287. doi: 10.1080/10550887.2012.694603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995;40(1):17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 21.Kakko J, Grönbladh L, Svanborg KD, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164(5):797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- 22.Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803–808. doi: 10.1007/s11606-010-1311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobell LC, Sobell MB, Maisto SA, Cooper AM. Time-line follow-back assessment method. In: Lettieri DJ, Sayers MA, Nelson JE, editors. NIAAA Treatment Handbook Series: Alcoholism Treatment Assessment Research Instruments. National Institute on Alcoholism and Alcohol Abuse; Washington, DC: 1979. pp. 530–534. [Google Scholar]
- 24.McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment: initial studies of the treatment services review. J Nerv Ment Dis. 1992;180(2):101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Efron B, Tibshirani R. An Introduction to the Bootstrap. Chapman & Hall; New York, NY: 1993. [Google Scholar]
- 26.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein MD, Anderson B. Injection frequency mediates health service use among persons with a history of drug injection. Drug Alcohol Depend. 2003;70(2):159–168. doi: 10.1016/s0376-8716(02)00344-7. [DOI] [PubMed] [Google Scholar]
- 28.Neumann AM, Blondell RD, Azadfard M, Nathan G, Homish GG. Primary care patient characteristics associated with completion of 6-month buprenorphine treatment. Addict Behav. 2013;38(11):2724–2728. doi: 10.1016/j.addbeh.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20(11):1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. doi:10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127–135. doi: 10.1016/j.drugalcdep.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koester KA, Morewitz M, Pearson C, et al. Patient navigation facilitates medical and social services engagement among HIV-infected individuals leaving jail and returning to the community. AIDS Patient Care STDS. 2014;28(2):82–90. doi: 10.1089/apc.2013.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]