Abstract
Background/Objectives
To validly assess quality-of-care differences among providers, performance measurement programs must reliably identify and exclude patients for whom the quality indicator may not be desirable, including those with limited life expectancy. We developed an algorithm to identify patients with limited life expectancy and examined the impact of limited life expectancy on glycemic control and treatment intensification among diabetic patients.
Design
We identified diabetic patients with coexisting congestive heart failure, chronic obstructive pulmonary disease, dementia, end-stage liver disease, and/or primary/metastatic cancers with limited life expectancy. To validate our algorithm, we assessed 5-year mortality among patients identified as having limited life expectancy. We compared rates of meeting performance measures for glycemic control between patients with and without limited life expectancy. Among uncontrolled patients, we examined the impact of limited life expectancy on treatment intensification within 90 days.
Setting
110 Veterans Administration facilities; October 2006 – September 2007
Participants
888,628 diabetic patients
Measurements
Hemoglobin A1c (HbA1c) <9%; treatment intensification within 90 days
Results
29,016 (3%) patients had limited life expectancy. Adjusting for age, 5-year mortality was 5 times higher among patients with limited life expectancy than those without. Patients with limited life expectancy had poorer glycemic control (77.1% vs. 78.1%) and less frequent treatment intensification (20.9% vs. 28.6%) than patients without, even after controlling for patient-level characteristics (odds ratio [OR]=0.84; 95% confidence interval [CI]=0.81-0.86 and OR=0.71; 95% CI=0.67-0.76, respectively).
Conclusion
Patients with limited life expectancy were slightly, but significantly less likely than those without to have HbA1c levels controlled and to receive treatment intensification, suggesting that providers treat these patients less aggressively. Quality measurement and performance-based reimbursement systems should acknowledge the different needs of this population.
Keywords: diabetes, quality of care, performance measurement, limited life expectancy
Introduction
Performance measurement programs aimed at improving chronic illness care are typically designed to encourage changes in provider behavior and to promote the provision of care in accordance with clinical practice guidelines. However, these guidelines often provide recommendations for care of individual conditions, thereby neglecting the complexities of caring for chronically ill patients1 and failing to account for illness severity, even in the setting of life-limiting conditions. While few guidelines concede that less stringent goals may be appropriate for chronically ill patients with life-limiting conditions, those targeted at treatment of diabetes in geriatric patients have recommended individualized targets for glycemic control among patients with limited life spans.2 Outside of geriatric patients or those with certain metastatic cancers, however, limited life expectancy rarely receives consideration when performance measures are developed.
The Veterans Health Administration (VA) is a nationwide health care system with an extensive process for measuring the quality of care delivered at their facilities. Diabetes guidelines, including those by the VA/US Department of Defense, recommend individualizing targets for glycemic control among patients with limited life expectancy, acknowledging that less stringent goals may better balance the risks and benefits of glycemic management in these patients.3 However, these recommendations have not been fully implemented in the VA's clinical performance measurement program.4 For example, although the VA excludes patients with documented life expectancy of less than 6 months from most performance measures, there are no explicit provisions addressing patients with chronic conditions that may lead to a limited life expectancy.4 For instance, the 1-year survival rate for hospitalized patients with severe congestive heart failure (CHF) has been reported to be as low as 35%,5 rivaling that of liver cancer at 39.3%.6 Despite similarly poor prognoses, patients with liver cancer are excluded from national VA performance measures while those with severe CHF are not. Failure to amend performance measures to account for such patients may lead providers to implement treatment plans that may be inappropriate for certain patients.7
We sought to determine the impact of limited life expectancy on rates of glycemic control meeting VA performance measurement criteria among a nationwide cohort of Veterans with diabetes. To examine whether patients with limited life expectancy were as likely as those without limited life expectancy to achieve guideline recommended thresholds for diabetes care, we assessed glycemic control among patients receiving primary care within the VA health care system. Further, we assessed the impact of limited life expectancy on treatment intensification to determine whether providers adjusted medications in response to uncontrolled hemoglobin A1c (HbA1c) levels at the same frequency among patients with and without a limited life span.
Methods
Study setting and study population
We assessed glycemic control for a nationwide cohort of diabetic Veterans with and without conditions associated with limited life expectancy. Using VA administrative databases, we identified Veterans with diabetes who received primary care in the VA health care system between October 1, 2006 and September 30, 2007 (fiscal year [FY] 2007). Veterans were classified as having diabetes if any of the following was documented in the VA National Patient Care Database (NPCD) or the VA fee-basis files in either FY 2006 or 2007: 2 outpatient diagnoses codes or 1 inpatient diagnosis code8 indicating diabetes, filled prescription of diabetes medication (oral hypoglycemic medications or insulin), or at least 2 outpatient blood glucose readings > 200 mg/dL. We extracted medication and blood glucose readings from the VA Decision Support System (DSS) National Data Extract pharmacy and laboratory files.
We included all eligible diabetic patients receiving care at facilities with complete HbA1c data recorded in VA clinical and administrative data sources (Figure 1). We excluded patients who did not have a qualifying primary care visit and those receiving hospice care during the study period. Because we were interested in provider actions in response to uncontrolled readings, we also excluded patients who died during the study interval or follow-up period to allow all patients in the cohort an equal opportunity to receive treatment intensification during the follow-up period.
Figure 1.
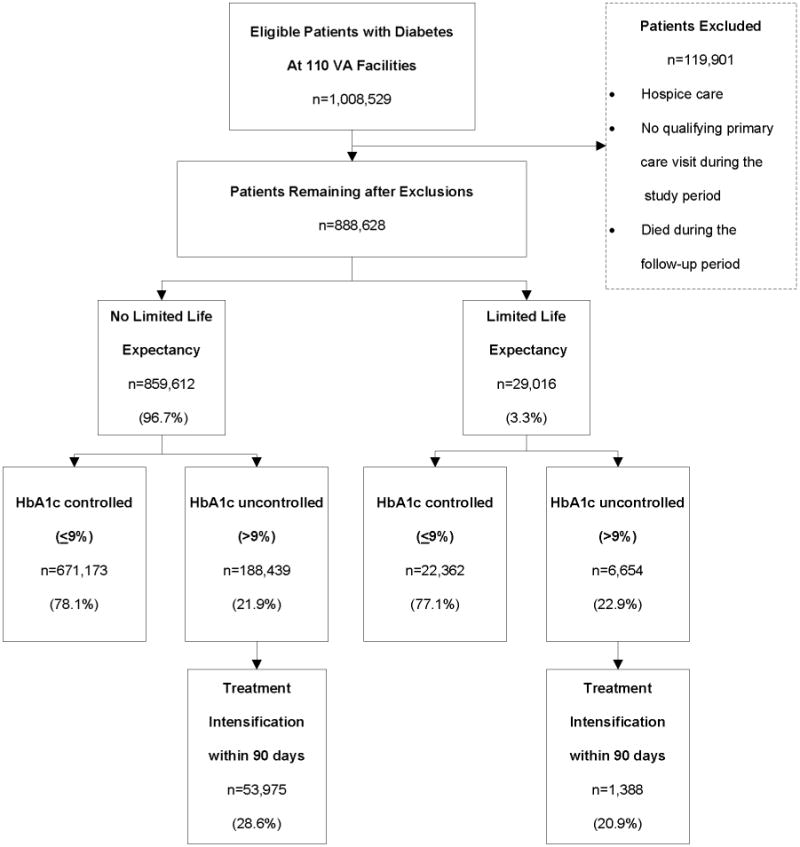
Patient Flow Diagram for Study Cohort and Exclusion Criteria. VA = Veterans Administration; HbA1c = hemoglobin A1c
We assigned each patient an index date to anchor the time period in which we assessed glycemic control and subsequent treatment intensification. The index date was the date of the patient's last HbA1c level recorded during the study interval. If no HbA1c level was documented, we used the patient's most recent primary care visit as the index date to anchor the follow-up period. If the patient did not have an HbA1c reading documented during the study period, we assessed whether the patient received a reading during the follow-up period. If that reading was uncontrolled, we determined whether the patient received treatment intensification. Each patient received 90 days of follow-up time regardless of when their index date occurred during FY 2007.
Identifying diabetic patients with limited life expectancy
We reviewed relevant literature and convened a multidisciplinary expert panel (the panel members are named in the acknowledgements) to identify conditions that health care providers frequently associate with limited life expectancy.9-12 Using VA administrative data and available Medicare data, we developed condition-specific algorithms (Appendix Table) to identify seriously ill patients with the following chronic conditions: CHF, chronic obstructive pulmonary disease (COPD), dementia, end stage liver disease (ESLD), and certain primary or metastatic cancers. Patients who met the criteria for at least 1 of these conditions were considered to have limited life expectancy.
To validate the algorithm used to define limited life expectancy, we created a comparable cohort of diabetic patients who accessed VA care in FY 2003 and compared 5-year mortality rates among patients with and without limited life expectancy. We chose 5-year mortality because of findings from clinical trials indicating that it takes approximately 8 years for patients to see microvascular benefits from intensive glucose management.13-15
Outcomes
We assessed glycemic control at index and receipt of treatment intensification among those uncontrolled at index. Because the VA identifies poor diabetes control as having an HbA1c > 9%, we considered patients with HbA1c </=9% to be controlled at index.16 For uncontrolled patients, we examined a 90-day follow-up period to ascertain whether the patient received treatment intensification by a health care provider. We defined treatment intensification as starting or adding a diabetes medication to a patient's regimen or increasing the dosage of an existing medication. Using the days supply, quantity, and strength of the medication, we determined if the average daily dosage increased in the follow-up period. For insulin, we assessed increases in average daily units prescribed.
Statistical analysis
We used chi-square analyses to compare the proportions of diabetic patients with and without limited life expectancy that were controlled at index and that received treatment intensification within 90 days. We used logistic regression to determine the impact of having limited life expectancy on the two outcomes. The models were adjusted for patient's age, gender, illness burden, race, VA enrollment priority group, number of VA primary care visits during the 1 year prior to index, and insulin use within 100 days of index. We included the patient's home facility as a random effect in the models to account for nesting of patients within facilities. We used a likelihood ratio test to determine the amount of variation in the outcomes assessed were due to facility-level variation. Diagnostic Cost Group Relative Risk Scores (DCG RRS) represented patients' overall illness burden.17 VA enrollment priority groups reflect disability related to military service or economic hardship and were included in the analyses to represent socioeconomic status and service-connected medical problems.18 To determine whether our findings were unique to our choice of a 90-day follow-up period, we performed separate sensitivity analyses using a 60-day and 120-day follow-up period. Analyses were conducted using SAS version 9.1.3 (SAS Institute, Inc., Cary, North Carolina) and the GLLAMM package in Stata version 11.2 (StataCorp, College Station, Texas).19 Both the Institutional Review Board at Baylor College of Medicine and the Michael E. DeBakey VA Research and Development Committee approved this study.
Results
Of 131 eligible VA facilities, 21 did not have complete HbA1c data available for analysis and therefore, the data for patients cared for at these facilities were excluded. The remaining facilities accounted for 84% of all patients with diabetes in the VA. We identified 888,628 diabetic patients meeting study inclusion criteria who received care from 110 VA facilities in FY 2007. We identified 29,016 patients, approximately 3% of the study cohort, who had at least 1 coexisting condition that was associated with limited life expectancy. The most prevalent limited life expectancy conditions were primary or metastatic cancer (38.3%) and COPD (23.7%) (Table 1). High disability (VA priority groups 1 and 4) and low income (VA priority group 5) ratings were greater among the patients with limited life expectancy (39.5% vs. 24.0% and 34.7% vs. 31.0%, respectively; P < 0.001). Patients with limited life expectancy also had a higher proportion of insulin use (23.7% vs. 17.8%; P < 0.001), similar HbA1c (2.3 vs. 2.1; P < 0.001) laboratory tests performed, and more VA primary care visits within 1 year of index (6.2 vs. 4.0; P < 0.001) compared to patients without limited life expectancy. However, on a per visit basis, patients with limited life expectancy had fewer HbA1c results performed (0.8 vs. 1.1, p < 0.001) (data not shown).
Table 1. Demographics and Clinical Characteristics of Veterans with Diabetes by Limited Life Expectancy Status.
| Limited Life Expectancy Status* | |||
|---|---|---|---|
|
| |||
| Characteristics | No (n = 859,612) | Yes (n = 29,016) | p-value |
| Male, n (%) | 838,676 (97.6) | 28,368 (97.8) | 0.03 |
| Mean age (SD), y | < 0 .001 | ||
| < 65 | 396,868 (46.2) | 12,688 (43.7) | |
| 65-74 | 227,230 (26.4) | 7,371 (25.4) | |
| >/=75 | 235,514 (27.4) | 8,957 (30.9) | |
| Race, n (%) | |||
| Black | 119,352 (13.9) | 5,012 (17.3) | < 0.001 |
| Non-black | 668,084 (77.7) | 22,893 (78.9) | |
| Unknown | 72,176 (8.4) | 1,111 (3.8) | |
| VA priority group, n (%) | |||
| 1,4 (high disability) | 206,808 (24.1) | 11,455 (39.5) | < 0.001 |
| 2,3,6 (low/moderate disability) | 159,062 (18.5) | 4,071 (14.0) | |
| 5 (low income) | 266,472 (31.0) | 10,078 (34.7) | |
| 7,8 (lowest priority) | 227,270 (26.4) | 3,412 (11.8) | |
| Mean DCG Relative Risk Score (SD) | 1.45 (2.18) | 7.14 (6.17) | < 0.001 |
| Insulin use, n (%) | 152,738 (17.8) | 6,876 (23.7) | < 0.001 |
| Mean HbA1c test days (SD) | 2.1 (1.3) | 2.3 (1.6) | < 0.001 |
| Mean VA primary care visits within 1 year prior to index (SD) | 4.0 (3.4) | 6.2 (5.0) | < 0.001 |
| Limited life expectancy diagnosis, n (%) | |||
| Primary/metastatic cancers | 11,118 (38.3) | ||
| Chronic obstructive pulmonary disease | 6,875 (23.7) | ||
| Dementia | 8,891 (30.6) | ||
| End-stage liver disease | 2,781 (9.6) | ||
| Congestive heart failure | 1,666 (5.7) | ||
VA = Veterans Administration; DCG = Diagnostic Cost Group; HbA1c = hemoglobin A1c
Limited life expectancy status determined by condition-specific algorithms for congestive heart failure, chronic obstructive pulmonary disease, dementia, end-stage liver disease, and certain primary or metastatic cancers.
Validating algorithm to identify patients with limited life expectancy
We found that patients with limited life expectancy had a 55% 5- year mortality rate while patients without limited life expectancy had a 15% 5-year mortality rate (data not shown). Adjusting for age, mortality among patients with limited life expectancy was more than 5 times that of patients without limited life expectancy.
Glycemic control and treatment intensification among patients with and without limited life expectancy
Unadjusted comparisons showed that glycemic control at index was slightly lower among patients with limited life expectancy, 77.1% vs. 78.1% (P < 0.001) (Table 2). Providers intensified treatment in response to elevated HbA1c less often in patients with limited life expectancy, 20.9% vs. 28.6% (P < 0.001).
Table 2. Frequency of Control at Index and Treatment Intensification within 90 days among Veterans with Diabetes by Limited Life Expectancy Status.
| Limited Life Expectancy Status* | |||
|---|---|---|---|
|
| |||
| No (n = 859,612) | Yes (n = 29,106) | p-value | |
| Hemoglobin A1c | |||
| Controlled at index, </=9% | 671,173 (78.1) | 22,362 (77.1) | < 0.001 |
| Treatment intensification† | 53,975 (28.6) | 1,388 (20.9) | < 0.001 |
Limited life expectancy status determined by condition-specific algorithms for congestive heart failure, chronic obstructive pulmonary disease, dementia, end-stage liver disease, and certain primary or metastatic cancers.
Evaluated treatment intensification for patients who were uncontrolled or had no reading at index. Treatment intensification included initiation of a diabetes medication, adding medication to the patient's regimen, or increasing the dosage of an existing medication.
In analyses adjusted for age, gender, race, VA priority enrollment group, illness burden, VA primary care utilization, and insulin use, diabetic patients with limited life expectancy were less likely to have HbA1c levels controlled at index (odds ratio [OR]=0.84; 95% confidence interval [CI]=0.81-0.86]) compared to patients that did not have limited life expectancy (Table 3). Similarly, black patients were less likely than non-black patients to have index HbA1c levels controlled (OR=0.83; 95% CI=0.82-0.84). In contrast, the number of VA primary care visits in the prior year was positively associated with control at index (OR=1.13; 95% CI=1.13-1.13). Older patients age 64-75 (OR=1.40; 95% CI=1.38-1.42) and > 75 (OR=1.25; 95% CI=1.23-1.26) were also more likely have controlled HbA1c levels. Similarly, patients with high disability (OR=1.10; 95% CI=1.08-1.12) and low/moderate disability (OR=1.10; 95% CI=1.08-1.12) were more likely to be controlled. A likelihood ratio test revealed that a significant amount of variation in glycemic control was due to facility-level variation (p < 0.001).
Table 3. Predictors of Glycemic Control at Index and Treatment Intensification within 90 days among Veterans with Diabetes.
| Odds Ratio (95% CI) | ||
|---|---|---|
|
| ||
| Variable | HbA1c Controlled at Index (HbA1c </= 9%) | Treatment Intensification* within 90 Days |
| Limited life expectancy | 0.84 (0.81-0.86) | 0.71 (0.67-0.76) |
| Male | 1.19 (1.15-1.23) | 1.39 (1.31-1.49) |
| Age (years) | ||
| < 65 | 1.00 (Reference) | 1.00 (Reference) |
| 65-74 | 1.40 (1.38-1.42) | 0.49 (0.48-0.50) |
| >/=75 | 1.25 (1.23-1.26) | 0.28 (0.27-0.29) |
| Race | ||
| Non-black | 1.00 (Reference) | 1.00 (Reference) |
| Black | 0.83 (0.82-0.84) | 1.25 (1.22-1.29) |
| Unknown | 0.84 (0.83-0.86) | 0.99 (0.96-1.03) |
| VA priority group | ||
| 7,8 (lowest priority) | 1.00 (Reference) | 1.00 (Reference) |
| 1,4 (high disability) | 1.10 (1.08-1.12) | 0.97 (0.94-1.01) |
| 2,3,6 (low/moderate disability) | 1.10 (1.08-1.12) | 1.05 (1.02-1.09) |
| 5 (low income) | 1.03 (1.01-1.04) | 1.33 (1.29-1.37) |
| DCG Relative Risk Score | 0.98 (0.98-0.98) | 0.95 (0.94-0.95) |
| VA primary care visits within 1 year prior to index | 1.13 (1.13-1.13) | 1.06 (1.06-1.07) |
| Insulin use | 0.85 (0.84-0.87) | 2.28 (2.22-2.33) |
HbA1c = hemoglobin A1c; DCG = Diagnostic Cost Group; VA = Veterans Administration
Treatment intensification included initiation of a diabetes medication, adding medication to the patient's regimen, or increasing the dosage of an existing medication.
Among those not controlled at index, we found that patients with limited life expectancy were less likely than those without to receive treatment intensification (OR=0.71; 95% CI=0.67-0.76) within 90 days. Older patients were also less likely to receive treatment intensification, with the oldest age group (age > 75 years) being the least likely (OR=0.28; 95% CI=0.27-0.29). Although black patients were less likely to be controlled at index, they were more likely than non-black patients to receive treatment intensification in response to uncontrolled HbA1c levels (OR=1.25; 95% CI=1.22-1.29). Patients treated with insulin were also more likely than those not taking insulin to have their treatment intensified (OR=2.28; 95% CI=2.22-2.33). Similarly, patients classified by the VA as having low income (OR=1.33; 95% CI=1.29-1.37) and low/moderate disability (OR=1.05; 95% CI=1.02-1.09) were more likely to receive treatment intensification than those classified as having high income (low priority). Similar to control at index analyses, a significant amount of variation was due to variation at facilities (p < 0.001). The interpretation of our results did not change when assessing a 60-day or 120-day follow-up period.
Discussion
We compared glycemic control and receipt of treatment intensification in response to uncontrolled HbA1c levels among diabetic patients with and without conditions associated with limited life expectancy. To identify conditions that health care providers associate with limited life expectancy, we developed condition-specific algorithms based on relevant literature and input from a multidisciplinary expert panel. We found that patients identified as having limited life expectancy using this algorithm had a 55% 5-year mortality rate. After adjusting for age, 5-year mortality was approximately 5 times higher among patients with limited life expectancy compared to those without. Our choice of 5-year mortality reflects findings from clinical trials indicating that it takes approximately 8 years for patients to benefit from intense glycemic control in the reduction of microvascular complications.13-15 Use of such an algorithm could enable performance measurement programs to readily identify chronically patients for whom the benefits of intensive glycemic control is limited and for whom less aggressive care is reasonable.
We found that diabetic patients with limited life expectancy were less likely than those without limited life expectancy to achieve glycemic control at index, after adjusting for clinical and sociodemographic characteristics. Although diabetic patients with limited life expectancy were less likely to have controlled HbA1c levels at index, they had on average similar numbers of HbA1c tests performed annually and accessed VA primary care more frequently than patients without limited life expectancy. However, these patients were less likely to achieve glycemic control and providers intensified treatment less frequently in response to elevated levels, which may represent appropriate care in the setting of a life-limiting condition. Frequent glucose monitoring likely provides minimal benefit in this group of patients, particularly when coupled with the reluctance of providers to intensify treatment in response to uncontrolled levels. Further, aggressive treatment exposes patients with limited life expectancy to additional risks (e.g. hypoglycemia) with little likelihood of benefit while diminishing opportunities to focus on other aspects of care, such as pain control or other comfort measures, which may be more pertinent to these patients.
We also found that black patients were less likely than non-black patients to have HbA1c levels controlled at index, but were more likely to receive treatment intensification. Despite lower levels of control, this finding is promising given recent findings that treatment intensification is particularly effective in lowering HbA1c levels among black patients.20 Conversely, older patients were more likely than the youngest patients to be controlled at index, but were less likely to receive treatment intensification. This finding may reflect a reluctance of providers to intensify treatment in poorly controlled older patients whom they may believe will not benefit from the therapy.21,22 However, providers should be cautious in using age alone when making treatment decisions. Older patients often vary widely in functional status, self-management abilities, and diabetes-related complications. Patients whose benefits of outweigh the risks should be considered for more intensive diabetes management, regardless of age. Thus, such considerations should factor into a provider's decisions to intensify treatment among older patients.2
One approach to mitigating incentives for over-treatment that may result from performance measurement and performance-based reimbursement programs is exception reporting. In the United Kingdom (UK), exception reporting allows providers to use clinical judgment to exclude certain patients from performance measures.23 Despite concerns that UK providers would “game” the system by excluding patients for whom quality indicators would be difficult to attain, only a small proportion of patients were excluded from performance measures and there was minimal evidence of gaming.24 Additionally, the preservation of autonomy and the presence of exception reporting in the UK program contributed to higher provider satisfaction compared to providers in a similar California program, who expressed dissatisfaction with their perceived lack of discretion and inability to exclude patients from measures that may not be appropriate.25 In addition to improving provider satisfaction, studies suggest that developing measures of quality that focus less on strict guideline adherence and instead promote more comprehensive, individualized chronic illness care may result in higher patient satisfaction as well.26, 27 Thus, future iterations of performance measurement systems should consider including provisions such as those in place in the UK to exclude patients for whom a particular measure may be inappropriate.28, 29
Alternative explanations for our findings may exist. Studies have shown that health care providers are uncomfortable with end-of-life care discussions.30-32 Thus, our findings that providers have more frequent visits with and perform laboratory testing with similar frequency in the treatment of patients with limited life expectancy compared to those without may be explained by their tendency to provide usual care, rather than confronting what may be a poor prognosis. Alternatively, there is evidence that providers are often inaccurate when assessing the prognosis of chronically ill patients.33 It is possible that our findings reflect a lack of provider recognition of chronically ill patients who are near the end of life. However, the lower likelihood of in treatment intensification among those with limited life expectancy argues against this explanation.
Our study is limited in that it focused solely on care for a single, although highly prevalent, chronic condition and that it was conducted in the overwhelmingly male VA patient population. However, we are not aware of evidence suggesting that the care provided to female patients with limited life expectancy would differ significantly from that offered to male patients. Additionally, evidence shows that quality of care may be higher in the VA system than outside; thus, our findings may be different in a non-VA setting.34-36
In summary, we found that diabetic patients with limited life expectancy were less likely than those without limited life expectancy to achieve controlled HbA1c levels and to receive treatment intensification in response to abnormal levels. Despite these findings, patients with limited life expectancy received HbA1c testing with similar frequency and had higher numbers of VA primary care encounters than those without. Although the severity of illness in patients with limited life expectancy may necessitate more frequent visits, we posit that the frequency of HbA1c testing among this group may represent excessive care and consume resources unnecessarily. This could result in part from performance measurement and incentives that do not make appropriate considerations for patients with very low likelihood of benefiting from testing and treatment.37 In contrast, failure to achieve guideline-recommended levels and to intensify therapy in response to abnormal levels may, in fact, be appropriate in this population. Thus, as currently structured, performance measures could promote overuse of services while penalizing less stringent glycemic and lipid management, providing incentives that may reward inappropriate care for patients with life-limiting conditions. Policy initiatives advocating for the prevention of unnecessary and excessive treatment may be an important deterrent to overuse of testing and may encourage more patient-centered care in this population as opposed to care in strict accordance to guidelines. Further work is needed to design quality measurement and performance-based reimbursement systems that address the unique needs of this patient population. For performance measures to improve health care quality, they must account for patient-level factors such as life expectancy to ensure that providers are appropriately rewarded for providing individualized, patient-centered care.
Acknowledgments
Funding Sources and Related Paper Presentations: This work is supported in part by VA HSR&D PPO 09-316 (PI LeChauncy D. Woodard, MD, MPH), VA HSR&D SHP 08-166 (PI Laura A. Petersen, MD, MPH), and Houston VA HSR&D Center of Excellence HFP90-020 (PI Laura A. Petersen, MD, MPH). Dr. Petersen was a Robert Wood Johnson Foundation Generalist Physician Faculty Scholar (grant 045444) at the time this work was conducted. Dr. Profit's contribution is supported by NICHD K23 HD056298 (PI Jochen Profit). Dr. Virani is supported by a Department of Veterans Affairs Health Services Research and Development (HSR&D) Service Career Development Award (CDA-09-028). Parts of this manuscript were presented at the Department of Veterans Affairs annual Health Services Research and Development National Meeting in February 2009 in Washington, DC and at the Society of General Internal Medicine annual meeting in May 2009 in Miami, Florida.
Sponsor's Role: The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript
Appendix Table.
Algorithms for Identifying Patients with Limited Life Expectancy*
| Congestive Heart Failure (CHF) |
|---|
|
|
|
| Chronic Obstructive Pulmonary Disease (COPD) |
|
|
COPD ICD-9-CM diagnosis codes (1 inpatient or 2 outpatient codes during study interval or 5 years prior):
|
|
|
| Primary Cancers |
|
|
Primary Cancers of the esophagus, peritoneum, liver, gallbladder, pancreas, pleura, or stomachICD-9-CM diagnosis codes (1 outpatient or 1 inpatient code during study interval or 1 year prior):
|
|
|
| Metastatic Cancers |
|
|
Metastatic Cancers ICD-9-CM diagnosis codes (1 outpatient or 1 inpatient code during study interval or 1 year prior):
|
|
|
| Dementia |
|
|
Dementia ICD-9-CM diagnosis codes (1 inpatient or 2 outpatient codes during study interval or 5 years prior):
|
|
|
| End-Stage Liver Disease (ESLD) |
|
|
ESLD ICD-9-CM diagnosis codes for cirrhosis (1 inpatient or 2 outpatient codes during study interval or 5 years prior):
|
ICD-9-CM = International Classification of Diseases, Clinical Modification, 9th Revision; CPT = Current Procedural Terminology; DSS = Decision Support System
Patients need only to meet the criteria for one of the conditions CHF, COPD, Primary Cancers, Metastatic Cancers, Dementia, or ESLD to be classified as having limited life expectancy
Study interval = Veterans Administration fiscal year 2007 (October 1, 2006 – September 30, 2007) plus 8-week follow up period
All codes must appear on separate visits on separate days
Footnotes
The views expressed are solely of the authors, and do not necessarily represent those of the VA.
Conflicts of Interest: No author has any conflicts of interest to disclose.
Author Contributions: Dr. Woodard had full access to all of the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Woodard, Landrum, Urech, Virani, Petersen Acquisition of data: Woodard, Petersen Analysis and interpretation of data: Woodard, Landrum, Urech, Profit, Virani, Petersen, Pietz
Other contributions: The authors would like to acknowledge Mark Kuebeler, MS, Michael E. DeBakey VA Medical Center HSR&D Center of Excellence, for his programming effort and Kenneth Pietz, PhD, Michael E. DeBakey VA Medical Center HSR&D Center of Excellence, for his statistical expertise. The authors would also like to acknowledge the members of the expert panel that helped to define conditions associated with limited life expectancy: Neena S. Abraham, MD, MSCE, FACG, FASGE, John Chen, PhD, MD, MPH, Jessica Davila, PhD, Thomas Giordano, MD, MPH, Mark Kunik, MD, MPH, and Aanand Naik, MD, all of the Michael E. DeBakey VA Medical Center HSR&D Center of Excellence and Baylor College of Medicine. Dr. Woodard affirms that she has listed everyone who contributed significantly to the work and has obtained written consent from all contributors who are not authors and are named in the Acknowledgement section.
References
- 1.Boyd CM, Darer J, Boult C, et al. Clinical Practice Guidelines and Quality of Care for Older Patients with Multiple Comorbid Diseases. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 2.California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for Improving the Care of the Older Person with Diabetes Mellitus. Journal of the American Geriatrics Society. 2003;51(5 Suppl Guidelines):S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 3.Veterans Health Affairs, Departmentof Defense. VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus. 2010 Aug; Version 4.0. Release date: [Google Scholar]
- 4.Office of Quality and Performance. FY 2011 Q1 Clinical Measures Specification Manual. Vol. 2. Veterans Health Administration; Oct 1, 2010. [Google Scholar]
- 5.Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 6.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975-2006. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. [Google Scholar]
- 7.Hayward RA. Performance measurement in search of a path. N Engl J Med. 2007;346:951–952. doi: 10.1056/NEJMe068285. [DOI] [PubMed] [Google Scholar]
- 8.Solberg LI, Engebretson KI, Sperl-Hillen JM, et al. Are claims data accurate enough to identify patients for performance measures or quality improvement? The case of diabetes, heart disease, and depression. Am J Med Qual. 2006;21:238–245. doi: 10.1177/1062860606288243. [DOI] [PubMed] [Google Scholar]
- 9.Silveira MJ, Kazanis AS, Shevrin MP. Statins in the last six months of life: A recognizable, life-limiting condition does not decrease their use. J Palliat Med. 2008;11:685–693. doi: 10.1089/jpm.2007.0215. [DOI] [PubMed] [Google Scholar]
- 10.Braun UK, McCollough LB, Beyth RJ, et al. Racial and ethnic differences in the treatment of seriously ill patients: A comparison of African-American, Caucasian and Hispanic veterans. J Natl Med Assoc. 2008;100:041–1051. doi: 10.1016/s0027-9684(15)31442-5. [DOI] [PubMed] [Google Scholar]
- 11.Casarett DJ, Quill TE. “I'm not ready for hospice”: Strategies for timely and effective hospice discussions. Ann Intern Med. 2007;146:443–449. doi: 10.7326/0003-4819-146-6-200703200-00011. [DOI] [PubMed] [Google Scholar]
- 12.Fox E, Landrum-McNiff K, Zhong Z, et al. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA. 1999;282:1638–1645. doi: 10.1001/jama.282.17.1638. [DOI] [PubMed] [Google Scholar]
- 13.United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 14.Goddihn PP, Bilo HJ, Feskens EJ, et al. Longitudinal study on glycemic control and quality of life in patients with type 2 diabetes mellitus referred for intensified Control. Diabet Med. 1999;16:23–30. doi: 10.1046/j.1464-5491.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 15.Shorr RI, Franse LV, Resnick He. Glycemic control of older adults with type 2 diabetes: Findings from the Third National Health and Nutrition Examination Survey, 1988-94. J Am Geriatr Soc. 2000;48:264–267. doi: 10.1111/j.1532-5415.2000.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 16.Office of Quality and Performance. FY 2007, Q3 Technical Manual. VHA Performance Measurement System. 2007 Mar 16; [Google Scholar]
- 17.Petersen LA, Pietz K, Woodard LD, et al. Comparison of the predictive validity of diagnosis-based risk adjusters for clinical outcomes. Med Care. 2005;43:61–67. [PubMed] [Google Scholar]
- 18.Petersen LA, Byrne MM, Daw CN, et al. Relationship between clinical conditions and use of Veterans Affairs health care among Medicare-enrolled Veterans. Health Serv Res. 2010;45:762–791. doi: 10.1111/j.1475-6773.2010.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabe-Hesketh S, Skrondal A, Pickles A. GLLAMM Manual. University of California; Berkley: 2004. Paper 160. [Google Scholar]
- 20.McEwen LN, Bilik D, Johnson SL, et al. Predictors and impact of intensification of antihyperglycemic therapy in type 2 diabetes. Diab Care. 2009;32:971–976. doi: 10.2337/dc08-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: The treatment-risk paradox. JAMA. 2004;291:1864–1870. doi: 10.1001/jama.291.15.1864. [DOI] [PubMed] [Google Scholar]
- 22.Virani SS, Woodard LD, Chitwood SS, et al. Frequency and correlates of treatment intensification for elevated cholesterol levels in patients with cardiovascular disease. Am Heart J. 2011;162:725–732.e1. doi: 10.1016/j.ahj.2011.07.013. Epub 2011 Aug 25. [DOI] [PubMed] [Google Scholar]
- 23.British Medical Association. Quality and outcomes framework guidance: Section 2: clinical indicators. [Accessed July 26, 2011]; Available at http://www.bma.org.uk/employmentandcontracts/independent_contractors/quality_outcomes_framework/qof06.jsp?page=6#Exceptionreporting.
- 24.Doran T, Fullwood C, Reeves D, et al. Exclusion of patients from pay-for-performance targets by english physicians. N Engl J Med. 2008;359:274–284. doi: 10.1056/NEJMsa0800310. [DOI] [PubMed] [Google Scholar]
- 25.McDonald R, Rolan M. Pay for performance in primary care in England and California: Comparison of unintended consequences. Ann Fam Med. 2009;7:121–127. doi: 10.1370/afm.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang ES, Zhang Q, Gandra N, et al. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: A decision analysis. Ann Intern Med. 2008;149:11–19. doi: 10.7326/0003-4819-149-1-200807010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayliss EA, Edwards AE, Steiner JF, et al. Processes of care desired by elderly patients with mulitmorbidities. Fam Pract. 2008;25:287–293. doi: 10.1093/fampra/cmn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choe HM, Bernstein SJ, Standiford CJ, et al. New diabetes HEDIS blood pressure quality measure: potential for overtreatment. Am J Manag Care. 2010;16:19–24. [PubMed] [Google Scholar]
- 29.Wegner MS, Solomon DH, Amin A, et al. Application of assessing care of vulnerable elders-3 quality indicators to patients with advanced dementia and poor prognosis. J Am Geriatr Soc. 2007;55(Suppl 2):S457–463. doi: 10.1111/j.1532-5415.2007.01375.x. [DOI] [PubMed] [Google Scholar]
- 30.Weiner JS, Roth J. Avoiding iatrogenic harm to patient and family while discussing goals of care near the end of life. Palliat Care Rev. 2006;9:451–463. doi: 10.1089/jpm.2006.9.451. [DOI] [PubMed] [Google Scholar]
- 31.Larson DG, Tobin DR. End-of-life conversations: Evolving practice and theory. JAMA. 2000;284:1573–1578. doi: 10.1001/jama.284.12.1573. [DOI] [PubMed] [Google Scholar]
- 32.Curtis JR, Patrick DL, Caldwell ES, et al. Why don't patients and physicians talk about end-of-life care? Barriers to communication for patients with acquired immunodeficiency syndrome and their primary care clinicians. Arch Intern Med. 2000;160:1690–1696. doi: 10.1001/archinte.160.11.1690. [DOI] [PubMed] [Google Scholar]
- 33.Christakis NA, Lamont EB. Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–473. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Executive Summary. American Diabetes Association. Standards of Medical Care in Diabetes – 2009. Diabetes Care. 2009;32(supplement 1):S6–S12. doi: 10.2337/dc09-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr EA, Gerzoff RB, Krein SL, et al. diabetes care quality in the Veterans Affairs Health Care System and Commercial Managed Care: The TRIAD Study. Ann Intern Med. 2004;141:272–281. doi: 10.7326/0003-4819-141-4-200408170-00007. [DOI] [PubMed] [Google Scholar]
- 36.Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and Patients in a National Sample. Ann Intern Med. 2004;141:938–945. doi: 10.7326/0003-4819-141-12-200412210-00010. [DOI] [PubMed] [Google Scholar]
- 37.Walter LC, Davidowtiz NO, Heineken PA, et al. Pitfalls of converting practice guidelines into quality measures: lessons learned from a VA performance measure. JAMA. 2004;291:2466–2470. doi: 10.1001/jama.291.20.2466. [DOI] [PubMed] [Google Scholar]


