Abstract
Inherited red blood cell (RBC) membrane disorders, such as hereditary spherocytosis, elliptocytosis and hereditary ovalocytosis, result from mutations in genes encoding various RBC membrane and skeletal proteins. The RBC membrane, a composite structure composed of a lipid bilayer linked to a spectrin/actin-based membrane skeleton, confers upon the RBC unique features of deformability and mechanical stability. The disease severity is primarily dependent on the extent of membrane surface area loss. RBC membrane disorders can be readily diagnosed by various laboratory approaches that include RBC cytology, flow cytometry, ektacytometry, electrophoresis of RBC membrane proteins and genetics. The reference technique for diagnosis of RBC membrane disorders is the osmotic gradient ektacytometry. However, in spite of its recognition as the reference technique, this technique is rarely used as a routine diagnosis tool for RBC membrane disorders due to its limited availability. This may soon change as a new generation of ektacytometer has been recently engineered. In this review, we describe the workflow of the samples shipped to our Hematology laboratory for RBC membrane disorder analysis and the data obtained for a large cohort of French patients presenting with RBC membrane disorders using a newly available version of the ektacytomer.
Keywords: Red blood cell membrane, Spherocytosis, Ektacytometer, LoRRca, EMA
1. Introduction
The red blood cell (RBC) membrane is not a static structure but is highly dynamic, enabling it to undergo the extensive deformations necessary for traversing the vascular bed to perform its main function of oxygen delivery. The complex structural organization of the various RBC membrane components is responsible for its unique features of extensive deformability and mechanical stability [1–4]. The RBC membrane is composed of two important complexes, the ankyrin and the 4.1R complexes, which link various proteins embedded in the phospholipid bilayer to the α/β spectrin heterodimers underlying the lipid bilayer [1,3,5]. Alterations in the structural membrane organization due to various protein defects are responsible for a large panel of human disorders either constitutional or acquired. Inherited RBC membrane disorders, such as hereditary spherocytosis (HS), hereditary elliptocytosis (HE) and its severe form known as hereditary pyropoikilocytosis (HPP), hereditary ovalocytosis (SAO), and hereditary stomatocytosis (HSt) are commonly responsible for hemolytic anemia. The severity of the disease is variable and depends on the extent of surface area loss, ranging from asymptomatic forms to severe neonatal or prenatal forms responsible for rare hydrops fetalis cases requiring transfusion in utero.
HS and HE [1,6–10] are the most common RBC membrane disorders worldwide with a prevalence of 1 out of 2000 cases in North America and Northern European countries. HS has been linked to defects in the ankyrin (ANK1), α-spectrin (SPTA1), β-spectrin (SPTB), band 3 (SLC4A1) or protein 4.2 (EPB42) genes [11–15] and HE to defects in the SPTA1, SPTB or protein 4.1R genes [9,16–21]. In both HS and HE, RBC life span is shortened as a result of splenic sequestration of RBCs with increased sphericity. The abnormal RBCs with decreased membrane surface area and increased sphericity are trapped in the billroth canals in the spleen and phagocytozed by the splenic reticulo-endothelial system [22,23], resulting in regenerative hemolytic anemia, splenomegaly, and ictera with increased free bilirubin level. Hereditary ovalocytosis, also designated as South-east Asian Ovalocytosis (SAO), has a geographical distribution mostly in Indonesia, the Philippines, Melanesia and Southern Thailand [24–26]. The molecular defect responsible for SAO has been identified as a deletion of the 27 nucleotides encoding amino acids 400 to 408 in the SLC4A1 gene [27,28]. Hereditary stomatocytosis is a rare RBC disorder divided into two different entities: xerocytosis or dehydrated hereditary stomatocytosis (DHSt) and overhydrated hereditary stomatocytosis (OHS) [6,7,9,29,30]. In both cases, RBCs exhibit a leak to the univalent cations Na+ and K+, resulting in altered intracellular cation content and cell volume alterations. Although PIEZO1 and RhAG gene mutations have been identified in xerocytosis [31–33] and the overhydrated form [34], respectively, the molecular basis for both forms of hereditary stomatocytosis is still under investigation.
The RBC membrane disorders described above can be usually diagnosed easily, sometimes even without any specialized laboratory tests besides a meticulous cytologic microscopic examination and measurement of RBC and reticulocyte indices. In 2011, Bolton-Maggs et al. [35] defined guidelines for the diagnosis and the management of hereditary spherocytosis. Indeed, patients with a family history of HS and typical HS biological manifestations (hemolytic anemia with high Cell Hemoglobin Concentration Mean (CHCM) >36 g/dl on Siemens/Advia hematological analyzers, high percentage (>4%) of hyperdense cells (gated RBCs with a CHCM >41 g/dL) and the presence of spherocytic cells in blood smears) do not require any additional test (grade 1 recommendation, grade A evidence). However, more specific biological tests may be required in cases where the HS diagnosis is not readily evident including: lack of HS family history, lack of typical biological manifestation (in particular normal osmotic fragility and iron deficiency, which may mask the regeneration, the increased CHCM, and the increased reticulocyte count) or severe forms of elliptocytosis and stomatocytosis, in which the diagnosis may be difficult in particular in the dehydrated form. It is critical to accurately diagnose hereditary stomatocytosis in order to exclude splenectomy as part of patient treatment because, for reasons that are still unclear, splenectomy in the case of stomatocytosis leads to lethal thrombosis. Various specialized laboratory tests are available to clinicians to help them to select for the most pertinent ones based upon sensitivity and specificity. Flow cytometry measurement of the mean RBC fluorescence, after labeling of RBCs with the dye eosin-5′ maleimide (EMA), is often used to document surface area loss and is a test of choice for screening of HS [35–45]. This test is able to detect HS with a sensitivity of 92.7% and a specificity of 99.1%, with a positive predictive value of 97.8% and a negative predictive value of 96.9%. However, this EMA-based test fails to identify some HS cases associated with ankyrin defects and is not as reliable for other RBC membrane disorders such as elliptocytosis, pyropoikilocytosis, stomatocytosis and SAO. Osmotic gradient ektacytometry fills this gap and has therefore been considered a reference technique and the diagnostic test for HS and the other red cell membrane disorders. However, until now, the use of ektacytometry has not been widespread in the routine hematology laboratory due to the limited availability of the ektacytometer, originally designed in the seventies. Ektacytometry has not even been evaluated in the guidelines published in 2011. Recently, a new generation ektacytometer, the Osmoscan LoRRca MaxSis, has been engineered by Mechatronics Instruments BV® (Zwaag, The Netherlands), which measures RBC deformability under a defined shear stress as a function of suspending medium osmolality. The differences between this new generation laser diffraction viscometer and the previous ektacytometer (Technicon®) reside mostly in the analysis of the diffraction images acquired: while the Technicon model measures the extent of light scattering by a photodiode to define ellipticity of the diffraction images, the LoRRca MaxSis scans images of acquired diffraction patterns using a digital camera and analyzes the images with the gray nuance from 0 to 128 of 256,000 pixels. In both systems, a value for ellipticity of the diffraction images, a measure of cellular deformability defined as the deformability index (or IE) is generated. The 3 key features of the osmotic gradient ektacytometer profile (Omin, DImax and Hyper or O′) can be calculated and analyzed. In this review, we describe the results of the analysis of RBC deformability features in patients presenting with various RBC membrane disorders and other diseases obtained over a period of 20 months in our hematology laboratory using this new generation ektacytometer and usual diagnostic tests.
2. Methods
2.1. Characteristics of the population studied for red blood cell membrane disorders
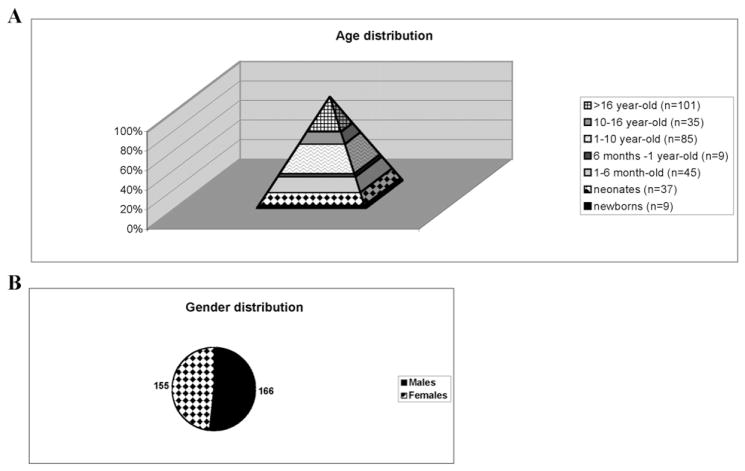
A total of 321 patients have been referred to our hematology clinical diagnosis laboratory, at the Robert Debré hospital in Paris (France), for RBC membrane disorder diagnosis during this 20-month study. Some of them have been diagnosed before and have been sent to us for the purpose of the study. The vast majority of the patients have been studied in the regular work-flow of the laboratory. The population studied included 166 male patients and 155 female patients. As expected, the population pyramid was in favor of infants with 220 out of 321 samples obtained from patients under 16 years of age, 185 of which were from individuals less than 10 years old (Fig. 1A–B). In most cases, the underlying pathologies were diagnosed between birth and 10 years of age, with only 35 patients being diagnosed between 10 and 16 years of age. Of particular interest, 3 blood cord samples have been successfully studied leading to an early diagnosis of HS in one of these cases. This study was carried out in accordance with the Declaration of Helsinki.
Fig. 1.
Age distribution (A) and gender distribution (B) of the populations studied.
2.2. Work-flow of a laboratory specialized in diagnosis of red blood cell membrane disorders
2.2.1. RBC indices, reticulocyte count and biochemical analysis
RBC indices including hemoglobin concentration, hematocrit, mean cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean hemoglobin content (MHC), RBC volume distribution (RDW) and reticulocyte count were measured for each sample using a hematological analyzer (XE 2100, Sysmex, Kobe, Japan). Blood smears stained with May Grümwald Giemsa (MGG) were carefully examined and diagnosis of RBC morphology abnormalities were validated independently by two cytologists prior to additional analysis. All blood samples were collected on EDTA (used as anticoagulant) and shipped at 4 °C as soon as possible after the blood draw along with a blood smear. Samples were delivered to our laboratory within 48 h after blood collection. The blood smears sent with the tubes of blood were used as a reference for optimal RBC morphology analysis to rule out that blood smears performed upon arrival might identify RBC feature artefacts (echinocytes, acanthocytes, speculated dense RBCs) that could arise during shipment. In addition, direct agglutination test (DAT), analysis of iron deficiency or overload (iron, ferritin, transferrin receptor, transferrin saturation percentage, total capacity of transferrin fixation), evaluation of hemolysis markers (indirect bilirubin, haptoglobin, LDH), hemoglobin electrophoresis and glucose-6 phosphate dehydrogenase (G6PD) as well as pyruvate kinase (PK) enzymatic activity measurements were performed.
2.2.2. Eosin-5′-maleimide (EMA) dye test by flow cytometry
After performing routine analysis with the hematology analyzer and ektacytometry analysis on fresh patient samples, samples were stored at 4 °C for a maximum of 7 days to be tested by flow cytometry, with no differences being observed over that time frame in accordance with previously published work [46]. All 321 samples were subjected to an EMA-test, consisting of EMA dye labeling of RBCs and subsequent mean RBC fluorescence measurement by flow cytometry (Beckman-Dickinson, BD Biosciences, Qume Drive San José, CA, USA) as previously described by King et al. [38,40,41]. EMA binds predominantly to band 3 (the anion exchanger) through covalent binding to Lys430 in the first extracellular loop of the band 3 protein but it can also interacts with sulfhydryl groups expressed by the Rh, RhAG and CD47 antigens, thus allowing to evaluate protein copy number and conformation. This robust technique has been used now for more than a decade as a first screen for HS diagnosis with a sensitivity of 92.7% and a specificity of 99.1%. Additionally, as EMA assay requires only a small amount of blood (5 μl of washed packed RBCs) it can therefore be easily performed along with a routine CBC test including RBC indices and reticulocyte count measurement (180 μl of blood) and an ektacytometry analysis (100 μl of blood). The mean fluorescence intensity (MFI) for each sample was compared to 6 age-matched controls collected on the same day. A ratio of the mean fluorescence for patient RBCs to the mean fluorescence for the 6 controls was derived (mean of 6 age-matched control MFI – patient MFI/mean of 6 age-matched control MFI). The cut-off value above which the test is considered positive is much debated. In our study, we used the cut-off value chart described by Girodon et al. [46]: when the mean fluorescence ratio was decreased by >21%, the test was considered positive while a value of <16% was considered negative. Values between 16 and 21% were considered as indeterminate and additional studies were needed to either confirm or rule out the diagnosis of HS for such samples [46]. We further evaluated optimal cut-off taking advantage of our large cohort.
2.2.3. Ektacytometry
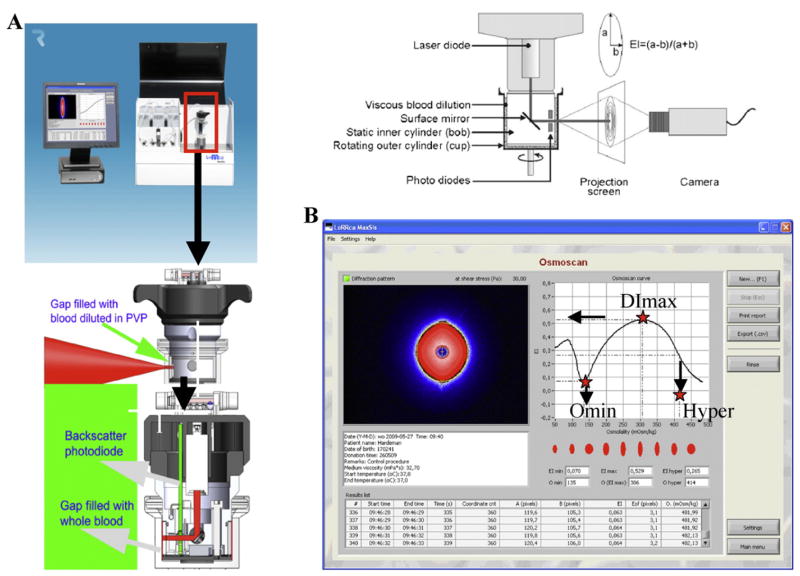
Blood samples (minimum of 100 μl), either fresh or within 48 h after blood collection, were analyzed by osmotic gradient ektacytometry. Briefly, blood samples were subjected to a defined value of shear stress and an osmotic gradient and the laser diffraction pattern generated by the RBC suspension was recorded. The RBC shape goes from circular to elliptical as shear stress increases. From these measurements a deformability index for the cells can be derived. Ektacytomers, including the LoRRca, use a Couette geometry with a static cylinder and a rotating cylinder to create a known shear flow (Fig. 2A). Thus, the ektacytometer is a laser diffraction viscometer, in which the deformation of RBCs suspended in a viscous polyvinylpyrrolidone (PVP) solution at defined values of applied shear stress of 30 Pa and at a constant temperature of 37 °C are monitored as a continuous function of medium osmolality. Three distinct features of the osmotic gradient ektacytometry profiles are the Omin, the DImax and the O′ or hyper points. The Omin point corresponds to the osmolarity at the minimal deformability in hypo-osmolar region and corresponds to the osmolarity at which 50% of the RBCs hemolyzed in the conventional osmotic fragility test. It reflects the surface area to volume ratio of red cells. DImax corresponds to the maximal deformability index or elongation index (EI) and is a reflection of the membrane surface. The hyper point or O′ corresponds to the osmolarity at which reaches 50% of the DImax in the hyper-osmolar area and reflects the hydration state of the cells (Fig. 2B). In the case of HS [47,48], the constant and characteristic features are a decrease in the DImax in conjunction with a shift of the Omin point to the right (reduced surface to volume ratio of RBCs) and a shift of the O′ or hyper point to the left (increased dehydration of RBCs). The amount of blood required to perform the test is small (100 μl) and, as for the EMA dye test, ektacytometry analysis can be performed on blood samples from neonates. The limitation of ektacytometry is the restricted availability of the instrument and the need to perform the analysis within 48 h of blood sampling. Importantly, the ektacytometer generates distinct osmotic deformability profiles, thus enabling diagnosis of not only HS but also other RBC membrane disorders such as elliptocytosis, HPP, stomatocytosis and SAO.
Fig. 2.
A. Principle of ektacytometry. A laser beam is scattered by a suspension of RBCs stressed between a static inner cylinder (bob) and a rotating outer cylinder (cup). The shape of the diffraction pattern, projected on a small screen, is a measure of the average RBC deformability and changes from circular at rest to elliptical at a high shear stress. The major (a) and minor (b) axes of the ellipse serve to calculate the elongation index, EI = (a − b)/(a + b). B. Deformability pattern obtained using the new generation ektacytometer and the derivation of the three ektacytometry derived parameters (DImax, Omin and Hyper points).
The LoRRca MaxSis is a new generation ektacytometer. It is a unique instrument, designed for hemo-rheological research and clinical application, that combines RBC ektacytometry with an osmotic gradient and aggregometry. A laser diode, mounted in the bob, serves as light source. The reflected light is sensed by a photodiode. The laser beam diffraction pattern is detected with a video camera and analyzed by a computer. It enables simultaneous analysis of three major RBC properties, RBC cell geometry, viscosity and deformability, under the osmoscan application.
Mean, CI 95%, variance, standard deviation, median, minimum/maximum, 5–95 percentile, Jouden J index and ROC curves were calculated using the Medcalc software (http://www.medcalc.org; Ostend, Belgium).
3. Results
3.1. Red blood cell indices and reticulocyte count in the population
Hemoglobin concentration, hemoglobin content, hematocrit, mean cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC) and reticulocyte count were determined for all 321 samples except for 8 samples. The MCV, MCHC and the hemoglobin content varied among patients depending on the extent of the RBC membrane disorder (Table 1).
Table 1.
Red cell indices and reticulocyte count (mean ± S).
| Hb (g/L) | MCV (fL) | MHC (pg/cell) | MCHC (g/dL) | RDW (%) | Reticulocyte (×109/L) | ||
|---|---|---|---|---|---|---|---|
| HS | Mean | 11.1 | 83.9 | 29.2 | 34.8 | 19.2 | 313 |
| (n = 75) | S | ±2.4 | ±10.1 | ±3.2 | ±2.2 | ±4.5 | ±198 |
| HE | Mean | 11.5 | 77.3 | 26.0 | 33.5 | 16.5 | 80 |
| (n = 44) | S | ±1.9 | ±10.2 | ±4.0 | ±1.6 | ±3.8 | ±48 |
| ABO incompatibility/AIHA | Mean | 10.9 | 100.9 | 33.7 | 33.4 | 20.5 | 381 |
| (n = 18) | S | ±2.9 | ±16.8 | ±5.8 | ±1.8 | ±4.3 | ±213 |
| Stomatocytosis | Mean | 11.3 | 92.4 | 30.7 | 33.4 | 15.6 | 225 |
| (n = 4) | S | ±1.8 | ±18.1 | ±5.6 | ±2.5 | ±1.8 | ±244 |
| SAO | Mean | 13.7 | 83.5 | 29.1 | 35.0 | 16.7 | 142 |
| (n = 5) | S | ±2.8 | ±19.1 | ±5.7 | ±1.4 | ±3.5 | ±155 |
| Pyknocytosis | Mean | 7.8 | 98.8 | 33.0 | 33.5 | 17.9 | 185 |
| (n = 7) | S | ±1.5 | ±6.1 | ±1.2 | ±1.5 | ±2.5 | ±110 |
HS: Hereditary spherocytosis; HE: hereditary elliptocytosis; HPP: pyropoikilocytosis; AIHA: auto-immune hemolytic anemia; SAO: South east Asian Ovalocytosis; Hb: hemoglobin; MCV: mean corpuscular volume; MHC: mean hemoglobin content; MCHC: mean corpuscular hemoglobin concentration; RDW: red cell volume distribution.
S: standard deviation.
Other biological parameter characteristic of hemolysis, including high indirect bilirubin, low haptoglobin and high LDH activity level could be obtained in 66 out of the 321 patients. In addition, 71 patients were tested for hemoglobinopathies, 15 of whom exhibited abnormal hemoglobin electrophoresis. Six of these 15 patients were diagnosed with sickle cell anemia (one homozygote (SS) and five heterozygotes (AS)), two with HbF persistence syndrome (one of whom bearing a S/deltaBeta thalassemia mutation), two with C hemoglobinopathy (one of whom carrying as well an alpha thalassemia trait), two with alpha-thalassemia and three with beta-thalassemia. DAT was performed in 59 patients and was deemed positive in 12 out these 59 patients. Enzymatic activities were measured in 65 patients. We identified 4 patients with defects in G6PD activity; among them, 3 exhibited a partial defect and 1 patient a GPI defect. Iron metabolism was tested in 70 patients and iron deficiency was found in 8 out of these 70 patients.
3.2. The EMA test is still the diagnostic tool of choice for HS screening
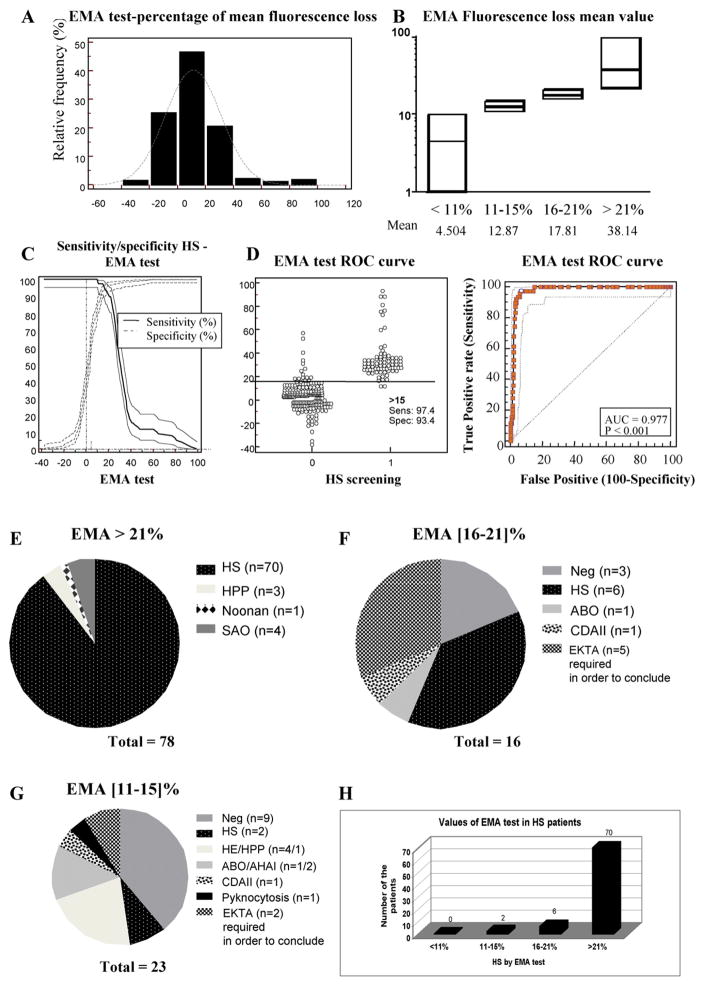
A total of 321 patients were referred to our laboratory for RBC membrane disorder diagnosis. They were all subjected to a flow cytometry-based EMA test after labeling of RBCs with EMA. Results of the EMA test in our population followed a Gaussian (bell curve) distribution (Fig. 3A). Mean fluorescence intensity (MFI) for each sample was compared to that of 6 age-matched controls collected on the same day. The mean EMA in the category under 11% of mean fluorescence decrease was 4.5%, in the 11–15% category, 12.9%, in the 16–21% category, 17.8% and in the category above 21%, 38.1%, respectively (Fig. 3B). We determined the sensitivity and specificity of the EMA test in our population of 321 patients (Fig. 3C), and established the receiver operating characteristic (ROC) curve in order to determine the optimal cut-off point of the EMA test for diagnosis of HS (Fig. 3D). The ROC curve was used to evaluate the ability of this biomarker to classify disease status. We determined the Youden J index, which corresponds to the maximum potential effectiveness of a biomarker defined as J = max [Se (c) + Sp (c) −1] [49]. The cut-off point (c) used in this equation is the maximum since this value optimizes biomarker differentiating ability when equal weight is given to sensitivity and specificity. We estimated this cut-off point at 0.9080 in the case of the EMA test, corresponding to an EMA test >15% with a sensitivity of 97.4% and a specificity of 93.4%. The area under the curve has determined to be 0.977 (standard error = 0.008; 95% confidence interval = 0.953–0.991; significance level p < 0.0001) (Fig. 3C–D).
Fig. 3.
A. Percentage distribution of the mean fluorescence of RBCs labeled with the dye eosin-5′ maleimide (EMA) by flow cytometry in all the 321 samples studied. B. Values of the EMA-labeled RBC mean fluorescence percentage decrease in the different categories (below 11%, 11–15%, 16–21% and above 21% of mean fluorescence loss). The line represents the median value for each category. The mean value of the mean fluorescence loss is also indicated in each category. C. Sensitivity and specificity profiles and calculation of the cut-off of the EMA test for HS diagnosis. D. EMA test ROC curve. The cut-off of the EMA test for HS diagnosis for the 321 samples tested has been estimated at 15% (p < 0.001) with the highest sensitivity of 97.4% and a specificity of 93.4%. Only 2 out of 21 HS patients have been diagnosed below this 15% cut-off. E. Diagnosis of the patients based on EMA test with a setting of decrease in mean fluorescence of >21% (78 cases). There were 8 false positive cases including 3 HPP cases, 4 SAO cases and 1 Noonan syndrome affected patient. F. Diagnosis of the patients based on EMA test with a setting of decrease in mean fluorescence of between 16 and 21% (16 cases) including 6 HS cases, one case of ABO incompatibility and one CDAII-affected patient. In 3 patients, there was no hint for a RBC membrane disorder from complete family history, clinical assessment, RBC and reticulocyte indices, cytology and biochemistry data analyses. For 5 patients, the ektacytometer-based analysis was performed in order to confirm the presence of a RBC membrane disorder. G. Diagnosis of the patients based on EMA test with a setting of decrease in mean fluorescence between 11 and 15% (23 cases) including 2 HS cases, 4 HE cases, 1 HPP case, 3 immune hemolytic anemia cases, 1 CDAII case and 1 pyknocytosis case. In 9 patients RBC membrane disorder was ruled out with the same criteria as in (F). H. Number of patients with confirmed HS diagnosis in each category of the EMA test mean fluorescence loss percentage. No HS case was diagnosed below 11% of mean fluorescence decrease of EMA labeled RBCs, while 2 HS cases were diagnosed below the calculated 15% cut-off, 6 cases in the “gray zone” between 16% and 21% and 70 out of 78 cases were true positive HS cases with an EMA test up to 21%.
78 out of the 321 patients analyzed exhibited a positive EMA test >21%, whereas 16 fell into the ‘gray zone’ between 16 and 21% of mean fluorescence decrease and 227 patients exhibited a negative EMA test <16% of mean fluorescence loss according to Girodon et al. [46]. Among the 78 patients with a positive EMA test >21%, HS was confirmed in 70 cases (89.7%). Out of the 8 remaining patients, three were diagnosed with HPP, four with SAO, and unexpectedly one with a Noonan syndrome (Fig. 3E). Among the 16 patients falling into the gray zone between of 16% and 21% decrease in RBC mean fluorescence, 6 patients exhibited a HS, one an ABO incompatibility, one a type II congenital dyserythropoiesis (CDAII), whereas 3 patients did not exhibit any RBC membrane disorder and 5 could not be fully diagnosed in the laboratory at the time of the study (Fig. 3F). Since the cut-off of the positive EMA test is still a matter of debate [36,50], we analyzed the number of patients between 11 and 15% and below the limit of 11%, a threshold under which no HS has been diagnosed in previous Italian [36] and French [50] studies. We identified 23 patients with a loss of RBC mean fluorescence between 11 and 15%. Among them, 2 patients were diagnosed with HS, 11 had no RBC disorder and 9 other forms of RBC membrane disorders including 5 HE/HPP, 3 immune hemolytic anemia, 1 pyknocytosis and 1 CDAII (Fig. 3G). When we analyzed the EMA test value for the 78 patients with confirmed diagnosis of HS, 70 exhibited a positive EMA test >21%, 6 between 16% and 21% and 2 between 11% and 15%. Confirming findings from previous studies, no HS was diagnosed below 11% of mean fluorescence decrease in RBCs labeled with EMA (Fig. 3H).
3.3. Determination of the repeatability and reproducibility, and normal values for each of the distinct ektacytometer parameters
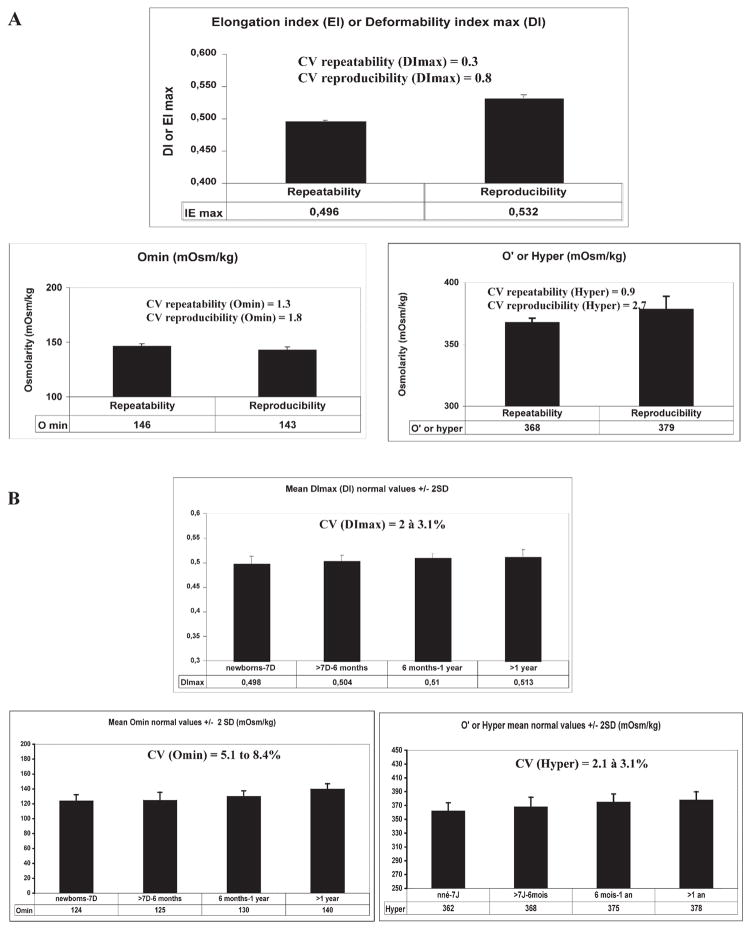
We determined the repeatability and the reproducibility for each of the distinct osmotic deformability profile parameters, Omin, Hyper and DImax, on the LoRRca MaxSis. The repeatability consists of testing the same sample thirty times in the same series by the same operator and using the same PVP solution. The repeatability coefficient of variation (CV) obtained for the DImax, Omin and hyper or O′ points were calculated as 0.3%, 1.3% and 0.9%, respectively (Fig. 4A). The reproducibility consists of testing the same sample thirty times but with different operators, different PVP solutions and at different times of the day. The reproducibility CV obtained for the DImax, Omin and hyper or O′ points were calculated as 0.8%, 1.8%, and 2.7%, respectively (Fig. 4A). Both repeatability and reproducibility CVs were less than <3% for all experimental points. We then established normal values in 90 normal controls for each age category: 1) newborn and less than 7 days of age, 2) >7 days to 6 months of age, 3) >6 months to 1 year of age, and 4) >1 year of age (Table 2). These age categories have been chosen based upon the changes in the RBC properties at these different ages. The DImax varied from 0.498 to 0.513 with a CV of 2% to 3.1% in the 4 different age categories; the Omin point from 124 to 140 mOsm/kg with a CV of 5.1% to 8.4%; and the Hyper or O′ point from 362 to 378 mOsm/kg with a CV of 3.1% to 3.8% (Fig. 4B).
Fig. 4.
A. Values for the three ektacytometry derived parameters (DImax, Omin and Hyper points) obtained during validation of the new generation LoRRca MaxSis ektacytometer. The CV of the repeatability and reproducibility tests for each of the derived parameters was evaluated. B. Mean values and standard deviations (±2SD) and CV for each of the three ektacytometry derived parameters (DImax, Omin and Hyper points) in normal controls in different age categories (newborns to 7 day-old; 7 days to 6 month-old; 6 month-old to one year-old; and more than 1 year old).
Table 2.
Normal values established in a control population for each age category for the three ektacytometry derived parameters (DImax, Omin and Hyper points) (n = 90 normal controls).
| EI max |
O min |
O hyper |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Newborns–<7 days | >7 days–6 months | >6 months–1 year | >1 year | Newborns–<7 days | >7 days–6 months | >6 month–1 year | >1 year | Newborn–<7 days | >7 days–6 months | >6 months–1 year | >1 year | |
| M | 0.498 | 0.504 | 0.51 | 0.513 | 124 | 125 | 130 | 140 | 362 | 368 | 375 | 378 |
| S | 0.015 | 0.012 | 0.010 | 0.013 | 8.4 | 10.5 | 7.4 | 7.1 | 12.0 | 13.9 | 11.7 | 11.9 |
| CV % | 3.1 | 2.5 | 2.0 | 2.5 | 6.7 | 8.4 | 5.7 | 5.1 | 3.3 | 3.8 | 3.1 | 3.2 |
| Maxi | 0.519 | 0.504 | 0.525 | 0.543 | 142 | 125 | 146 | 158 | 383 | 368 | 395 | 411 |
| Mini | 0.470 | 0.484 | 0.494 | 0.489 | 111 | 93 | 118 | 119 | 333 | 336 | 355 | 346 |
| M + 2S | 0.529 | 0.528 | 0.490 | 0.538 | 141 | 146 | 145 | 155 | 386 | 396 | 399 | 402 |
| M − 2S | 0.468 | 0.479 | 0.490 | 0.487 | 108 | 104 | 115 | 126 | 338 | 340 | 352 | 354 |
M: mean; S: standard deviation.
3.4. LoRRca MaxSis allows improved diagnosis of red blood cell membrane disorders
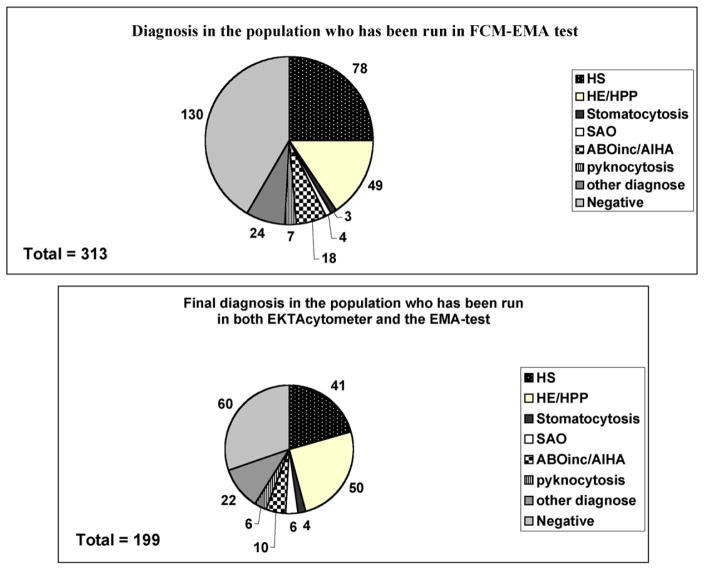
Having validated the method, we then compared the capacity and accuracy of the new generation LoRRca MaxSis ektacytometer to diagnose RBC membrane disorders with those of the previous version of LoRRca that did not have the ability to perform osmoscans. We performed cytologic analysis of RBC morphology on blood smears for all cases and compared the cytology/EMA test/ektacytometer/other hemolysis markers and clinical data before final diagnosis. Few patients had been evaluated using the previous version of ektacytometer since these patients had been diagnosed in other hospitals prior to referral to our hospital: in all cases we confirmed the initial diagnosis established with the older generation ektacytometer using the new generation ektacytometer. 199 out of the 321 patients were subjected to both EMA test and ektacytometry analysis using the LoRRca MaxSis. Among these 199 patients, we were able to diagnosed 41 HS, 50 HE/HPP, 3 stomatocytosis, 6 SAO, 10 immune hemolytic anemia (AIHA/ABO incompatibility) and 7 pyknocytosis. 22 other patients were affected by other diseases including hemoglobinopathies, iron deficiency, RBC enzyme defect, CDAII or non RBC-related diseases. In 60 cases, no RBC membrane anomaly or other RBC disorder could be diagnosed (Fig. 5).
Fig. 5.
Top panel: Final diagnosis of RBC membrane disorders for all the 313 samples studied. Other diagnoses included hemoglobinopathies as well as measurements of iron deficiency and enzymatic activity defects. “Negative” corresponds to patients with no RBC pathology. Bottom panel: Final diagnosis of RBC membrane disorders for the 199 samples analyzed both by EMA test-based flow cytometry and by LoRRca MaxSis ektacytometer. Other diagnoses included search for hemoglobinopathies as well as measurements of iron deficiency and enzymatic activity defects. “Negative” corresponds to patients with no RBC pathology.
The LoRRca MaxSis ektacytometer was able to generate the characteristic HS curve with two main features (Fig. 6A): 1) abnormal values for the three points including a decrease in DImax (the main and constant feature of HS reflecting a decrease in RBC deformability due to surface area loss), a shift to the right of the Omin (reflecting a decrease in the surface area/volume ratio) and a shift to the left of the Hyper point (illustrating the dehydration state of the RBCs) in 21 HS patients; and 2) abnormal values for two points, i.e. a decrease in DImax and a shift to the right of Omin in 13 patients or a decrease in DImax and a shift to the left of the Hyper point in 4 patients. Surprisingly, in 3 patients, only one abnormal point was observed, i.e. a decrease in the DImax. Of particular note, none of the patients with confirmed HS exhibited a normal DImax as previously reported. The correlation between the cytologic and ektacytometry analysis was 100%. Of particular interest, the EMA test was able to discriminate between immune and the hereditary origin of the generation of spherocytes in all 10 cases except one. Indeed, the characteristics of the ektacytometer curves (decreased DI max, shift or not to the right of the Omin and shift or not to the left of the hyper point) were the same for the AIHA/ABO incompatibility and HS cases as already reported with previous ektacytometers. Only the EMA test was able to diagnose specifically the HS with a loss in the RBC mean fluorescence up to 21%, the EMA test being in the normal range or in the ‘gray zone’ in the case of immune hemolytic anemia [46]. Furthermore, as previously reported, the ektacytometer curve in one patient affected with CDAII (and confirmed to bear a mutation in the SEC23B gene) reproduced the feature of HS. Strikingly, in one neonate case, the EMA test was highly positive at birth with a DAT-positive ABO incompatibility, the same sample controlled one week later exhibited a normal EMA test profile.
Fig. 6.
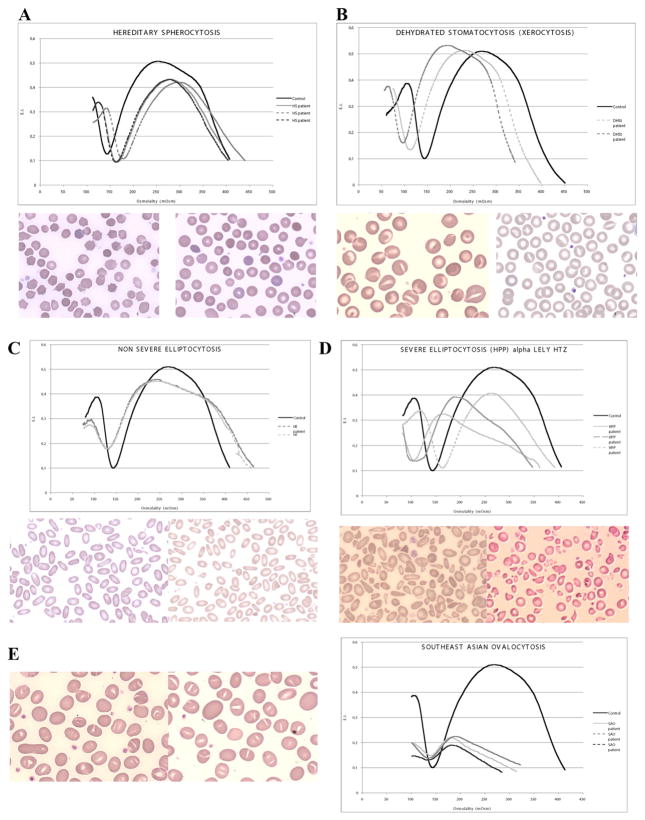
RBC membrane disorders diagnosed by the LoRRca MaxSis ektacytometer: A. Hereditary spherocytosis ektacytometry curve and blood smear B. Dehydrated stomatocytosis ektacytometry curve and blood smear C. Hereditary elliptocytosis ektacytometry curve and blood smear D. Pyropoikilocytosis ektacytometry curve and blood smear E. South east Asian Ovalocytosis ektacytometry curve and blood smear.
The LoRRca MaxSis ektacytometer was also able to diagnose stomatocytosis; however the amplitude of the shift of both the Omin and Hyper points, either to the right in the case of overhydrated stomatocytosis or to the left in the case of dehydrated stomatocytosis or xerocytosis, was less than the one typically observed with the previous Technicon® ektacytometer. We diagnosed 3 cases with stomatocytosis in its dehydrated form/xerocytosis (Fig. 6B). Mutation in the PIEZO1 gene [33] was identified in one of them. The mutation screening analysis in the two other patients did not show any of the previously reported mutations associated with stomatocytosis [33].
The LoRRca MaxSis ektacytometer was able to generate a trapezoidal curve characteristic of HE with a decrease in DImax and a normal value for the Omin and Hyper points in 26 out of 39 patients affected with isolated HE (Fig. 6C). No RBC pathologies were associated with HE in these patients. The 13 HE patients with an ektacytometer curve that did not recapitulate completely the canonical trapezoidal ektacytometer curve characteristic of HE, often exhibited a shift close to the limit of normal range of values for either the Omin or the Hyper point, with no clear explanation. Unfortunately, we could not perform the additional tests necessary for investigating other causes of hemolytic anemia or RBC-associated pathologies, including DAT, enzyme activity measurements (G6PD, PK), hemoglobin electrophoresis, and iron analyses, in these patients. The 5 HE patients who were diagnosed with iron deficiency, hemoglobinopathies or enzyme defects exhibited as expected a modified Omin or Hyper point or both. Of particular interest, the trapezoidal curve shape was the most constant feature of HE along with the decrease in DImax. Strikingly, the 6 patients who exhibited the most severe form of HE, pyropoikilocytosis, based on the RBC fragmentation on the blood smear, the decrease in Hb value and MCV, and a positive alpha-Lely polymorphism revealed by molecular biology [19,51], did not show the characteristic ektacytometer curve (decrease in DImax, shift to the right for the Omin point and to the left for the Hyper point) on the LoRRca MaxSis, except for one patient. The ektacytometer curve in the 5 other HPP cases exhibited either a left shift of the Omin point or a classical HE curve, with notably a major decrease in DImax (Fig. 6D).
The LoRRca MaxSis ektacytometer was able to generate a curve typical of SAO characterized by a dramatic decrease in the RBC deformability in all the 6 patients tested (Fig. 6E). All the SAO patients, except one female neonate, were as expected asymptomatic and the discovery was fortuitous on the blood smear during a consultation visit or hospitalization for a disease with no link with SAO or any other RBC disorder. The SAO diagnosis in the 6 patients was confirmed by molecular analysis revealing a 27 base pair deletion (encoding codons 400 to 408) in the band 3 (SLC4A1) gene [52,53].
The LoRRca MaxSis ektacytometer was able to diagnose pyknocytosis although only 3 out of 7 cases exhibited the normal expected osmotic gradient ektacytometer profiles. In the 4 other cases, we observed either a slight decrease in DImax (2 cases) or an abnormal Omin and Hyper point (2 other cases). Unfortunately, we could not perform measurements of the G6PD and PK enzymatic activities in the cases for which the ektacytometer curve was abnormal. Therefore, we cannot rule out an association of both defects since these enzymatic activity defects are often associated with bite cells and “pyknocytes-like” RBCs. However, no ghosts or hemighosts, characteristic of G6PD deficiency, have been identified in any of these cases.
4. Discussion
In the present study, we report the diagnosis of various RBC disorders in the workflow of a hematology laboratory in France over a 20 month period since 2012 using the usual diagnosis tools and using the new generation LoRRca MaxSis ektacytometer. The routine analysis included RBC and reticulocyte indices, RBC morphology analysis on a blood smear stained with MGG, flow cytometry-based measurement of mean fluorescence of eosin-5′-maleimide labeled RBCs (EMA test), molecular tests including alpha-LELY polymorphism in HPP, band 3 deletion screening in SAO and PIEZO1 gene mutation screening in xerocytosis.
Osmotic fragility, glycerol lysis test or Pink tests may be used as a first line of clinical laboratory tests. However, the sensitivity of these tests for diagnosis purpose is low [36,48,54]. Flow cytometry-based measurement of the mean RBC fluorescence, following labeling of RBCs with the dye eosin-5′ maleimide (EMA), is a method of choice to screen surface area loss for diagnosis of HS [35–46,50]. The cut-off above which the test is considered positive is much debated. Whereas Girodon et al. [51] initially reported an optimal cut-off value of 16% for the decrease in mean RBC fluorescence, later on Crisp et al. [45] reported a 17% cut-off value. More recently, Mayeur-Rousse et al. [50] and Bianchi et al. [36] recommended a lower cut-off of 11%, this cut-off being necessary to discriminate 150 HS patients from normal controls. One may argue that this latter low threshold may likely lead to an over-diagnosis of HS if the laboratories base their diagnosis solely on flow cytometry-based analysis. In our study, we analyzed the sensitivity and specificity of the EMA test in our cohort of 321 patients and determined the optimal cut-off value that would provide the highest sensitivity and specificity for diagnosis of HS. We determined that this optimal cut-off value was 15%, a value close to the 16% reported in the initial study [46]. Although this cut-off enabled to identify 21 patients with confirmed HS, 2 HS patients exhibited an EMA test between 11% and 15% of mean RBC fluorescence loss. Of note, no HS patients were diagnosed below 11% decrease in mean RBC fluorescence. This test enables to detect HS due to defects in band 3, spectrin and protein 4.2 but is less effective in identifying HS due to ankyrin defects [40]. The test can also be positive in pyropoikilocytosis (HPP) due to a significant extent of cell fragmentation leading to the generation of RBCs with decreased surface area. In our study, 4 patients diagnosed with HPP exhibited a positive EMA, three with an EMA test value of >21% and one with an EMA test value between 11% and 15%. However, for all patients with HPP, if fragmented RBCs were gated out by their distinct forward scatter signal, the non-fragmented RBCs did not exhibit any decrease in membrane-associated fluorescence, in contrast with HS in which the entire population of RBCs exhibited decreased membrane-associated fluorescence. Furthermore, the blood smear examination enabled reliable and clear-cut differential diagnosis between HS and HPP. The EMA test may lead to false-positive results in the case of SAO as a result of the failure of the truncated band 3 (characteristic of SAO) to bind to EMA. In our study, all 4 SAO patients subjected to the EMA test exhibited a positive EMA test >21%. Two other SAO patients were not analyzed since we have now validated the flow chart in the laboratory and we no longer perform an EMA test in identified SAO and HE/HPP cases (Fig. 7). Patients with congenital dyserythropoietic anemia type II (CDAII), a condition that results from abnormal glycosylation of band 3, and patients with cryohydrocytosis have been previously reported to exhibit a positive EMA test [40]. In our experience, none of the CDAII patients nor the patients diagnosed with stomatocytosis exhibited an EMA positive test >21%, but one CDAII patient exhibited a decrease of 18%. Furthermore, one neonate diagnosed with ABO incompatibility exhibited an EMA test value of 18%. Unexpectedly, one patient with Noonan syndrome (referred to our laboratory for spherocytic RBCs on the blood smear without hemolytic anemia) exhibited an EMA test value of 22%.
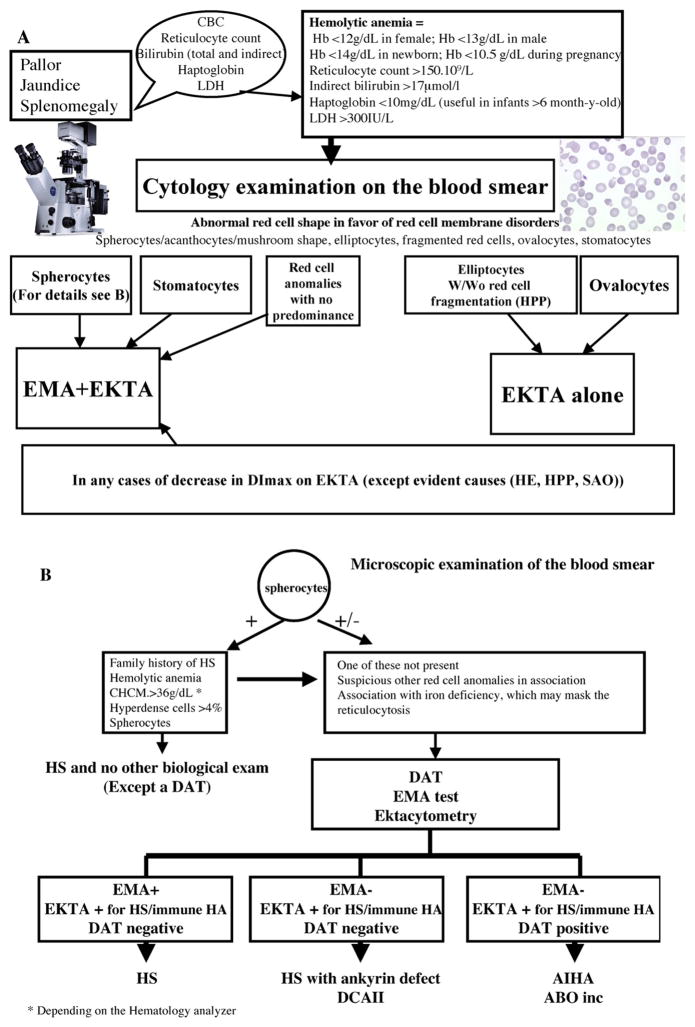
Fig. 7.
Proposed work flow chart for laboratory diagnosis of HS and other RBC membrane disorders.
* Depending on the Hematology analyzer
EMA binding is not dependent on the severity of the HS and is also positive in compensated HS. However, the sensitivity is increased in splenectomized patients with HS compared to non-splenectomized patients with HS [36]. Unfortunately, we could not conduct the necessary analyses to confirm these data in our study. EMA binding is also independent of the molecular defect in the affected RBC membrane protein although it has been reported to be less sensitive for diagnosis of HS with undefined molecular defects or with ankyrin defects [36,40]. However, despite the limitations described above, the EMA screening test should nonetheless replace the much lower sensitive and specific tests since: 1) it is easy to perform particularly in neonates, since only 5 μl of peripheral blood is needed and since a flow cytometer is available in most hematology laboratories; 2) the test results are available in 2–3 h; 3) the samples could be analyzed up to 7 days after blood sampling [46]; and 4) the gating on the abnormal RBCs, if feasible, eliminates the bias due to fragmented RBCs in HPP and the presence of transfused RBCs, enabling the diagnosis of HS in patients, even after a recent transfusion [41]. However, it is recommended to wait for 3 months after the last transfusion if possible or at least perform the blood collection just before a transfusion when most of the cells are of patient origin if the patient is regularly transfused. In any case, cytology analysis, focusing on RBC morphology, should remain an obligatory step towards diagnosis of RBC membrane disorders. An EMA test should never be interpreted without a cytology examination of the blood smear performed on the same tube, because of the potential for false-positive results due to various RBC membrane disorders as described above. Furthermore, if the blood is shipped, a blood smear should always be performed before shipment of the blood sample and shipped along with the sample in order to rule out RBC morphology artifacts (including spiculated RBCs, echinocytes, stomatocytes) related to the delay between blood collection and the preparation of blood smear.
Ektacytometry should still be considered as the diagnostic test and is still acknowledged as the reference technique for diagnosis of RBC membrane disorders [1–3,47,48,54–56]. The technology is based on major physics properties of RBCs including RBC geometry, cytoplasmic viscosity and cell volume regulation and membrane deformability. The amount of blood required to perform the ektacytometry test is small (100 μl) and, as in the case of the EMA dye test, ektacytometry can be performed on blood samples from neonates and from cord blood. However, the limitations of ektacytometry are: 1) the complexity of the instrumentation, 2) the limited access to the ektacytometer, 3) the need of specialized staff members in order to process the samples and analyze the data, 4) the need to delay the analysis after transfusion (ideally 3 months) for the same reasons evoked for the EMA test and finally, and 5) the need to perform the analysis within 48 h of blood sampling.
In the present study, we evaluated the new generation LoRRca MaxSis osmoscan in the context of our laboratory workflow by testing a large number of samples sent to us for diagnosis of RBC membrane disorders. Method validation tests, including repeatability and reproducibility with CV calculation for each of the 3 remarkable ektacytometer points (DImax, Omin and O′ or hyper points), were all satisfactory. We then established the normal range of values for each of the parameters, DImax, Omin and Hyper for each age category. Of note, comparing our settings with those of other users, it became clear that the settings established upon evaluation/installation of the LoRRca osmoscan should be defined by each individual user. Indeed, although we established our reference range of values, the settings may be different from one osmoscan to another one (Table 2). Furthermore, each sample from a patient analyzed on the ektacytometer should always be compared to an age-matched control drawn the same day.
As with the Technicon® ektacytometer, the new generation ektacytometer was able to accurately diagnose all major RBC membrane disorders, including hereditary spherocytosis, elliptocytosis, stomatocytosis, and South eastern Melanesian ovalocytosis (SAO). It generated deformability curves for RBCs from patients with HS/immune hemolytic anemia, stomatocytosis, and SAO similar to those obtained with the Technicon® ektacytometer. It was able to generate the characteristic HS curve in 21 patients. None of the patients with confirmed HS exhibited a normal DImax. In all 10 patients diagnosed with an immune hemolytic anemia (ABO incompatibility or AIHA), the ektacytometer curve generated by the new generation ektacytomer exhibited the HS features described above [47,48]. The patient affected with CDAII exhibited an ektacytometer curve with features characteristics of a HS curve, confirming previous observations with the Technicon® ektacytometer.
It need to be noted that the RBC deformation curves generated by the new generation LoRRca ektacytometer exhibited some notable differences when compared to those obtained with the Technicon® ektacytometer: 1) the trapezoidal curve of HE patients was “less trapezoidal”, 2) the Omin and hyper points in HE patients were not always in the normal values as seen with the Technicon® ektacytometer but were rather shifted (14 out 44 HE samples showed a shift in the Omin and/or hyper points), and 3) the RBCs from infants with pyknocytosis did not exhibit the normal classical ektacytometer curve but rather a decrease in DImax associated with normal or abnormal Omin and hyper points in 3 out of 6 patients.
Having now used and compared both ektacytometers, we can ascertain that the noted differences are likely due to differences in the design of diffraction image analysis. The Technicon® ektacytometer uses photodiodes to measure accurately the extent of light scatter while the LoRRca MaxSis osmoscan® uses a digital camera. The LoRRca may therefore have difficulties in deconvoluting complex diffraction patterns. However, a recent study performed by Finkelstein et al. [57] confirmed that, although both techniques do not provide the same values of DImax, the features of the ektacytometer curves are very similar and the authors validated the use of the digital image acquisition.
Despite the great benefit of conducting several types of analyses to confirm diagnoses of RBC membrane disorders and the availability of a reliable new generation ektacytometer, it remains that in routine practice, analysis of RBC morphology on a blood smear should be the first clue of diagnosis and the gold standard in order to guide the diagnosis of RBC membrane disorders. The final diagnosis should then take into account the EMA test, an ektacytometer analysis (the reference technique for diagnosis of RBC membrane disorders) as well as molecular analyses and analyses specifically designed to discriminate from other causes of hemolytic anemia (DAT, enzyme defect analysis, Hemoglobin electrophoresis) and importantly patient family and medical history. Indeed, the patient medical records should be analyzed by multidisciplinary teams in order to finalize the diagnosis. One must keep in mind that other diseases may be associated with a primary RBC membrane disorder and may affect the EMA test result and/or the ektacytometer curve profile. Based on our findings, we propose an optimized flow chart for diagnosis of RBC membrane disorders in the context of a hematology laboratory (Fig. 7).
In conclusion, RBC membrane disorders should be diagnosed after conducting a panel of complementary analyses including ektacytometer profiling and discussion of the results by multidisciplinary teams. The future development of NGS molecular biology will undoubtedly constitute an invaluable tool that will revolutionize diagnosis of RBC membrane disorders. Functional analyses will still remain an important component of patient care and perhaps become even more necessary to validate the diagnosis and to assess the functional consequences of identified mutations.
Acknowledgments
Funding
This work was supported by the BQR funding (Université Paris Diderot Sorbonne Paris Cité) and by Université Paris VII-Denis Diderot-Sorbonne Paris cité and the INTS. The Labex GR-Ex was supported by the «investissement d’Avenir» program, ANR.
We would like to deeply thank Dr. Yves Colin (Institut National de Transfusion Sanguine (INTS), Paris, France) and the Université Paris VII-Denis Diderot, Sorbonne Paris Cité for the funding of the LoRRca MaxSis. We thank Philippe Gascard (Department of Pathology, University of California San Francisco, USA) for his constructive suggestions. We thank the patients, their families and the physicians in charge of these patients for their support and for sending us samples.
Abbreviations
- AIHA
auto-immune hemolytic anemia
- CBC
complete blood count
- CDA
congenital dyserythropoietic anemia
- CHCM
Cell Hemoglobin Concentration Mean
- CV
coefficient of variation
- DAT
direct agglutination test
- DHSt
dehydrated hereditary stomatocytosis (xerocytosis)
- DI
deformability index
- EDTA
ethylenediamine tetraacetic acid
- EI
elongation index
- EMA
eosin-5′ maleimide
- G6PD
glucose-6 phosphate dehydrogenase
- GPI
glucose 6 phosphate isomerase
- Hb
hemoglobin
- HE
hereditary elliptocytosis
- HPP
hereditary pyropoikilocytosis
- HS
hereditary spherocytosis
- Hst
hereditary stomatocytosis
- LDH
lactate dehydrogenase
- MCV
mean corpuscular volume
- MFI
mean fluorescence intensity
- MGG
May Grümwald Grünwald
- MHC
mean hemoglobin content
- OHS
overhydrated hereditary stomatocytosis
- PBS
phosphate-buffered saline
- PK
pyruvate kinase
- PVP
polyvinylpyrrolidone
- RBC
red blood cell
- RDW
red blood cell distribution width
- SAO
South-east Asian Ovalocytosis
- SD
standard deviation
Footnotes
Author contributions: LS and TP collected the data. LS, JG, LDC performed the statistical tests of the ROC analysis. JG performed the ektacytometry analysis of the samples, the validation of the ektacytometry method, and analyzed the ektacytometry data. OF and LDC analyzed RBC morphology in blood smears. AB performed the flow cytometry analysis of EMA-labeled RBCs. NC carried out the Hemoglobin electrophoresis, enzymatic activity measurements and G6PD gene mutation screening. LDC designed the study, analyzed RBC morphology in blood smears with OF, analyzed the EMA-tests and the ektacytometry results, validated the final diagnosis with OF, LS, NC and pediatricians and hematologists from the SFH and the SHIP, who sent us patient samples. LDC and NM wrote the article.
None of the authors have any conflicts of interest to declare.
References
- 1.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohandas N, Chasis JA, Shohet SB. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol. 1983;20:225–242. [PubMed] [Google Scholar]
- 3.Mohandas N, Chasis JA. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993;30:171–192. [PubMed] [Google Scholar]
- 4.Mohandas N, Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- 5.Mohandas N, An X. New insights into function of red cell membrane proteins and their interaction with spectrin-based membrane skeleton. Transfus Clin Biol. 2006;13:29–30. doi: 10.1016/j.tracli.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher PG. Red cell membrane disorders. Hematol Am Soc Hematol Educ Prog. 2005;13–18 doi: 10.1182/asheducation-2005.1.13. [DOI] [PubMed] [Google Scholar]
- 7.An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141:367–375. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 8.Iolascon A, Piscopo C, Boschetto L. Red cell membrane disorders in pediatrics. Pediatr Ann. 2008;37:295–301. doi: 10.3928/00904481-20080501-05. [DOI] [PubMed] [Google Scholar]
- 9.Iolascon A, Perrotta S, Stewart GW. Red blood cell membrane defects. Rev Clin Exp Hematol. 2003;7:22–56. [PubMed] [Google Scholar]
- 10.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411–1426. doi: 10.1016/S0140-6736(08)61588-3. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher PG, Ferreira JD, Costa FF, Saad ST, Forget BG. A recurrent frameshift mutation of the ankyrin gene associated with severe hereditary spherocytosis. Br J Haematol. 2000;111:1190–1193. doi: 10.1046/j.1365-2141.2000.02441.x. [DOI] [PubMed] [Google Scholar]
- 12.Bracher NA, Lyons CA, Wessels G, Mansvelt E, Coetzer TL. Band 3 Cape Town (E90K) causes severe hereditary spherocytosis in combination with band 3 Prague III. Br J Haematol. 2001;113:689–693. doi: 10.1046/j.1365-2141.2001.02800.x. [DOI] [PubMed] [Google Scholar]
- 13.Alloisio N, Maillet P, Carre G, Texier P, Vallier A, Baklouti F, Philippe N, Delaunay J. Hereditary spherocytosis with band 3 deficiency. Association with a nonsense mutation of the band 3 gene (allele Lyon), and aggravation by a low-expression allele occurring in trans (allele Genas) Blood. 1996;88:1062–1069. [PubMed] [Google Scholar]
- 14.Gallagher PG, Forget BG. Hematologically important mutations: band 3 and protein 4.2 variants in hereditary spherocytosis. Blood Cells Mol Dis. 1997;23:417–421. doi: 10.1006/bcmd.1997.0160. [DOI] [PubMed] [Google Scholar]
- 15.Wichterle H, Hanspal M, Palek J, Jarolim P. Combination of two mutant alpha spectrin alleles underlies a severe spherocytic hemolytic anemia. J Clin Invest. 1996;98:2300–2307. doi: 10.1172/JCI119041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarolim P, Wichterle H, Hanspal M, Murray J, Rubin HL, Palek J. Beta spectrin PRAGUE: a truncated beta spectrin producing spectrin deficiency, defective spectrin heterodimer self-association and a phenotype of spherocytic elliptocytosis. Br J Haematol. 1995;91:502–510. doi: 10.1111/j.1365-2141.1995.tb05330.x. [DOI] [PubMed] [Google Scholar]
- 17.Delaunay J, Nouyrigat V, Proust A, Schischmanoff PO, Cynober T, Yvart J, Gaillard C, Danos O, Tchernia G. Different impacts of alleles alphaLEPRA and alphaLELY as assessed versus a novel, virtually null allele of the SPTA1 gene in trans. Br J Haematol. 2004;127:118–122. doi: 10.1111/j.1365-2141.2004.05160.x. [DOI] [PubMed] [Google Scholar]
- 18.Dhermy D, Galand C, Bournier O, King MJ, Cynober T, Roberts I, Kanyike F, Adekile A. Coinheritance of alpha-and beta-spectrin gene mutations in a case of hereditary elliptocytosis. Blood. 1998;92:4481–4482. [PubMed] [Google Scholar]
- 19.Wilmotte R, Marechal J, Morle L, Baklouti F, Philippe N, Kastally R, Kotula L, Delaunay J, Alloisio N. Low expression allele alpha LELY of red cell spectrin is associated with mutations in exon 40 (alpha V/41 polymorphism) and intron 45 and with partial skipping of exon 46. J Clin Invest. 1993;91:2091–2096. doi: 10.1172/JCI116432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maillet P, Alloisio N, Morle L, Delaunay J. Spectrin mutations in hereditary elliptocytosis and hereditary spherocytosis. Hum Mutat. 1996;8:97–107. doi: 10.1002/(SICI)1098-1004(1996)8:2<97::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Delaunay J. Molecular basis of red cell membrane disorders. Acta Haematol. 2002;108:210–218. doi: 10.1159/000065657. [DOI] [PubMed] [Google Scholar]
- 22.Warkentin TE, Barr RD, Ali MA, Mohandas N. Recurrent acute splenic sequestration crisis due to interacting genetic defects: hemoglobin SC disease and hereditary spherocytosis. Blood. 1990;75:266–270. [PubMed] [Google Scholar]
- 23.Yang YM, Donnell C, Wilborn W, Goodman SR, Files B, Moore RB, Mohandas N, Mankad VN. Splenic sequestration associated with sickle cell trait and hereditary spherocytosis. Am J Hematol. 1992;40:110–116. doi: 10.1002/ajh.2830400207. [DOI] [PubMed] [Google Scholar]
- 24.Mohandas N, Lie-Injo LE, Friedman M, Mak JW. Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood. 1984;63:1385–1392. [PubMed] [Google Scholar]
- 25.Mohandas N, An X. Malaria and human red blood cells. Med Microbiol Immunol. 2012 doi: 10.1007/s00430-012-0272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosanas-Urgell A, Lin E, Manning L, Rarau P, Laman M, Senn N, Grimberg BT, Tavul L, Stanisic DI, Robinson LJ, Aponte JJ, Dabod E, Reeder JC, Siba P, Zimmerman PA, Davis TM, King CL, Michon P, Mueller I. Reduced risk of Plasmodium vivax malaria in Papua New Guinean children with Southeast Asian Ovalocytosis in two cohorts and a case–control study. PLoS Med. 2012;9:e1001305. doi: 10.1371/journal.pmed.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura M, Shimizu Y, Settheetham-Ishida W, Soemantri A, Tiwawech D, Romphruk A, Duangchan P, Ishida T. Twenty-seven base pair deletion in erythrocyte band 3 protein gene responsible for Southeast Asian ovalocytosis is not common among Southeast Asians. Hum Biol. 1998;70:993–1000. [PubMed] [Google Scholar]
- 28.Jarolim P, Palek J, Amato D, Hassan K, Sapak P, Nurse GT, Rubin HL, Zhai S, Sahr KE, Liu SC. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci U S A. 1991;88:11022–11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaunay J, Stewart G, Iolascon A. Hereditary dehydrated and overhydrated stomatocytosis: recent advances. Curr Opin Hematol. 1999;6:110–114. doi: 10.1097/00062752-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Delaunay J. The hereditary stomatocytoses: genetic disorders of the red cell membrane permeability to monovalent cations. Semin Hematol. 2004;41:165–172. doi: 10.1053/j.seminhematol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, Cahalan S, Garcon L, Toutain F, Simon Rohrlich P, Delaunay J, Picard V, Jeunemaitre X, Patapoutian A. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andolfo I, Alper SL, De Franceschi L, Auriemma C, Russo R, De Falco L, Vallefuoco F, Esposito MR, Vandorpe DH, Shmukler BE, Narayan R, Montanaro D, D’Armiento M, Vetro A, Limongelli I, Zuffardi O, Glader BE, Schrier SL, Brugnara C, Stewart GW, Delaunay J, Iolascon A. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood. 2013;121:3925–3935. S3921–3912. doi: 10.1182/blood-2013-02-482489. [DOI] [PubMed] [Google Scholar]
- 33.Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, Rinehart J, Gallagher PG. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120:1908–1915. doi: 10.1182/blood-2012-04-422253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genetet S, Ripoche P, Picot J, Bigot S, Delaunay J, Armari-Alla C, Colin Y, Mouro-Chanteloup I. Human RhAG ammonia channel is impaired by the Phe65Ser mutation in overhydrated stomatocytic red cells. Am J Physiol Cell Physiol. 2012;302:C419–C428. doi: 10.1152/ajpcell.00092.2011. [DOI] [PubMed] [Google Scholar]
- 35.Bolton-Maggs PH, Langer JC, Iolascon A, Tittensor P, King MJ. Guidelines for the diagnosis and management of hereditary spherocytosis–2011 update. Br J Haematol. 2012;156:37–49. doi: 10.1111/j.1365-2141.2011.08921.x. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi P, Fermo E, Vercellati C, Marcello AP, Porretti L, Cortelezzi A, Barcellini W, Zanella A. Diagnostic power of laboratory tests for hereditary spherocytosis: a comparison study in 150 patients grouped according to molecular and clinical characteristics. Haematologica. 2012;97:516–523. doi: 10.3324/haematol.2011.052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Alcamo E, Agrigento V, Sclafani S, Vitrano A, Cuccia L, Maggio A, Perrotta S, Capra M, Rigano P. Reliability of EMA binding test in the diagnosis of hereditary spherocytosis in Italian patients. Acta Haematol. 2011;125:136–140. doi: 10.1159/000322253. [DOI] [PubMed] [Google Scholar]
- 38.King MJ, Smythe JS, Mushens R. Eosin-5-maleimide binding to band 3 and Rh-related proteins forms the basis of a screening test for hereditary spherocytosis. Br J Haematol. 2004;124:106–113. doi: 10.1046/j.1365-2141.2003.04730.x. [DOI] [PubMed] [Google Scholar]
- 39.King MJ, Jepson MA, Guest A, Mushens R. Detection of hereditary pyropoikilocytosis by the eosin-5-maleimide (EMA)-binding test is attributable to a marked reduction in EMA-reactive transmembrane proteins. Int J Lab Hematol. 2011;33:205–211. doi: 10.1111/j.1751-553X.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- 40.King MJ, Behrens J, Rogers C, Flynn C, Greenwood D, Chambers K. Rapid flow cytometric test for the diagnosis of membrane cytoskeleton-associated haemolytic anaemia. Br J Haematol. 2000;111:924–933. [PubMed] [Google Scholar]
- 41.King MJ, Telfer P, MacKinnon H, Langabeer L, McMahon C, Darbyshire P, Dhermy D. Using the eosin-5-maleimide binding test in the differential diagnosis of hereditary spherocytosis and hereditary pyropoikilocytosis. Cytometry B Clin Cytom. 2008;74:244–250. doi: 10.1002/cyto.b.20413. [DOI] [PubMed] [Google Scholar]
- 42.Kar R, Mishra P, Pati HP. Evaluation of eosin-5-maleimide flow cytometric test in diagnosis of hereditary spherocytosis. Int J Lab Hematol. 2010;32:8–16. doi: 10.1111/j.1751-553X.2008.01098.x. [DOI] [PubMed] [Google Scholar]
- 43.Kedar PS, Colah RB, Kulkarni S, Ghosh K, Mohanty D. Experience with eosin-5′-maleimide as a diagnostic tool for red cell membrane cytoskeleton disorders. Clin Lab Haematol. 2003;25:373–376. doi: 10.1046/j.0141-9854.2003.00557.x. [DOI] [PubMed] [Google Scholar]
- 44.Tachavanich K, Tanphaichitr VS, Utto W, Viprakasit V. Rapid flow cytometric test using eosin-5-maleimide for diagnosis of red blood cell membrane disorders. Southeast Asian J Trop Med Public Health. 2009;40:570–575. [PubMed] [Google Scholar]
- 45.Crisp RL, Solari L, Vota D, Garcia E, Miguez G, Chamorro ME, Schvartzman GA, Alfonso G, Gammella D, Caldarola S, Riccheri C, Vittori D, Venegas B, Nesse A, Donato H. A prospective study to assess the predictive value for hereditary spherocytosis using five laboratory tests (cryohemolysis test, eosin-5′-maleimide flow cytometry, osmotic fragility test, autohemolysis test, and SDS-PAGE) on 50 hereditary spherocytosis families in Argentina. Ann Hematol. 2011;90:625–634. doi: 10.1007/s00277-010-1112-0. [DOI] [PubMed] [Google Scholar]
- 46.Girodon F, Garcon L, Bergoin E, Largier M, Delaunay J, Feneant-Thibault M, Maynadie M, Couillaud G, Moreira S, Cynober T. Usefulness of the eosin-5′-maleimide cytometric method as a first-line screening test for the diagnosis of hereditary spherocytosis: comparison with ektacytometry and protein electrophoresis. Br J Haematol. 2008;140:468–470. doi: 10.1111/j.1365-2141.2007.06944.x. [DOI] [PubMed] [Google Scholar]
- 47.Da Costa L, Mohandas N, Sorette M, Grange MJ, Tchernia G, Cynober T. Temporal differences in membrane loss lead to distinct reticulocyte features in hereditary spherocytosis and in immune hemolytic anemia. Blood. 2001;98:2894–2899. doi: 10.1182/blood.v98.10.2894. [DOI] [PubMed] [Google Scholar]
- 48.Cynober T, Mohandas N, Tchernia G. Red cell abnormalities in hereditary spherocytosis: relevance to diagnosis and understanding of the variable expression of clinical severity. J Lab Clin Med. 1996;128:259–269. doi: 10.1016/s0022-2143(96)90027-x. [DOI] [PubMed] [Google Scholar]
- 49.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50:419–430. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayeur-Rousse C, Gentil M, Botton J, Thibaut M, Guitton C, Picard V. Testing for hereditary spherocytosis: a French experience. Haematologica. 2012;97:e48–e49. doi: 10.3324/haematol.2012.074070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alloisio N, Morle L, Marechal J, Roux AF, Ducluzeau MT, Guetarni D, Pothier B, Baklouti F, Ghanem A, Kastally R, et al. Sp alpha V/41: a common spectrin polymorphism at the alpha IV-alpha V domain junction. Relevance to the expression level of hereditary elliptocytosis due to alpha-spectrin variants located in trans. J Clin Invest. 1991;87:2169–2177. doi: 10.1172/JCI115250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kittanakom S, Cordat E, Reithmeier RA. Dominant-negative effect of Southeast Asian ovalocytosis anion exchanger 1 in compound heterozygous distal renal tubular acidosis. Biochem J. 2008;410:271–281. doi: 10.1042/BJ20070615. [DOI] [PubMed] [Google Scholar]
- 53.Cheung JC, Cordat E, Reithmeier RA. Trafficking defects of the Southeast Asian ovalocytosis deletion mutant of anion exchanger 1 membrane proteins. Biochem J. 2005;392:425–434. doi: 10.1042/BJ20051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guitton C, Garcon L, Cynober T, Gauthier F, Tchernia G, Delaunay J, Leblanc T, Thuret I, Bader-Meunier B. Hereditary spherocytosis: guidelines for the diagnosis and management in children. Arch Pediatr. 2008;15:1464–1473. doi: 10.1016/j.arcped.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 55.Mohandas N, Clark MR, Health BP, Rossi M, Wolfe LC, Lux SE, Shohet SB. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982;59:768–774. [PubMed] [Google Scholar]
- 56.Mohandas N, Clark MR, Jacobs MS, Groner W, Shohet SB. Ektacytometric analysis of factors regulating red cell deformability. Blood Cells. 1980;6:329–334. [PubMed] [Google Scholar]
- 57.Finkelstein A, Talbot H, Topsu S, Cynober T, Garçon L, Havkin G, Kuypers F. Comparison between a camera and a four quadrant detector, in the measurement of red blood cell deformability as a function of osmolality. J Med Biogr. 2013;2:62. [Google Scholar]