Abstract
Chronic asthma is associated with airway remodeling and decline in lung function. Here we show that follistatin like 1 (Fstl1), a mediator not previously associated with asthma is highly expressed by macrophages in the lungs of severe human asthmatics. Chronic allergen challenged Lys-Cretg/Fstl1Δ/Δ mice in whom Fstl1 is inactivated in macrophages/myeloid cells had significantly reduced airway remodeling and reduced levels of oncostatin M (OSM) a cytokine previously not known to be regulated by Fstl1. The importance of the Fstl1 induction of OSM to airway remodeling was demonstrated in murine studies in which administration of Fstl1 induced airway remodeling and increased OSM, while administration of an anti-OSM antibody blocked the effect of Fstl1 on inducing airway remodeling, eosinophilic airway inflammation, and airway hyperresponsiveness all cardinal features of asthma. Overall, these studies demonstrate that the Fstl1/oncostatin M pathway may be a novel pathway to inhibit airway remodeling in severe human asthma.
INTRODUCTION
Asthma is a disease characterized by airway inflammation, airway remodeling, and airway hyperresponsiveness1. Features of airway remodeling in humans with asthma include increases in epithelial mucus cells, peribronchial fibrosis, and smooth muscle1 which can be modeled in mice subjected to chronic allergen challenge2. To identify potential novel mediators of airway remodeling in asthma, we measured levels of mediators in lungs from wild type (WT) mice acutely challenged with allergen (which is not associated with induction of airway remodeling), and compared this to levels of mediators in lungs from WT mice chronically challenged with allergen (which is associated with induction of airway remodeling). This strategy identified that follistatin like 1 (Fstl1), a mediator not previously associated with either asthma, or airway remodeling in asthma, is highly expressed in WT mice chronically but not acutely challenged with allergen.
Fstl1 is a 308 amino acid extracellular glycoprotein that shares 94% identity in man and mouse3,4. Although Fstl1 is part of the follistatin family it has very low protein sequence homology to follistatin (7%) as well as several key structural and functional differences. Fstl1 was initially identified as a TGF-β inducible gene and has been cloned in mouse3, and man4. Fstl1 is generated in particular by non-hematopoetic cells such as cells of the mesenchymal lineage (fibroblasts, chondrocytes, osteocytes, adipocytes, cardiomyocytes) by stimuli including TGFβ, IL1β, TNFα, IL6, and LPS5. Fstl1 released from these mesenchymal cells targets immune cells (monocytes, macrophages and T cells) to express pro-inflammatory cytokines (IL1β, TNFα, IL6, IFNγ) and chemokines (IL8, MCP1, IP10)5. Fstl1 binds to target cells through at least one defined cell surface receptor (i.e. DIP2A or disco-interacting protein 2 homolog A)6. The mechanism by which Fstl1 influences signaling events in target cells has been the focus of several studies which have identified at least five potential Fstl1 signaling pathways including Fstl1 influencing the bone morphogenic protein (BMP) pathway to inhibit pSmad 1, 5, 8 signaling7, as well as Fstl1 influencing the AKT (or protein kinase B) pathway, the AMP activated protein kinase pathway, the TLR4/CD14 pathway, and Na/K-ATPase membrane potential8,9. Fstl1 has been implicated in cellular functions including survival, proliferation, differentiation, migration, and organ development5. Fstl1 has not previously been reported in asthma or allergy but has been studied in embryogenesis10,11, tumor development12, cardiac disease13,14, arthritis5,15,16, and wound healing17. The predominant effect of Fstl1 appears to be pro-inflammatory15,16,18, although anti-inflammatory effects of Fstl1 have also been described (inhibits MMP1, MMP2, MMP3, MMP9 and prostaglandin E2 expression)8,19. Fstl1 transfection into cells increases levels of IL-1β, TNF, and IL-615.
Here we show that Fstl1, a mediator not previously associated with asthma is highly expressed by macrophages in the lungs of severe human asthmatics as compared to normal control subjects. Although severe human asthmatics only comprise approximately 5% of all asthmatics they utilize approximately 50% of the $20 billion dollars/year in direct and indirect healthcare costs spent on all asthmatics in the USA20, underscoring the need for novel therapies in these severe asthmatics. Utilizing a mouse model of chronic asthma, we demonstrate that lung macrophages in mice, like lung macrophages in severe human asthmatics, highly express Fstl1, and that chronic allergen challenged Lys-Cretg/Fstl1Δ/Δ mice in whom Fstl1 is inactivated in macrophages/myeloid cells had significantly reduced airway remodeling and significantly reduced expression of oncostatin M (OSM) a cytokine previously unrecognized to be downstream of Fstl1 that we demonstrate mediates Fstl1’s effect on airway remodeling, eosinophilic inflammation, and airway responsiveness. The importance of the Fstl1 induction of OSM to airway remodeling was demonstrated in studies in which administration of Fstl1 to the mouse airway induced airway remodeling and increased OSM, while administration of an anti-OSM Ab blocked the effect of Fstl1 on inducing airway remodeling. Overall, these studies demonstrate that the Fstl1/OSM pathway may be a novel pathway to inhibit airway remodeling in severe human asthma.
MATERIALS AND METHODS
Expression of Fstl1 in human asthma and control lungs
Human asthma lungs
Postmortem human lungs from asthmatics were obtained from National Disease Research Interchange in a protocol approved by the UCSD Human Research Protections program. Lung sections were immunostained with an anti-Fstl1 antibody (R&D) or species and isotype control antibody. To determine the contribution of lung macrophages to levels of Fstl1 detected, lung sections were co-immunostained with Abs to Fstl1(R&D) as well as to CD68 (R&D) a macrophage marker. In these experiments, the two different primary antibodies were detected using two different horseradish peroxidase (HRP) enzyme-labeled secondary antibodies with tyramide signal amplification (Molecular Probes) according to the manufacturer’s instructions as previously described35.
Bronchial biopsy
The protocols for utilizing bronchoscopy to obtain bronchial biopsies from patients with asthma (severe, mild) and control non-asthmatics at McGill University and Universite de Montreal with the approval of the respective institutional review boards has previously been described36. The methods for processing the bronchial biopsies, and immunostaining lung sections using the ABC immunoperoxidase method has also been described36. In this study, biobanked bronchial biopsy sections from severe asthmatics (n=10), mild asthmatics (n=10), and non-asthmatic controls (n=10) were immunostained with an anti-Fstl1 antibody (R&D) or species and isotype control antibody. The number of bronchial biopsy Fstl1 positive cells was quantitated with image analysis (Image-Pro) in each subject and results expressed as Fstl1 positive cells/mm2.
Animal care and use
All the mouse experimental protocols described in the online methods were approved by the UCSD Institutional Animal Care and Use Committee.
Wild Type (WT) mice acute and chronic OVA challenge and lung Fstl1 expression
We performed initial experiments to detect whether Fstl1 was expressed in WT mouse lung following acute or chronic OVA allergen challenge using two groups of mice (WT OVA; WT no OVA; 8 female C57BL/6 mice/group aged 12 weeks). In the acute OVA protocol WT mice were sensitized and challenged with OVA (Worthington, Lakewood, NJ) as previously described37. In brief, mice were sensitized i.p. with 100 μg OVA and 2 mg aluminum hydroxide (Imject Alum; Thermo Fisher Scientific, Waltham, MA) in a total volume of 200 μl PBS on days 0 and 10 followed by intranasal administration of 200 μg OVA in 20 μl PBS on days 21, 23, and 25. In the acute OVA protocol mice were sacrificed on day 26 as previously described2,37. In the chronic OVA protocol, mice were initially sensitized and challenged with OVA as described for the acute OVA protocol, and from day 28 mice continued challenges with intranasal OVA twice a week for an additional one month2,37. Non-OVA challenged mice were sensitized and challenged with PBS only. Twenty-four hours after the last challenge, bronchoalveolar lavage (BAL) fluid and lungs were collected as previously described2,37 to assess levels of Fstl1 by immunohistochemistry, RT-PCR, and Elisa.
WT Mouse BAL Fluid collection
BAL fluid was collected by lavaging the lung with 1 ml PBS via a tracheal catheter as previously described2. BAL fluid was centrifuged, and the supernatant frozen at −80 °C for subsequent Fstl1 analysis. BAL total and differential cell counts were quantitated in Wright Giemsa stained slides.
WT mouse lung processing to detect Fstl1
Lungs were processed for immunohistology (paraffin-embedded lung sections), as well as protein and RNA extraction as previously described in this laboratory30,31. For protein and RNA extractions, lungs were initially snap-frozen in liquid nitrogen and stored at −80°C.
WT mouse lung immunohistology-to detect Fstl1 and M2 macrophages
For paraffin-embedded sections, lungs were equivalently inflated with an intra-tracheal injection of the same volume of 4% paraformaldehyde solution (Sigma Chemicals, St. Louis, MO) to preserve the pulmonary architecture. Lung sections were processed for immunohistochemistry to detect Fstl1 (anti-mouse Fstl1 Ab, Abcam). To determine the contribution of lung macrophages to levels of Fstl1 detected, lung sections were co-immunostained with Abs to Fstl1 as well as to F4/80 (anti-mouse F4/80 Ab, AbD Serotec). In these experiments, the two different primary antibodies were detected using two different horseradish peroxidase (HRP) enzyme-labeled secondary antibodies with tyramide signal amplification (Molecular Probes) according to the manufacturer’s instructions as previously described35. The anti-Fstl1 Ab was detected with an HRP-labeled secondary Ab (Alexa 546, red), while the anti-F4/80 Ab was detected with a different HRP-labeled secondary Ab (Alexa 488, green). Cells co-expressing F4/80 and Fstl1 stained a blended yellow color. To determine whether the macrophages in the lung had an M2 phenotype, we similarly used double immunostaining combining an antibody to a mouse macrophage M2 marker [i.e. arginase 1 (Arg-1)]21(Abcam) with an anti-F4/80 Ab to detect macrophages. The number of individual cells staining positive for different cell types in the peribronchial space was counted using a light microscope. Results are expressed as the number of peribronchial cells staining positive per bronchiole with 150–200 μm of internal diameter. At least ten bronchioles were counted in each slide.
WT mouse lung RT-PCR to detect Fstl1 mRNA
qRT/PCR was performed as previously described in this laboratory30,31. In brief, total RNA was extracted with RNA-STAT-60 (Tel-Test) and reverse transcribed with Oligo-dT and SuperScript II kit (Life Technologies). qPCR was performed with TaqMan PCR Master Mix and Fstl1 primers (all from Applied Biosystems). The relative amounts of transcripts were normalized to those of housekeeping gene (GAPDH) mRNA and compared between the different genes by the ΔΔCt method as previously described in this laboratory30,31.
WT mouse lung ELISA to detect Fstl1 protein
We utilized an Fstl1 ELISA (MyBio Source) to detect Fstl1 in BAL fluid.
Detection of lung cells that express Fstl1
As previous studies have not reported that macrophages or airway epithelium are significant sources of Fstl15, we examined levels of Fstl1 by qRT-PCR in unstimulated and stimulated macrophages and epithelial cells compared to fibroblasts a known source of Fstl15. Pure populations of bone marrow derived macrophages were cultured from mouse bone marrow as previously described38. Pure populations of mouse primary lung fibroblasts and mouse lung epithelial cells were obtained from Sciencell. Stimuli used for all cell types included TGFβ1 (50 ng/ml)(R&D Systems) a known inducer of Fstl1 in fibroblasts5 and Fstl1 (100 ng/ml)(Sino Biological) which has not previously been investigated as an autocrine stimulus for its production by macrophages. Macrophages were also stimulated with either IL-4 (100 ng/ml) (R&D Systems), or IL-13 (100 ng/ml) (R&D Systems). Cells (106/well) were cultured for 24 hrs at 37°C in complete media (Sciencell) with or without the above stimuli at which time RNA from the cells was extracted and processed for RT-PCR to quantitate Fstl1 mRNA expression.
WT mice challenged with Fstl1
To determine whether Fstl1 administration to the mouse airway can influence either airway inflammation, airway remodeling (mucus, fibrosis, smooth muscle changes), or airway responsiveness (AHR), we utilized two groups of WT mice (WT mice administered Fstl1; WT mice no Fstl1)(8 female C57BL/6 mice aged 12 weeks/group). The Fstl1 challenged WT mice were administered 10 μg Fstl1 (Sino Biological) in 50 μl PBS intranasally daily for 15 days and sacrificed on day 16.
WT mice challenged with Fstl1 and administered an anti-OSM antibody
In these experiments, WT mice challenged intranasally with 10 μg Fstl1 (n= 4 mice/group)(female C57BL/6 mice aged 12 weeks/group) received either 0 μg or 10 μg anti-OSM neutralizing Ab (R&D) administered i.p. in 100 μl of sterile PBS every other day 4 hours before each of the 15 daily Fstl1 challenges described above. A control group of WT mice received 10 μg anti-OSM neutralizing Ab and no Fstl1. Mice were sacrificed on day 16.
Detection of airway remodeling in WT mice challenged with Fstl1
Airway mucus expression-PAS
To quantitate the level of mucus expression in the airway, the number of periodic acid Schiff (PAS)-positive and PAS-negative epithelial cells in individual bronchioles was counted as previously described in this laboratory30,31. At least ten bronchioles were counted in each slide. Results are expressed as the percentage of PAS-positive cells per bronchiole, which is calculated from the number of PAS-positive epithelial cells per bronchus divided by the total number of epithelial cells of each bronchiole.
Airway mucus expression- lung qPCR Muc5AC
qPCR was performed as described above for Fstl1 to detect the mouse lung mucus gene Muc5AC using Muc5AC primers (Applied Biosystems).
Peribronchial fibrosis-lung trichrome staining
To detect peribronchial fibrosis, the area of peribronchial trichrome staining in paraffin-embedded lungs was outlined and quantified under a light microscope (Leica DMLS, Leica Microsystems) attached to an image analysis system (Image-Pro plus, Media Cybernetics) as previously described30,31. Results are expressed as the area of trichrome staining per μm length of basement membrane of bronchioles 150–200 μm of internal diameter.
AHR in WT mice challenged with Fstl1
Airway responsiveness to methacholine was assessed in intubated and ventilated mice (WT Fstl1; WT no Fstl1)(n = 8 mice/group) (flexiVent ventilator; Scireq) anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) i.p. as previously described30,31. The dynamic airway resistance was determined using Scireq software in mice exposed to nebulized PBS and methacholine (0 and 24 mg/ml). The following ventilator settings were used: tidal volume (10 ml/kg), frequency (150/min), and positive end-expiratory pressure (3 cmH2O).
IgE levels in WT mice challenged with Fstl1
Serum total IgE was quantitated with an IgE ELISA kit (BD Biosciences). ELISA plates were read with a BioRad Model 680 microplate reader.
In vitro effects of Fstl1 on target structural cells in lung (i.e. epithelium, fibroblasts, and smooth muscle)
As Fstl1 administration to WT mouse lung in vivo induced mucus, peribronchial fibrosis, and AHR, we examined whether any of the effects of Fstl1 could be occurring by direct effects of Fstl1 on either fibroblasts, epithelial cells, or smooth muscle. In these in vitro experiments either primary mouse lung fibroblasts, primary human bronchial epithelial cells (we used primary human bronchial epithelial cells as we were not able to obtain sufficient numbers of primary mouse bronchial epithelial cells for in vitro studies), or primary mouse lung smooth muscle cells (all obtained from Sciencell) were incubated with Fstl1 (100 ng/ml) for 24 hours. End points measured for lung fibroblasts were collagen synthesis (collagen genes I, III, V by qPCR), for lung epithelial cells (mucus gene Muc5AC by qPCR), and for smooth muscle (contraction). For each cell type a positive control was used (TGFβ1).
Lung smooth muscle contraction assay
Primary mouse lung smooth muscle (SM) cells (ScienCell) were cultured according to the manufacturer’s instructions for use in an in vitro SM gel contraction assay, which we have adapted from studies of human airway SM39, as well as from our studies with esophageal smooth muscle contraction22. SM cells were cultured in basal medium without growth factors for 24 hours before seeding in collagen gels free of LPS (Advanced BioMatrix, San Diego, Calif). After overnight incubation in collagen gels, SM cells were cultured in the presence or absence of Fstl1 (100 ng/ml). Control mediators used in the gel contraction assay included TGF-β1 (50 ng/mL)(R&D Systems) a cytokine we have previously demonstrated to induce slow onset SM contraction in this assay22. With agonist-induced SM contraction, the area of the gel decreases significantly, as described in studies of airway SM39. The area of the gels was quantitated by using a Bio-Rad ImageDR transilluminator and Versadoc scanner (Bio-Rad Laboratories, Hercules, Calif) with an accompanying image-capture and analysis program to generate area in square millimeters.
Fstl1flox/flox and Lys-Cretg mice
As our initial studies in WT mice challenged with OVA allergen demonstrated that lung macrophages were a significant source of Fstl1, we utilized cre-lox techniques as previously described in this laboratory40 to inactivate Fstl1 in macrophages and myeloid cells. Fstl1F/F floxed mice were kindly provided by X Zhang (Shanghai Institute for Biological Sciences, CAS), and X Gao (Nanjing University, Nanjing, China) as described7,9. Lys-Cretg mice (Cre expression in macrophages and myeloid cells) were acquired from Jackson labs. To delete the Fstl1F allele in myeloid cells, Fstl1F/F mice (background strain C57/Bl6) were crossed with transgenic Lys-Cretg mice to generate Lys-Cretg/Fstl1Δ/Δ progeny in which the Fstl1F allele is selectively deleted in macrophages and myeloid cells. Mice were genotyped with cre- and Fstl1 specific primers and the PCR product was run on a 1.5% agarose gel.
Lys-Cretg/Fstl1Δ/Δ OVA allergen challenge
Lys-Cretg/Fstl1Δ/Δ and littermate control mice (hereafter referred to as WT mice)(n=8 mice/group) were sensitized and challenged with OVA as described above for the chronic OVA challenge protocol. Outcomes measured included lung eosinophils, features of remodeling (mucus, fibrosis, smooth muscle), and remodeling mediators (MMP9, oncostatin M). Eosinophils were quantitated by image analysis in lung sections immunostained with an anti-MBP Ab (Jamey Lee PhD, Mayo). MMP9 and oncostatin M were quantitated by qRT-PCR.
Statistical Analysis
All results are presented as mean ± SEM. A statistical software package (Graph Pad Prism, San Diego, CA) was used for the analysis. P values of < 0.05 were considered statistically significant.
RESULTS
Fstl1 is highly expressed in severe asthmatics
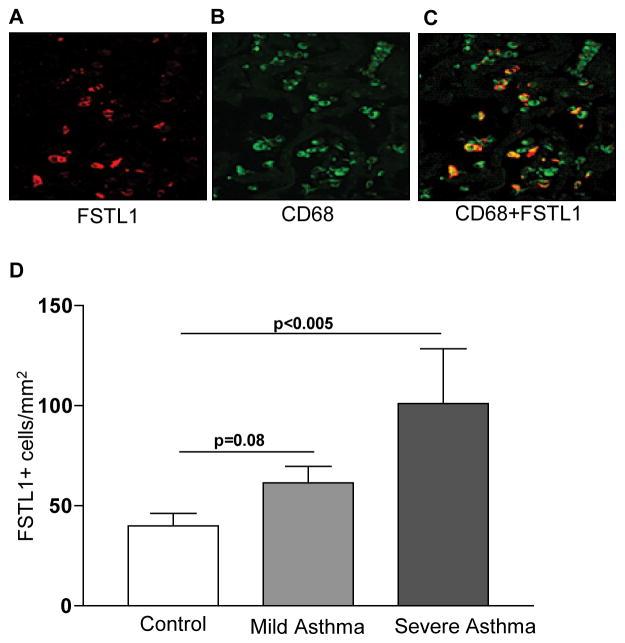
Immunofluorescence microscopy of lungs from human asthmatics demonstrated that Fstl1 was highly expressed (Fig. 1a) and that many of the Fstl1+ cells co-expressed CD68 a macrophage marker (Fig. 1b–c). We also used immunohistochemistry to quantitate levels of expression of Fstl1 in bronchial biopsies obtained from the lungs of severe asthmatic compared to control subjects. These studies demonstrated that the number of Fstl1 positive cells in the lungs of severe asthmatics was significantly greater than the number of Fstl1 positive lung cells in normal control subjects (P<0.005)(Fig. 1d).
Figure 1. Fstl1 is highly expressed in lungs of severe human asthma.
Lungs from human asthmatics were processed for immunofluorescence microscopy to detect either Fstl1 (Fig. 1a), CD68 (Fig. 1b), or Fstl1 and CD68 (Fig. 1c). Bronchial biopsies from human subjects with severe asthma, mild asthma, or no asthma were processed for immunohistochemistry with an anti-Fstl1 Ab (n=10 subjects/group)(Fig 1d). The number of peribronchial Fstl1 positive cells were quantitated by light microscopy and image analysis in each group.
M2 macrophages highly express Fstl1
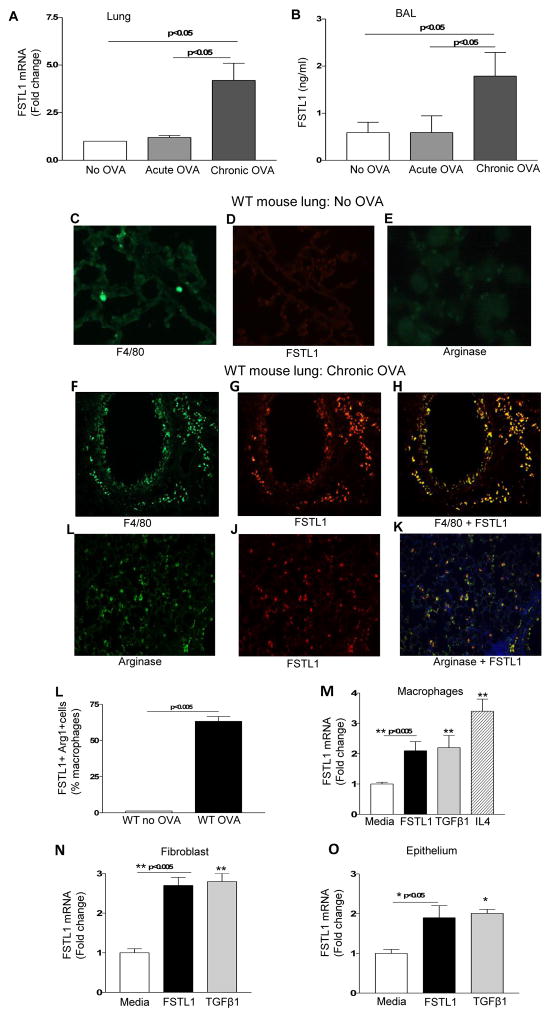
Having detected high levels of expression of Fstl1 in humans with severe chronic asthma we used a mouse model of chronic asthma to determine the role of Fstl1 in contributing to the pathogenesis of asthma. WT mice challenged chronically with OVA allergen have a significant increase in lung Fstl1 mRNA as assessed by qPCR (p<0.05; chronic OVA vs no OVA)(Fig. 2a) and bronchoalveolar lavage (BAL) Fstl1 protein as assessed by ELISA (p <0.05; chronic OVA vs no OVA)(Fig. 2b). In contrast, WT mice challenged acutely with OVA allergen do not have increases in lung Fstl1 mRNA (Fig. 2a) or BAL Fstl1 protein (Fig. 2b). We have previously demonstrated that chronic OVA, but not acute OVA challenge, induces airway remodeling2.
Figure 2. M2 macrophages highly express Fstl1 in response to chronic allergen.
WT mice were sensitized to OVA and challenged acutely or chronically with OVA allergen (8 mice/group). Lungs were processed to detect Fstl1 mRNA by qPCR (Fig. 2a). BAL fluid levels of Fstl1 protein was measured by ELISA (Fig. 2b). Lungs from non-OVA challenged mice were processed for immunofluorescence microscopy to detect either F4/80 (Fig. 2c), Fstl1 (Fig. 2d), or arginase (Fig. 2e). Lungs from chronic OVA challenged WT mice were processed for immunofluorescence microscopy to detect either F4/80 (Fig. 2f), Fstl1 (Fig. 2g), or F4/80 and Fstl1 (Fig. 2h), as well as to detect arginase (Fig. 2i), Fstl1 (Fig. 2j), or arginase and Fstl1 (Fig. 2k). Image analysis quantitated the % of Fstl1 positive cells that co-expressed arginase (Fig. 2l). Levels of Fstl1 mRNA were quantitated by qPCR in mouse macrophages (Fig. 2m), fibroblasts (Fig. 2n), or epithelial cells (Fig. 2o) incubated with either TGFβ1 a known stimulator of Fstl1 expression, IL-4, or Fstl1.
Lung sections from WT mice not challenged with OVA had low numbers of F4/80 positive cell (Fig. 2c) which did not express Fstl1 (Fig. 2d) or the M2 macrophage marker arginase21(Fig. 2e) as assessed by immunofluorescence microscopy. In contrast, lung sections of WT mice challenged chronically with OVA demonstrated a significant increase in the number of Fstl1- positive cells which co-expressed the macrophage marker F4/80 (Fig. 2f–h). The Fstl1 positive cells also co-expressed the M2 macrophage marker arginase (Fig. 2i–k). The percentage of lung Fstl1 positive cells that co-expressed arginase was significantly higher in WT mice chronically challenged with OVA compared to non-OVA challenged mice (p<0.005)(Fig. 2l).
Prior studies have not reported macrophages as a significant source of Fstl15. We therefore performed in vitro studies with macrophages to compare their ability to express Fstl1 with that of fibroblasts a known source of Fstl15. Macrophages stimulated in vitro with TGFβ1 (a known stimulus for Fstl1 induction in fibroblasts)5 expressed a 2.1 fold increase in Fstl1 mRNA levels as assessed by qPCR (p<0.005)(Fig. 2m), which was similar in range to that of fibroblasts stimulated with TGFβ1 which expressed a 2.7 fold increase in Fstl1 mRNA levels (Fig. 2n). Interestingly, IL-4 induced macrophages to express a significant increase in Fstl1 mRNA (p<0.05) (Fig 2m). IL-13 did not induce macrophages to express Fstl1 mRNA (data not shown). As lung epithelial cells have not previously been demonstrated to express Fstl15, we also examined whether lung epithelial cells expressed Fstl1. Like macrophages and fibroblasts, epithelial cells also expressed Fstl1 mRNA when incubated with either Fstl1 (p<0.05), or TGFβ1 (p<0.05)(Fig. 2o).
We also made the novel observation that macrophages (as well as fibroblasts and epithelium) express Fstl1 mRNA in response to Fstl1 stimulation (Fig. 2m, 2n, 2o). Thus, macrophages, fibroblasts, and epithelium are both cellular sources of Fstl1 which can subsequently through an autocrine or paracrine pathway induce further Fstl1 expression by macrophages, fibroblasts, or epithelium (Fig. 2m, 2n, 2o).
Inhibition of macrophage Fstl1 expression inhibits airway remodeling
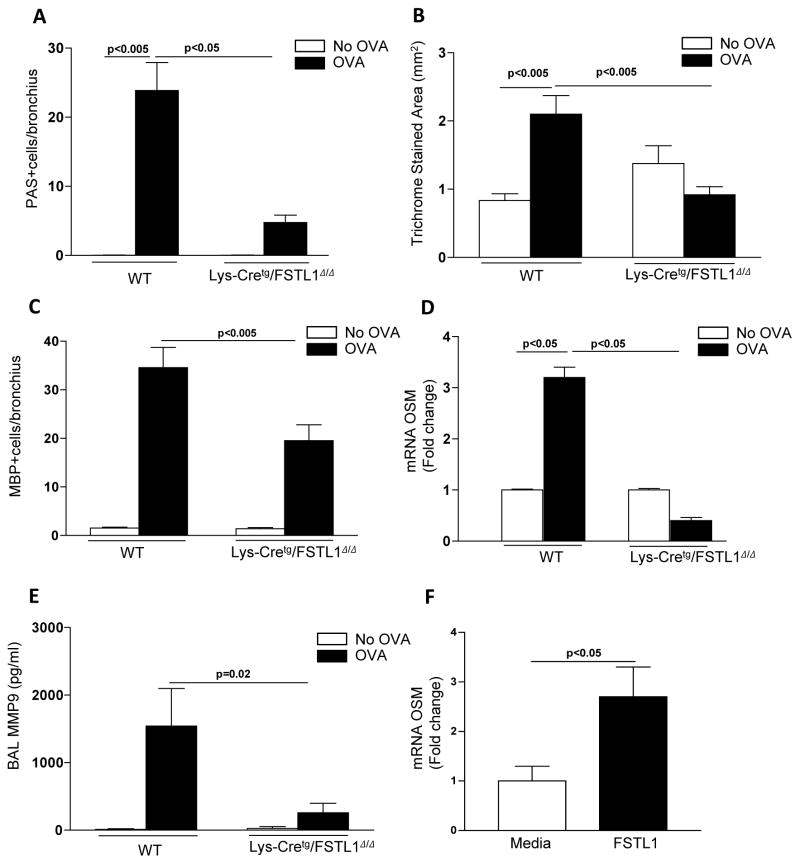
As we had made the novel observation that lung macrophages express high levels of Fstl1 in vitro (Fig. 2m) and in vivo (Fig. 2l), to determine the role of macrophage derived Fstl1 in mediating the features of airway remodeling noted in chronic allergen challenged WT mice, we chronically allergen challenged Lys-Cretg/Fstl1Δ/Δ mice in whom Fstl1 is inactivated in macrophages and myeloid cells. These studies demonstrated that chronically allergen challenged Lys-Cretg/Fstl1Δ/Δ mice had reduced features of airway remodeling including reduced mucus (p<0.05) (Fig. 3a), reduced peribronchial fibrosis (p<0.005)(Fig. 3b), as well as reduced eosinophilic lung inflammation (p<0.005)(Fig. 3c), and reduced expression of remodeling pathways including reduced lung oncostatin M (p<0.05)(Fig. 3d), and reduced BAL MMP9 (p<0.02) (Fig. 3e). There was no change in AHR (data not shown). In separate in vitro experiments, we demonstrated that Fstl1 directly induces WT macrophages from bone marrow to express OSM (Fig. 3f).
Figure 3. Inhibition of macrophage Fstl1 expression inhibits airway remodeling.
Lys-Cretg/Fstl1Δ/Δ or WT mice (8 mice/group) were sensitized with OVA allergen followed by chronic exposure to OVA allergen. Levels of lung mucus were quantitated by PAS staining (Fig. 3a). Levels of peribronchial trichrome staining were quantitated by image analysis (Fig 3b). The number of peribronchial eosinophils was quantitated by MBP immunostaining and image analysis (Fig. 3c). Levels of oncostain M (OSM) were quantitated by qPCR (Fig. 3d). Levels of BAL MMP9 were quantitated by ELISA (Fig. 3e). In separate experiments, WT mouse bone marrow derived macrophages were incubated for 24 hrs with either Fstl1 (100 ng/ml) or media and levels of OSM mRNA quantitated by qPCR (Fig. 3f).
Chronic Fstl1 induces airway remodeling
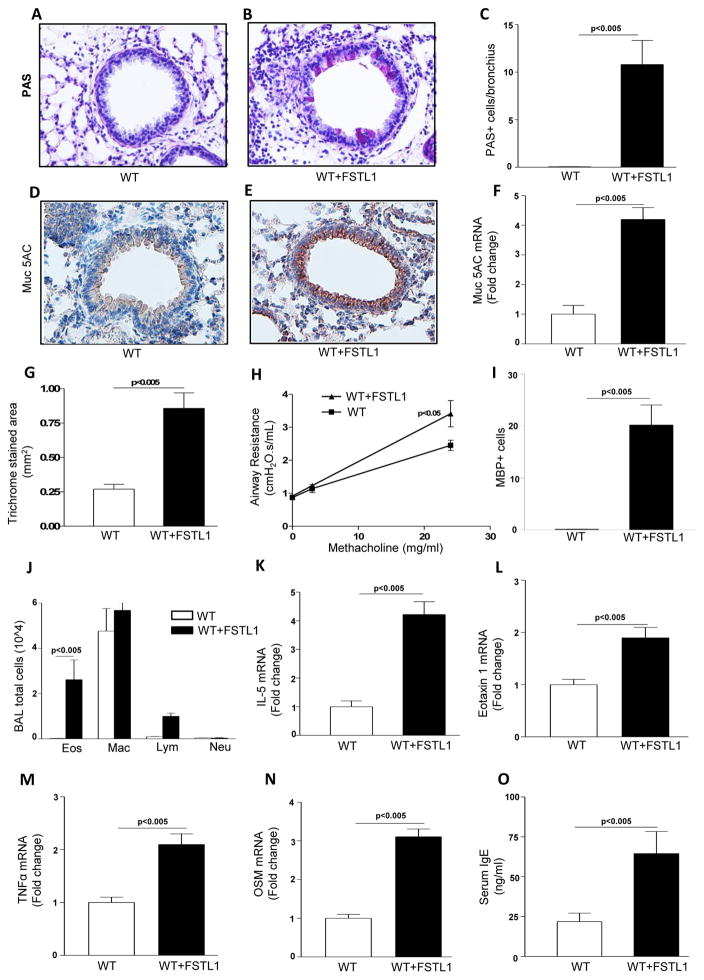
Chronic administration of Fstl1 to WT mice induced a significant increase in mucus as assessed by PAS staining (p<0.005; chronic Fstl1 vs no Fstl1)(Fig. 4a–c), Muc5AC immunostaining (p<0.005)(Fig. 4d–e), and by qPCR for the mucus gene Muc5AC (p<0.05)(Fig. 4f), as well as increased peribronchial fibrosis as assessed by image analysis of the peribronchial area of trichrome staining (p<0.005)(Fig. 4g), and AHR (p<0.05; chronic Fstl1 vs no Fstl1)(Fig. 4h).
Figure 4. Chronic Fstl1 induces airway remodeling.
WT mice (8 mice/group) were administered Fstl1 intranasally daily for 15 days prior to sacrifice (WT+ Fstl1 group). A control WT group did not receive Fstl1 (WT). Lungs from the different groups of WT mice were processed for PAS staining (Fig. 4a–c), Muc5ac immunostaining (Fig. 4d–e), assessment of expression of the mucus gene Muc5AC by qPCR (Fig. 4f), quantitation of peribronchial fibrosis by trichrome staining and image analysis (Fig. 4g), measurement of AHR (Fig. 4h), quantitation of lung MBP positive eosinophils (Fig. 4i), BAL inflammatory cells (Fig. 4j), and assessment of cytokine gene expression by qPCR including IL-5 (Fig. 4k), eotaxin-1 (Fig. 4l), TNFα (Fig. 4m), and oncostatin M (OSM) (Fig. 4n). Levels of serum IgE were quantitated by Elisa (Fig. 4o).
Chronic Fstl1 challenged WT mice had increased numbers of lung MBP+ eosinophils (p<0.0001)(Fig. 4i), and BAL eosinophils (p<0.005) (Fig. 4j), without changes in the numbers of BAL macrophages, lymphocytes, or neutrophils (p=ns)(Fig. 4j). The increase in lung and BAL eosinophils was associated with increased lung levels of the eosinophil active cytokines IL-5 mRNA (p<0.05)(Fig. 4k), and the eosinophil chemo-attractant eotaxin-1 mRNA (also known as CCL11) (p<0.05)(Fig. 4l) as assessed by qPCR. In addition, chronic Fstl1 challenged WT mice had increased levels of TNFα mRNA (p<0.05)(Fig. 4m), lung oncostatin M mRNA (p<0.05) (Fig. 4n), and IgE (p<0.05) (Fig. 4o) with no change in levels of TGFβ1 (data not shown).
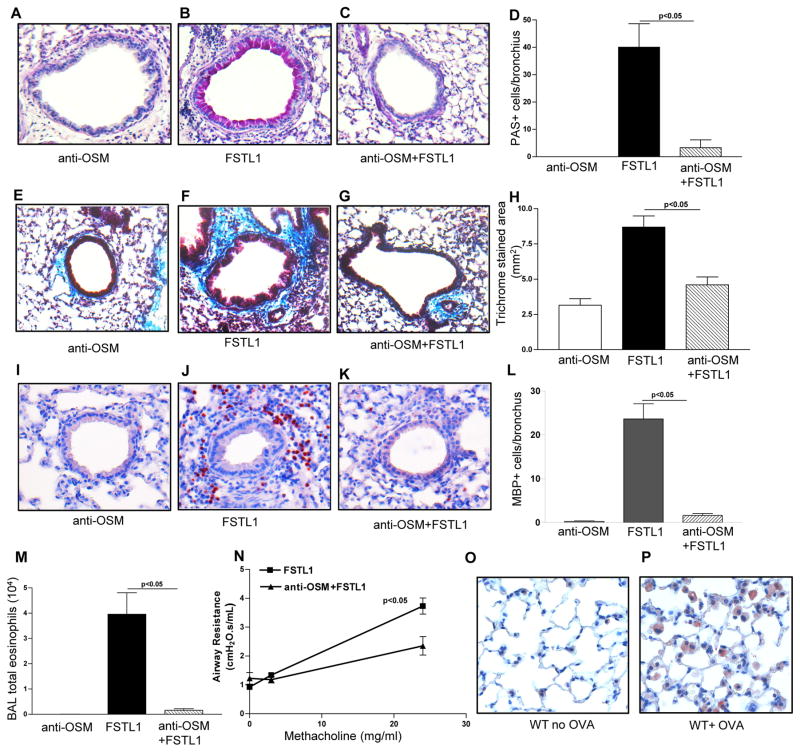
Blocking Oncostatin M inhibits Fstl1 induced airway remodeling, eosinophilic inflammation, and AHR
Chronic administration of Fstl1 to WT mice pre-treated with an anti-OSM antibody resulted in a significant reduction in airway remodeling (mucus and fibrosis) as assessed by PAS staining (p<0.005; chronic Fstl1 + anti-OSM Ab vs chronic Fstl1)(Fig. 5a–d), as well as significantly reduced peribronchial fibrosis as assessed by image analysis of the peribronchial area of trichrome staining (p<0.005)(Fig. 5e–h), lung eosinophilic inflammation (p<0.05)(Fig. 5i–l), BAL eosinophils (p<0.05)(Fig. 5m), and AHR (p<0.05)(Fig. 5n). Immunohistochemistry demonstrated that OSM was highly expressed by lung macrophages in chronic OVA challenged, but not in non-OVA challenged WT mice (Fig 5o–p).
Figure 5. Blocking Oncostain M inhibits Fstl1 induced airway remodeling.
WT mice (4 mice/group) were administered Fstl1 intranasally daily for 15 days, with or without pre-treatment with an anti-oncostatin M antibody (anti-OSM). A control WT group received the anti-oncostatin M antibody and no Fstl1. Levels of lung mucus were quantitated by PAS staining (Fig. 5a–d). Levels of peribronchial trichrome staining were quantitated by image analysis (Fig. 5e–h). The number of MBP+ peribronchial eosinophils were quantitated by image analysis (Fig. 5i–l). The number of Wright-Giemsa stained BAL eosinophils was quantitated by light microscopy (Fig. 5m). Levels of airway responsiveness to methacholine was assessed by flexivent (Fig. 5n). In a separate experiment, lungs from either WT mice subjected to chronic OVA challenge (WT+OVA), or WT mice not challenged with OVA (WT+ No OVA), were immunostained with an anti-OSM Ab to detect OSM positive cells in the lung.
Fstl1 can also directly induce remodeling in vitro
In vitro Fstl1 directly induced lung fibroblasts to express collagen genes known to be expressed in asthma1, including collagen I (p<0.05), collagen III (p<0.05), and collagen V (p<0.05) (Fig. 6a) suggesting potential direct effects of Fstl1 in vivo on inducing peribronchial fibrosis. Fstl1 also induced lung epithelial cells to express the mucus gene Muc5AC mRNA as assessed by qPCR (p<0.05) (Fig. 6b), as well as RANTES (p<0.05) (Fig. 6c), but not eotaxin-1 (data not shown). Fstl1 increased lung smooth muscle contractility (Fig. 6d, 6e), with slow onset kinetics, similar to what we have previously demonstrated for TGFβ1 induced esophageal smooth muscle contraction22, and TGFβ1 induced lung smooth contraction in this study (Fig. 6d, 6e).
Figure 6. Fstl1 can also directly induce remodeling in vitro.
Mouse lung fibroblasts (Fig. 6a), human airway epithelial cells (Fig. 6b, 6c), or mouse lung smooth muscle cells (Fig. 6d), were incubated for 24 hrs with either Fstl1 (100 ng/ml), TGFβ1 (50 ng/ml), or media. Levels of collagen mRNA (Col1α1; Col3α1; Col5α1) expressed by fibroblasts were assessed by qPCR (Fig. 6a). Levels of mucus gene (Muc5AC)(Fig. 6b) and RANTES mRNA expression (Fig. 6c) were assessed by qPCR in human bronchial epithelial cell incubated with Fstl1, TGFβ1, or media. Levels of smooth muscle contraction (Fig. 6d, 6e) were assessed at baseline (time 0 or 0h), as well as 24 hours (24h) after incubation with either Fstl1, TGFβ1, or media (Fig. 6d, 6e). With agonist-induced smooth muscle contraction the area of the gel decreases significantly.
DISCUSSION
In this study we have identified that Fstl1 is highly expressed by macrophages in the lungs of humans with severe asthma, and based on our studies using a mouse model of chronic asthma that Fstl1 is a novel mediator of airway remodeling in asthma via induction of OSM a previously unknown downstream pathway of Fstl1. There are several additional novel observations in this study in particular the demonstration using Lys-Cretg/Fstl1Δ/Δ mice that macrophages/myeloid cells are a significant source of Fstl1 in asthma (previous studies have not noted that macrophages/myeloid cells are a significant source of Fstl1 in other diseases)5, that administration of Fstl1 to WT mice induces airway remodeling in a mouse model of asthma, and that humans with severe asthma have increased expression of Fstl1 in bronchial biopsies compared to mild asthmatics and non-asthmatic controls, underscoring the relevance of the findings in a mouse model of asthma to human disease.
Our study is the first to report that OSM, a member of the IL-6 family of cytokines23, is induced by Fstl1. We demonstrated this in vitro (i.e. that Fstl1 can directly induce macrophages to express OSM), as well as in vivo in studies using an anti-OSM antibody that inhibited the ability of Fstl1 to induce airway remodeling (peribronchial fibrosis, mucus) in WT mice. Our studies of Lys-Cretg/Fstl1Δ/Δ mice demonstrated that macrophage and myeloid cells expressing Fstl1 are key in vivo regulators of OSM expression, as chronic allergen challenged Lys-Cretg/Fstl1Δ/Δ mice did not generate OSM. The pro-fibrotic effect of OSM has been appreciated in studies showing that OSM stimulates human lung fibroblast proliferation and collagen production23,24. In addition, adenoviral mediated over-expression of OSM in the lungs of WT mice25 results in the features we have noted to be induced by Fstl1 in this study including increased fibrosis, goblet cell hyperplasia, eosinophilic inflammation, and AHR, supporting our observations that OSM mediates the effects of Fstl1 on airway remodeling, eosinophilic inflammation and AHR noted in this study. OSM upregulates VCAM and induces eotaxin expression which can contribute to eosinophilic inflammation26. Thus, overall our studies of Fstl1 and OSM suggest a model in which chronic allergen challenge induces lung macrophages to express Fstl1 which then through a subsequent autocrine or paracrine pathway induces lung macrophages to express OSM which stimulates fibroblast proliferation and collagen production24 as well as goblet cell hyperplasia, eosinophilic inflammation, and AHR as previously described25,26. Support for a role of OSM in human asthma and airway remodeling is derived from studies demonstrating increased levels of OSM in the sputum of asthmatics with incompletely reversible airway obstruction27. The OSM receptor has also been detected in the airways of fatal asthmatics23. Although our in vivo studies with an anti-OSM Ab demonstrated that it blocked the vast majority of the effect of Fstl1 on airway remodeling (peribronchial fibrosis, mucus), eosinophilic inflammation, and AHR, we also made the novel observation that Fstl1 in vitro can directly influence the lung fibroblast expression of collagen genes associated with remodeling in asthma (collagen I, III, V)1, mucus gene expression by airway epithelium, and lung smooth muscle contraction, suggesting a potential direct effect of Fstl1 on airway remodeling.
Our study also identified that the M2 macrophage was a significant source of Fstl1 in mouse models of chronic asthma. In contrast, most prior studies of Fstl1 have not considered the macrophage or hematopoietic lineage cells to be a source of Fstl15. Prior studies have demonstrated that mesenchymal cells (fibroblasts, synoviocytes, chondrocytes, osteocytes, adipocytes, cardiomyocytes, endotheliocytes) are a significant source of Fstl15. The reasons for our study, but not prior studies, demonstrating a significant contribution of macrophages to Fstl1 generation may relate to the diseases studied (asthma compared to past studies of arthritis, auto-immune disease, coronary disease), the stimulus studied (chronic allergen), the organ studied (lung vs joint or heart), or other factors. We demonstrated that in vitro macrophages expressed Fstl1 mRNA when stimulated with TGFβ1 (a known inducer of Fstl1) and that the level of Fstl1 induced by TGFβ1 in macrophages was not significantly different from levels of Fstl1 induced in fibroblasts (a major known source of Fstl1) by TGFβ15. We also demonstrated that in macrophages Fstl1 mRNA could be induced via an autocrine or paracrine pathway (Fstl1 stimulates macrophages to express Fstl1 mRNA). In addition, to demonstrating in vitro that macrophages express Fstl1, we also demonstrated that Lys-Cretg/Fstl1Δ/Δ mice in which Fstl1 is inactivated in macrophages and myeloid cells have significantly lower levels of Fstl1 expression confirming a significant macrophage and myeloid cell contribution to Fstl1 expression in asthma compared to other diseases (arthritis, auto-immune disease, coronary disease) in which macrophages and myeloid cells are not the source of Fstl15. Although M2 macrophages are a dominant macrophage subset in Th2 mouse models such as asthma, other macrophage populations are likely also to also produce Fstl1. In this study, arginase negative cells also produced Fstl1, though arginase positive cells were dominant. It is also possible for bone marrow derived macrophages to produce Fstl1 without differentiating to M2.
The use of homozygous Fstl1 deficient mice to study the role of Fstl1 in models of asthma or other diseases has not been possible as homozygous Fstl1 deficient mice die at birth because of respiratory failure7. Our study is the first to use conditional inactivation of Fstl1 in macrophage/myeloid cells to study its influence on a disease phenotype in vivo. Recent studies have used heterozygous Fstl1+/− deficient mice to demonstrate that inhibiting Fstl1 does not inhibit lung inflammation, but does attenuate bleomycin induced pulmonary fibrosis in mice through a TGFβ dependent pathway 28. In the study of bleomycin induced pulmonary fibrosis, the cellular source of Fstl1 was fibroblasts28 a well known mesenchymal source of Fstl15. Our study differs from the study of bleomycin induced pulmonary fibrosis in that we demonstrate that in chronic allergen induced asthma, non-mesenchymal cells such as macrophages (not considered a significant source of Fstl1) are a significant source of Fstl1, that inhibiting Fstl1 inhibits allergen induced airway eosinophilic inflammation (no effect on inhibiting bleomycin induced lung inflammation), and that the downstream pathway of Fstl1 in macrophages is OSM (in bleomycin model it is TGFβ). Furthermore, we use conditional Lys-Cretg/Fstl1Δ/Δ mice, an approach not used in prior Fstl1 research, to demonstrate the importance of macrophage and myeloid cell derived Fstl1 to asthma outcomes. Thus, pathways utilized by Fstl1 may differ in different diseases depending upon which cell expresses and responds to Fstl1. In asthma, the autocrine/paracrine macrophage Fstl1 oncostatin pathway is important, whereas in bleomycin induced pulmonary fibrosis fibroblast derived Fstl1 targets a different TGFβ dependent pathway in epithelial cells. In addition, our studies of chronic allergen challenged Lys-Cretg/Fstl1Δ/Δ mice demonstrate that these mice have reduced eosinophilic inflammation, whereas studies using bleomycin show no effect of Fstl1 on lung inflammation. Thus, this study has evidence for differentially activated Fstl1 downstream pathways (OSM vs TGFβ), with resultant differential Fstl1 effects on lung inflammation (Fstl1 mediates eosinophilic inflammation vs no effect of Fstl1 on lung inflammation in bleomycin induced fibrosis), depending upon the disease stimulus (chronic allergen induced asthma vs bleomycin induced lung fibrosis) and predominant cell expressing (macrophage vs fibroblast) or responding (macrophage vs epithelium) to a ligand such as Fstl1.
Although lung macrophages highly express Fstl1 in the mouse model of asthma as well as in the lungs of human asthmatics, other cell types such as airway epithelium, but not fibroblasts, were also noted to have lower levels of immunostaining for Fstl1 in both OVA challenged WT mice and Lys-Cretg/Fstl1Δ/Δ mice, as well as human lungs (data not shown). Although allergen challenged Lys-Cretg/Fstl1Δ/Δ mice had significantly reduced eosinophilic airway inflammation, mucus, and fibrosis, they did not have reduced AHR. As WT mice challenged with Fstl1 develop increased AHR, this suggests that alternative cellular sources of Fstl1 in Lys-Cretg/Fstl1Δ/Δ mice may be contributing alone or in combination with macrophages to Fstl1 induced AHR. Future studies could examine whether inactivating Fstl1 in epithelium or other cells known to express Fstl1 such as fibroblasts alone or in combination with inactivation of Fstl1 in macrophages had effects on AHR. The innate immune response is also implicated in the production of eosinophil active cytokines IL-5 and eotaxin-1 in WT mice administered Fstl1. At present we do not know the cellular source(s) of IL-5 and eotaxin-1 which will require further study.
Several cytokines/mediators have been implicated in airway remodeling in asthma including TGFβ, LIGHT, IL5, IL13, MMP9, and LTC41,29. More recently we have demonstrated that ORMDL3 an endoplasmic reticulum protein when expressed as a human transgene in mice can induce airway remodeling in the absence of inflammation30,31. Thus, while it is increasingly appreciated that there are several asthma clinical endotypes (e.g. Th2, Th17, etc)32,33, it is also likely that there are several different asthma endotypes that contribute to airway remodeling through either direct effects on target structural cells (epithelium, smooth muscle, fibroblast) or through indirect effects on inflammatory cells which subsequently influence target cells. In this regard, Fstl1 is an example of a cytokine that we have demonstrated induces airway remodeling, eosinophilic inflammation, and AHR through OSM, and may also have direct effects on target structural cells (epithelium, fibroblasts, smooth muscle).
In summary, we demonstrated that Fstl1, a mediator not previously associated with asthma is highly expressed by macrophages in the lungs of humans with severe asthma and by M2 macrophages in the lungs of mice with chronic allergen induced remodeling. Chronic allergen challenged Lys-Cretg/Fstl1Δ/Δ mice in whom Fstl1 is inactivated in macrophages/myeloid cells had significantly reduced airway remodeling and expression of OSM. The importance of the Fstl1 induction of OSM to airway remodeling was demonstrated in studies in which administration of Fstl1 to the mouse airway induced airway remodeling and increased levels of lung OSM, while administration of an anti-OSM antibody blocked the effect of Fstl1 on inducing airway remodeling, eosinophilic inflammation and AHR. The importance to human asthma is evident from the demonstration that Fstl1 is highly expressed in the lungs of severe human asthmatics compared to controls. Recent studies have also demonstrated that OSM levels are increased in bronchoalveolar lavage fluid of allergic asthmatic patients after segmental allergen challenge 41.
Overall, these studies demonstrate that the Fstl1/OSM pathway may be a novel pathway to inhibit airway remodeling in severe asthma, the subset of asthmatics most in need of novel therapies34.
Acknowledgments
Grant support: NIH grants AI 107779, AI 38425, AI 70535, AI 72115 to D.H.B
Abbreviations
- BAL
bronchoalveolar lavage
- Fstl1
follistatin like 1
- MBP
major basic protein
- OSM
oncostatin M
- OVA
ovalbumin
- PAS
periodic acid Schiff
- WT
wild-type
References
- 1.Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008;121:560–570. doi: 10.1016/j.jaci.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5 deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Ozaki S, Osakada F, Mori K, Okubo M, Nakao K. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol. 1998;10:1305–1314. doi: 10.1093/intimm/10.9.1305. [DOI] [PubMed] [Google Scholar]
- 5.Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin-like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–272. doi: 10.1007/s12026-014-8526-z. [DOI] [PubMed] [Google Scholar]
- 6.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. Disco-interacting protein 2 homolog A functions as a follistatin-like 1 receptor. J Biol Chem. 105:1147–1152. [Google Scholar]
- 7.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, Nakajima M, Furuichi T, Ikegawa S, Gao X, Chen YG, Jiang D, Ning W. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sylva M, Moorman AF, van den Hoff MJ. Follistatin-like 1 in vertebrate development. Birth Defects Res. 2013;99:61–69. doi: 10.1002/bdrc.21030. [DOI] [PubMed] [Google Scholar]
- 9.Li KC, Zhang FX, Li CL, Wang F, Yu MY, Zhong YQ, Zhang KH, Lu YJ, Wang Q, Ma XL, Yao JR, Wang JY, Lin LB, Han M, Zhang YQ, Kuner R, Xiao HS, Bao L, Gao X, Zhang X. Follistatin-like 1 suppresses sensory afferent transmission by activating Na+,K+-ATPase. Neuron. 2011;69:974–987. doi: 10.1016/j.neuron.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Adams D, Larman B, Oxburgh L. Developmental expression of mouse follistatin-like 1 (Fstl1): Dynamic regulation during organogenesis of the kidney and lung. Gene Expr Patterns. 2007;7:491–500. doi: 10.1016/j.modgep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umezu T, Yamanouchi H, Iida Y, Miura M, Tomooka Y. Follistatin-like-1, a diffusible mesenchymal factor determines the fate of epithelium. Proc Natl Acad Sci USA. 2010;107:4601–4606. doi: 10.1073/pnas.0909501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan QK, Ngan HY, Ip PP, Liu VW, Xue WC, Cheung AN. Tumor suppressor effect of follistatin-like 1 in ovarian and endometrial carcinogenesis: a differential expression and functional analysis. Carcinogenesis. 2009;30:114–121. doi: 10.1093/carcin/bgn215. [DOI] [PubMed] [Google Scholar]
- 13.Le Luduec JB, Condamine T, Louvet C, Thebault P, Heslan JM, Heslan M, Chiffoleau E, Cuturi MC. An immunomodulatory role for follistatin-like 1 in heart allograft transplantation. Am J Transplant. 2008;8:2297–2306. doi: 10.1111/j.1600-6143.2008.02398.x. [DOI] [PubMed] [Google Scholar]
- 14.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, Robbins P, Hirsch R. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758–4762. doi: 10.4049/jimmunol.177.7.4758. [DOI] [PubMed] [Google Scholar]
- 16.Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182:234–239. doi: 10.4049/jimmunol.182.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundaram GM, Common JE, Gopal FE, Srikanta S, Lakshman K, Lunny DP, Lim TC, Tanavde V, Lane EB, Sampath P. ‘See-saw’ expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature. 2013;495:103–106. doi: 10.1038/nature11890. [DOI] [PubMed] [Google Scholar]
- 18.Chaly Y, Marinov AD, Oxburgh L, Bushnell DS, Hirsch R. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64:1082–1088. doi: 10.1002/art.33422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, Mimori T, Ozaki S. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50:660–668. doi: 10.1002/art.20023. [DOI] [PubMed] [Google Scholar]
- 20.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–1235. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Dzik JM. Evolutionary roots of arginase expression and regulation. Front Immunol. 2014;5:544. doi: 10.3389/fimmu.2014.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-β1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–1204. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 23.O’Hara KA, Kedda MA, Thompson PJ, Knight DA. Oncostatin M: an interleukin-6-like cytokine relevant to airway remodelling and the pathogenesis of asthma. Clin Exp Allergy. 2003;33:1026–1032. doi: 10.1046/j.1365-2222.2003.01714.x. [DOI] [PubMed] [Google Scholar]
- 24.Scaffidi AK, Mutsaers SE, Moodley YP, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Oncostatin M stimulates proliferation, induces collagen production and inhibits apoptosis of human lung fibroblasts. Br J Pharmacol. 2002;136:793–801. doi: 10.1038/sj.bjp.0704769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritz DK, Kerr C, Fattouh R, Llop-Guevara A, Khan WI, Jordana M, Richards CD. A mouse model of airway disease: oncostatin M-induced pulmonary eosinophilia, goblet cell hyperplasia, and airway hyperresponsiveness are STAT6 dependent, and interstitial pulmonary fibrosis is STAT6 independent. J Immunol. 2011;186:1107–1118. doi: 10.4049/jimmunol.0903476. [DOI] [PubMed] [Google Scholar]
- 26.Fritz DK, Kerr C, Tong L, Smyth D, Richards CD. Oncostatin-M up-regulates VCAM-1 and synergizes with IL-4 in eotaxin expression: involvement of STAT6. J Immunol. 2006;176:4352–4360. doi: 10.4049/jimmunol.176.7.4352. [DOI] [PubMed] [Google Scholar]
- 27.Simpson JL, Baines KJ, Boyle MJ, Scott RJ, Gibson PG. Oncostatin M (OSM) is increased in asthma with incompletely reversible airflow obstruction. Exp Lung Res. 2009;35:781–794. doi: 10.3109/01902140902906412. [DOI] [PubMed] [Google Scholar]
- 28.Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y, Li X, Dong S, Liu X, Li X, Yang X, Zheng X, Xie T, Liang J, Dai H, Liu X, Yin Z, Noble PW, Jiang D, Ning W. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med. 2015;212:235–52. doi: 10.1084/jem.20121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, Zuraw BL, Ware CF, Broide DH, Croft M. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller M, Rosenthal P, Beppu A, Mueller JL, Hoffman HM, Tam AB, Doherty TA, McGeough MD, Pena CA, Suzukawa M, Niwa M, Broide DH. ORMDL3 transgenic mice have increased airway remodeling and airway responsiveness characteristic of asthma. J Immunol. 2014;192:3475–3487. doi: 10.4049/jimmunol.1303047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller M, Tam AB, Cho JY, Doherty TA, Pham A, Khorram N, Rosenthal P, Mueller JL, Hoffman HM, Suzukawa M, Niwa M, Broide DH. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc Natl Acad Sci U S A. 2012;109:16648–16653. doi: 10.1073/pnas.1204151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 34.Trejo Bittar HE, Yousem SA, Wenzel SE. Pathobiology of severe asthma. Annu Rev Pathol. 2015;10:511–545. doi: 10.1146/annurev-pathol-012414-040343. [DOI] [PubMed] [Google Scholar]
- 35.Le AV, Cho JY, Miller M, McElwain S, Golgotiu K, Broide DH. Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice. J Immunol. 2007;178:7310–7316. doi: 10.4049/jimmunol.178.11.7310. [DOI] [PubMed] [Google Scholar]
- 36.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, Ludwig MS, Martin JG, Hamid Q. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116:544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Pae S, Cho JY, Dayan S, Miller M, Pemberton AD, Broide DH. Chronic allergen challenge induces bronchial mast cell accumulation in BALB/c but not C57BL/6 mice and is independent of IL-9. Immunogenetics. 2010;62:499–506. doi: 10.1007/s00251-010-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzukawa M, Miller M, Rosenthal P, Cho JY, Doherty TA, Varki A, Broide D. Sialyltransferase ST3Gal-III regulates Siglec-F ligand formation and eosinophilic lung inflammation in mice. J Immunol. 2013;190:5939–5948. doi: 10.4049/jimmunol.1203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodman L, Siddiqui S, Cruse G, Sutcliffe A, Saunders R, Kaur D, Bradding P, Brightling C. Mast cells promote airway smooth muscle cell differentiation via autocrine up-regulation of TGFβ1. J Immunol. 2008;181:5001–5007. doi: 10.4049/jimmunol.181.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc Natl Acad Sci U S A. 2005;102:17723–17728. doi: 10.1073/pnas.0509235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pothoven KL, Norton JE, Hulse KE, Suh LA, Carter RG, Rocci E, Harris KE, Shintani-Smith S, Conley DB, Chandra RK, Liu MC, Kato A, Gonsalves N, Grammer LC, 3rd, Peters AT, Kern RC, Bryce PJ, Tan BK, Schleimer RP. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.01.043. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]