Abstract
A feature of multiple neuropsychiatric disorders is motor impulsivity. Recent studies have implicated serotonin (5-HT) systems in medial prefrontal cortex (mPFC) in mediating individual differences in motor impulsivity, notably the 5-HT2AR receptor (5-HT2AR) and 5-HT2CR. We investigated the hypothesis that differences in the ratio of 5-HT2AR:5-HT2CR protein expression in mPFC would predict the individual level of motor impulsivity and that the engineered loss of the 5-HT2CR would result in high motor impulsivity concomitant with elevated 5-HT2AR expression and pharmacological sensitivity to the selective 5-HT2AR antagonist M100907. High and low impulsive rats were identified in a 1-choice serial reaction time task. Native protein levels of the 5-HT2AR and the 5-HT2CR predicted the intensity of motor impulsivity and the 5-HT2AR:5-HT2CR ratio in mPFC positively correlated with levels of premature responses in individual outbred rats. The possibility that the 5-HT2AR and 5-HT2CR act in concert to control motor impulsivity is supported by the observation that high phenotypic motor impulsivity associated with a diminished mPFC synaptosomal 5-HT2AR:5-HT2CR protein:protein interaction. Knockdown of mPFC 5-HT2CR resulted in increased motor impulsivity and triggered a functional disruption of the local 5-HT2AR:5-HT2CR balance as evidenced by a compensatory upregulation of 5-HT2AR protein expression and a leftward shift in the potency of M100907 to suppress impulsive behavior. We infer that there is an interactive relationship between the mPFC 5-HT2AR and 5-HT2CR, and that a 5-HT2AR:5-HT2CR imbalance may be a functionally-relevant mechanism underlying motor impulsivity.
Keywords: 1-choice serial reaction time task, 5-HT2A receptor, 5-HT2C receptor, motor impulsivity, medial prefrontal cortex (mPFC), serotonin (5-HT)
INTRODUCTION
Impulsivity is a complex, multifaceted personality construct1 and is recognized as a symptomatic element of multiple neuropsychiatric disorders (e.g., attention deficit/hyperactivity disorder, autism, drug addiction).2 Motor impulsivity (difficulty in withholding a prepotent motor response) and impulsive choice (preference for small immediate rewards over large delayed rewards) are two primary facets of impulsivity which have been reliably assayed with self-report questionnaires and laboratory measures in humans and animals (for reviews).1,3 Analyses employing choice serial reaction time (CSRT) tasks in outbred rat strains implicate catecholamine, γ-aminobutyric acid (GABA), glutamate and serotonin (5-HT) systems in corticostriatal circuits in inherent motor impulsivity.4–10 The 5-HT2A receptor (5-HT2AR) and 5-HT2CR are G protein-coupled receptors (GPCR) demonstrated to control motor impulsivity. Systemic administration of selective 5-HT2AR antagonists (e.g., M100907)9,11–14 or selective 5-HT2CR agonists (e.g., Ro 60–0175, WAY163909)11,12,14–17 consistently reduce while the preferential 5-HT2AR agonist 2,5-dimethoxy-4-iodoamphetamine (DOI)9,18–26 or the 5-HT2CR antagonist SB242084 enhance motor impulsivity.12,27 The observation that motor impulsivity was synergistically suppressed by the combination of subthreshold doses of M100907 plus WAY163909 raises the possibility that the 5-HT2AR and 5-HT2CR may act in concert to regulate impulsive responding.15
The control of motor impulsivity by the 5-HT2AR and 5-HT2CR systems intersects within the medial PFC (mPFC), a critical neurobiological substrate of motor impulsivity.10,28–31 The mRNA and/or protein for both the 5-HT2AR and 5-HT2CR are found in glutamatergic and GABAergic neurons in the mPFC.32–36 Localized infusion of DOI into the mPFC enhances37 while intra-mPFC M10090738 suppresses premature responding assessed in the 5-CSRT task. The density of 5-HT2AR9 as well as 5-HT2CR6 protein expression in the mPFC predicts premature responses in the 1-CSRT task in outbred rats. High impulsive rats exhibit a greater 5-HT2AR-mediated head-twitch response and are more sensitive to the suppressive effects of the selective 5-HT2AR antagonist M1009079 while virally-mediated 5-HT2CR knockdown in the mPFC generates elevated premature responses in the 1-CSRT task.6 Taken together, these data suggest that dysregulation of 5-HT2AR and 5-HT2CR neuronal signaling in the mPFC contributed to high levels of inherent motor impulsivity.
The present study was designed to extend previous findings and investigate the hypothesis that the status and balance of the 5-HT2AR and 5-HT2CR in mPFC constitute neurobiological markers of inherent motor impulsivity in an outbred rodent population. We hypothesized that high impulsive (HI) rats, identified based upon levels of premature responses in the 1-CSRT task,6,9,13,15,17 would exhibit a higher ratio of 5-HT2AR to 5-HT2CR (5-HT2AR:5-HT2CR) expression in the mPFC, along with our previously observed higher and lower levels of 5-HT2AR9 and 5-HT2CR,6 respectively, and a disruption in the 5-HT2AR:5-HT2CR protein:protein interaction, relative to low impulsive (LI) rats. Lastly, we tested the hypothesis that the genetic knockdown of 5-HT2CR in the mPFC will evoke high motor impulsivity concomitant with elevated 5-HT2AR expression and pharmacological sensitivity to the suppressive effects of the selective 5-HT2AR antagonist M100907 relative to control rats. The observed differential ratio of native 5-HT2AR:5-HT2CR in high vs. low impulsive outbred rats as well as the observations from an engineered imbalance in the 5-HT2R system suggest that cortical 5-HT2AR and 5-HT2CR homeostasis is a key regulatory factor in motor impulsivity.
RESULTS AND DISCUSSION
Phenotypic stratification of motor impulsivity is achievable with the 1-CSRT task
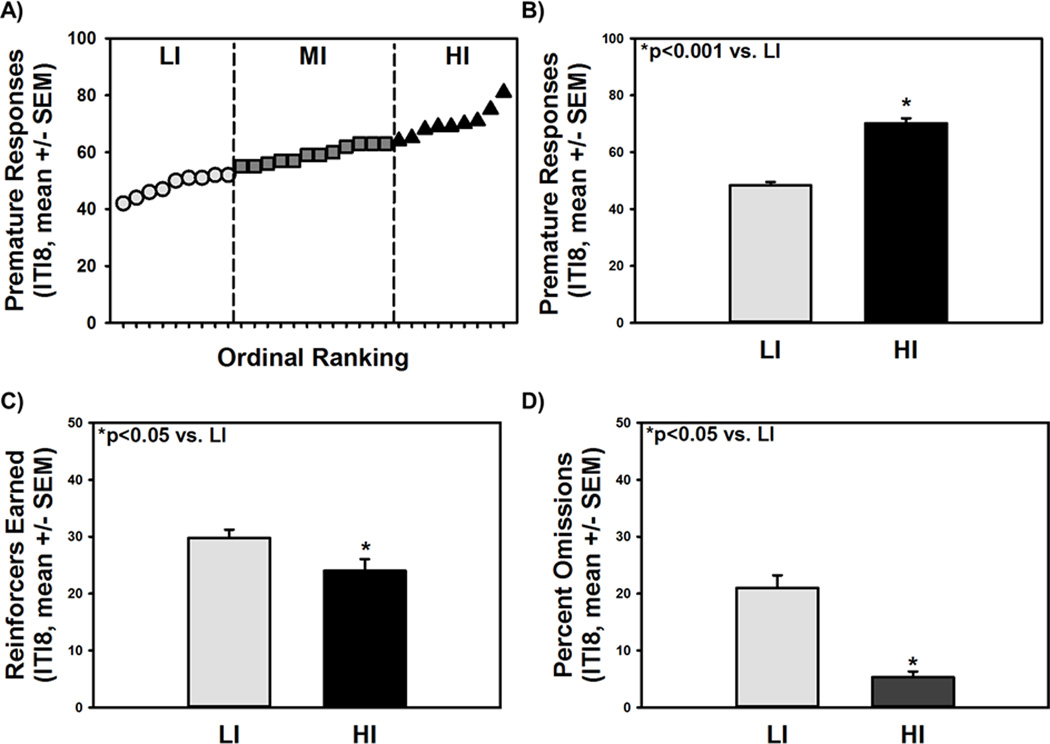
The motor impulsivity phenotype is reliably identifiable in CSRT tasks.4–9 Here, a cohort of outbred rats was stratified for the motor impulsivity phenotype using the 1-CSRT task. High (HI; n=9) and low impulsive rats (LI; n=9) were classified as the upper and lower quartile based upon premature responses on an ITI8 challenge session; the ITI8 challenge promotes premature responding and thus more easily allows for the detection of phenotypic differences.4–9 Accuracy on the ITI5 during task training and maintenance sessions and on the ITI8 challenge session averaged 97–98% indicating that rats detected the stimuli and performed effectively under both ITI conditions.6,9 Figure 1A illustrates the ordinal distribution of individual rats plotted by premature responses for the upper (HI) and lower (LI) quartile of rats relative to those in the mid (MI) range (middle two quartiles). HI rats engendered more premature responses vs. LI rats (Fig. 1B; p<0.001). HI rats earned fewer reinforcers (Fig. 1C; p<0.05) and exhibited lower percent omissions (Fig. 1D; p<0.05) vs. LI rats. No differences between LI and HI on ITI8 were observed in accuracy (97.3% ± 1.3 vs. 97.8% ± 1.1) or the latency to the first pellet (1.6 ± 0.7 sec vs. 0.6 ± 0.2). There was no significant difference in 1-CSRT task performance or phenotypic identification between the three cohorts of rats (data not shown) employed in the present studies (See Research Design).6,9 Thus, the natural variation in levels of motor impulsivity in an outbred rat population is quantifiable in the 1-CSRT task.6,9
Figure 1. Phenotypic stratification of motor impulsivity with the 1-CSRT task.
[A] The number of premature responses made during the ITI8 challenge session was used to stratify rats as high impulsive (HI, upper quartile) or low impulsive (LI, lower quartile) relative to mid impulsive (MI, middle two quartiles) rats. [B] HI rats displayed higher premature responses (*p<0.001 vs. LI), [C] earned fewer reinforcers (*p<0.05 vs. LI), and [D] made fewer omissions relative to LI rats (*p<0.05 vs. LI).
The mPFC 5-HT2AR:5-HT2CR synaptosomal protein profile distinguishes motor impulsivity phenotypes
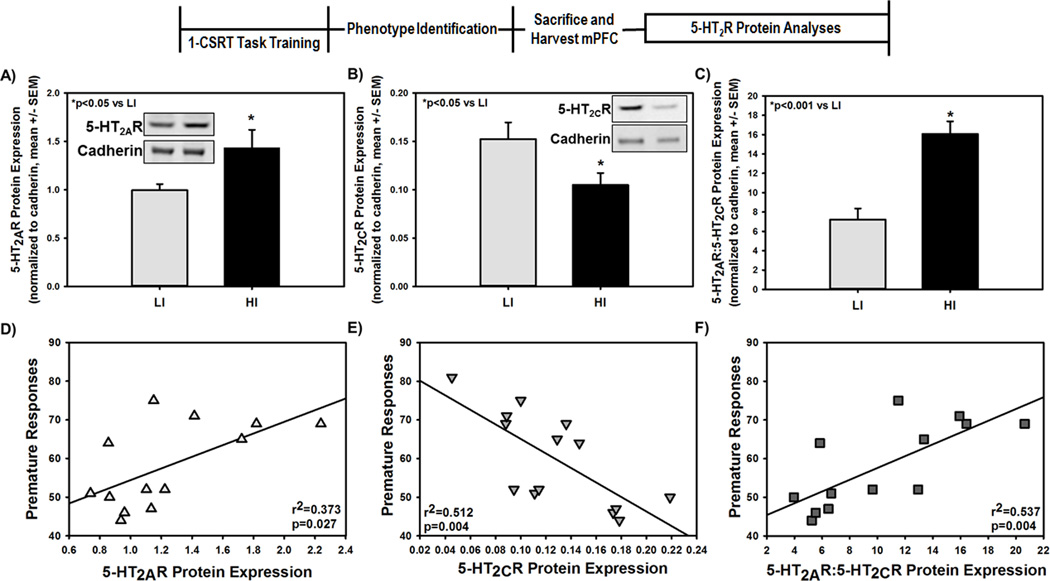
The 5-HT2AR and 5-HT2CR regulate the excitatory/inhibitory balance in the mPFC and the net consequence of 5-HT2AR and 5-HT2CR activation on cortical neurons is interactive as shown by the observation that constitutive knockout of the 5-HT2AR upregulates 5-HT2CR control over the excitability of mPFC pyramidal neurons.39 Rodent studies provide the opportunity to link individual differences in motor impulsivity with the functional capacity of cortical 5-HT2AR and 5-HT2CR ex vivo. The 5-HT2AR and 5-HT2CR are detected postsynaptically with a smaller proportion found in the presynaptic milieu in forebrain.40–42 To assess the available synaptosomal pool of receptors as a possible factor in differential receptor functionality and a neurobiological substrate of inherent motor impulsivity, we evaluated 5-HT2AR and 5-HT2CR synaptosomal protein expression in the mPFC in individual HI and LI rats. The synaptosomal protein fraction employed is enriched for the presynaptic and postsynaptic compartments.43 High impulsive rats (determined from the ITI8 challenge) demonstrated higher synaptosomal mPFC 5-HT2AR (Fig. 2A, p<0.05)9 and lower synaptosomal mPFC 5-HT2CR (Fig. 2B, p<0.05),6 resulting in a higher ratio of 5-HT2AR to 5-HT2CR (5-HT2AR:5-HT2CR; Fig. 2C, p<0.001). Premature responses positively correlated with mPFC 5-HT2AR synaptosomal protein levels (Fig. 2D; r2=0.373; p=0.027), inversely correlated with mPFC 5-HT2CR synaptosomal protein levels (Fig. 2E, r2=0.512; p=0.004) and positively correlated with the mPFC 5-HT2AR to 5-HT2CR ratio (Fig. 2F, r2=0.537; p=0.004). In contrast, the reinforcers earned on the ITI8 challenge did not correlate with either 5-HT2AR (r2=0.0647; p=0.380) or 5-HT2CR (r2=0.0684; p=0.367) protein expression in mPFC; a trend toward a correlation between reinforcers earned and the 5-HT2AR:5-HT2CR ratio was seen (r2=0.252; p=0.067). The percent omissions positively correlated with 5-HT2CR (r2=0.647; p<0.001) and inversely correlated with the 5-HT2AR:5-HT2CR ratio (r2=0.484; p=0.006), but not with 5-HT2AR expression (r2=0.101; p=0.267). Taken together, these data indicate that the 5-HT2AR:5-HT2CR balance is a neurobiological substrate underlying both prepotent responding and motivational drive in high inherent motor impulsivity.
Figure 2. Inherent motor impulsivity predicts mPFC 5-HT2AR:5-HT2CR synaptosomal protein profile.
Following 1-CSRT task training and phenotypic identification, the mPFC was collected for biochemical analysis. Immunoblots for the [A inset] 5-HT2AR, [B inset] 5-HT2CR and [A and B inset] cadherin loading control were performed using crude synaptosomal protein from the mPFC. Densitometric quantitation revealed [A] higher 5-HT2AR, [B] lower 5-HT2CR, and [C] a heightened ratio of 5-HT2AR:5-HT2CR protein expression in HI relative to LI rats (*p<0.05 vs. LI). There was a correlation between [D] premature responses and 5-HT2AR protein expression (r2=0.373; p<0.05), [E] premature responses and 5-HT2CR protein expression (r2=0.512; p<0.001), and [F] premature responses and the 5-HT2AR:5-HT2CR ratio (r2=0.537; p<0.001).
High motor impulsivity associates with lower 5-HT2AR:5-HT2CR protein complex formation in the mPFC
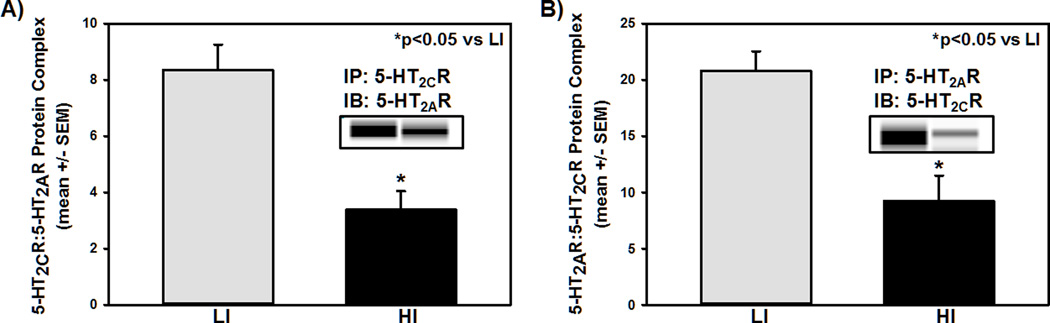
The 5-HT2AR and 5-HT2CR transcript and/or protein have been localized to both glutamate and GABA neurons in the mPFC,32–35,44–47 and has most recently been found to colocalize in GABA neurons, and perhaps pyramidal neurons of the mPFC.36 Given the association of 5-HT2AR and 5-HT2CR proteins in mPFC with levels of motor impulsivity (Fig. 2),6,9 we further considered the possibility that a 5-HT2AR:5-HT2CR protein complex may exist in the mPFC and that the pattern of complex formation may track with inherent motor impulsivity. Co-immunoprecipitation protocols6,9,17,40 were employed to assess the protein:protein interaction of the 5-HT2AR and 5-HT2CR in the mPFC. This technique is based on the ability of an antibody to capture the primary target (e.g., 5-HT2AR or 5-HT2CR) as well as other macromolecules that are bound to the target within a tissue lysate (in this case, mPFC crude synaptosomal protein). The co-immunoprecipitation assay was conducted under saturating antibody conditions, but not 100% efficiency, to control for the expression differences in 5-HT2AR and 5-HT2CR in HI vs. LI rats. We immunoprecipitated comparable amounts (~5 µg) of 5-HT2AR or 5-HT2CR from mPFC synaptosomal protein extracts of HI and LI rats and then equal amounts of protein (1 µg) were subjected to immunoblot analyses for the receptor (performed in duplicate) (Fig. 3). Immunoprecipitation (IP) of synaptosomal protein extracts of the mPFC using the 5-HT2CR antibody followed by immunoblot (IB) with the 5-HT2AR antibody yielded a band at the expected molecular weight (~55 kDa) for 5-HT2AR (Fig. 3A); the reciprocal experiment (5-HT2AR IP followed by 5-HT2CR IB) yielded a band at the expected molecular weight (~46 kDa) for 5-HT2CR (Fig. 3B). Intriguingly, the synaptosomal 5-HT2AR:5-HT2CR protein interaction was attenuated in the mPFC of HI relative to LI rats (Figs. 3A and 3B, p<0.05), suggesting that a 5-HT2AR:5-HT2CR complex exists and may be poised to confer regulatory control over the mPFC microcircuitry and output important in inherent motor impulsivity via actions at the single neuron level.
Figure 3. The 5-HT2AR:5-HT2CR mPFC protein complex is disrupted in high motor impulsivity.
Following 1-CSRT task training and phenotypic identification, the mPFC was collected for biochemical analysis. [A] Immunoprecipitation (IP) for 5-HT2CR followed by immunoblot (IB) for 5-HT2AR yielded 5-HT2AR immunoreactivity in both HI and LI rats. [B] Immunoprecipiation for 5-HT2AR and IB for 5-HT2CR yielded 5-HT2CR immunoreactivity in both HI and LI rats. [A and B] Qualitative (insets) and quantitative demonstration that synaptosomal 5-HT2CR associates with 5-HT2AR in the mPFC to a lesser extent in HI relative to LI rats (*p<0.05). The insets are representative electrophoretic bands. Arbitrary units (A.U.) of densitometry are presented.
Engineered loss of 5-HT2AR:5-HT2CR balance confers high motor impulsivity
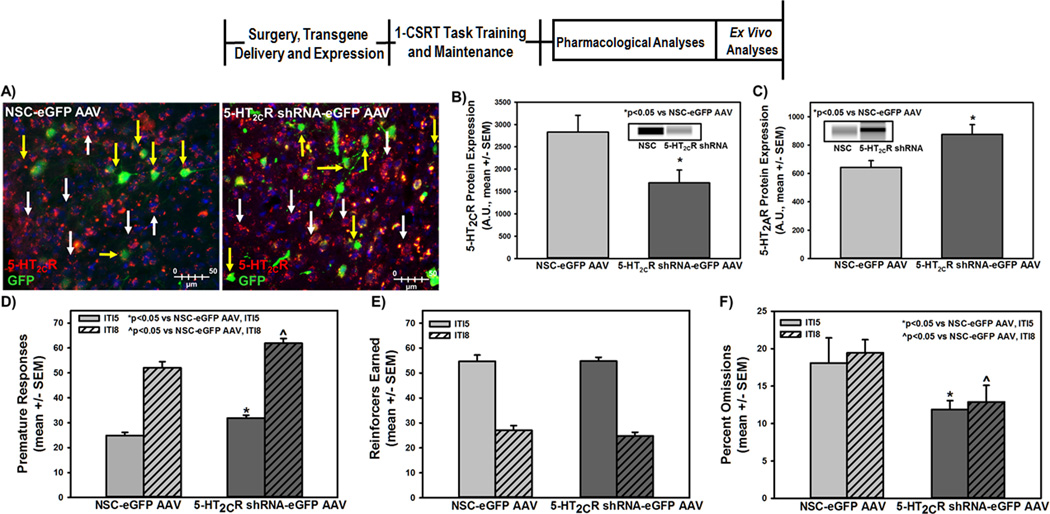
We next tested the hypothesis that, if the 5-HT2AR:5-HT2CR homeostasis in mPFC is important in the control of motor impulsivity, then loss of the 5-HT2CR in this region would tilt the 5-HT2AR:5-HT2CR balance towards a greater 5-HT2AR influence. In the present experiment, we targeted the mPFC comprising the ventral prelimbic and dorsal infralimbic subnuclei6 and employed adeno-associated viral (AAV) vectors to selectively suppress expression of the 5-HT2CR using RNA interference to silence gene expression and to curtail production of the protein. Details of the shRNA design, production and validation of the AAV vectors are published.6 The ex vivo analyses of microinfusion placements in individual rats illustrated that the viral infection was localized within the mPFC along the boundary of the ventral prelimbic/dorsal infralimbic subnuclei (data not shown). The mPFC of a rat infused with the non-silencing control (NSC)-eGFP AAV exhibited 5-HT2CR-immunoreactivity in infected neurons (yellow arrows) and non-infected neurons (white arrows; Fig. 4A, left) while that of a rat infused with the 5-HT2CR shRNA-eGFP AAV exhibited reduced 5-HT2CR-immunoreactivity in infected neurons (yellow arrows) relative to non-infected neurons (white arrows; Fig. 4A, right).
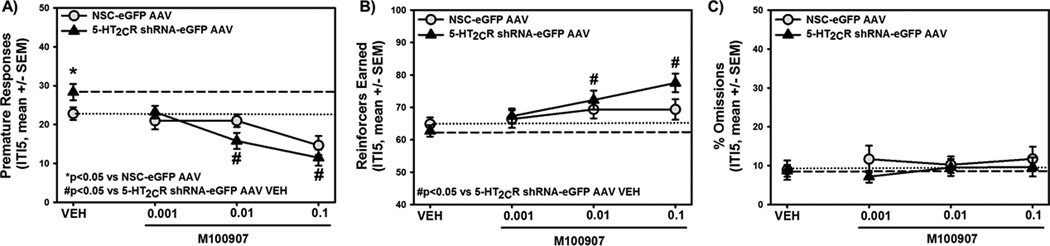
Figure 4. Knockdown of mPFC 5-HT2CR recapitulates high motor impulsivity.
Following intra-mPFC transgene delivery and stable viral vector expression, control and 5-HT2CR knockdown rats were subjected to the 1-CSRT task. [A] Neurons infected with the NSC-eGFP AAV (left) or 5-HT2CR shRNA-eGFP AAV (right) demonstrate green immunofluorescence; a subset of infected neurons are denoted by yellow arrows. A subset of noninfected neurons are denoted with white arrows. All infected neurons are not denoted. Red immunofluorescence indicates 5-HT2CR protein expression. The NSC-eGFP AAV (green) did not alter 5-HT2CR protein expression (red) in infected neurons (yellow arrows) relative to non-AAV infected neurons (white arrows). The 5-HT2CR shRNA-eGFP AAV (green) induced a knockdown of 5-HT2CR protein (red) in infected neurons (yellow arrows) relative to non-infected neurons (white arrows). Ex vivo biochemical analyses indicate that 5-HT2CR knockdown rats display [B] lower mPFC 5-HT2CR protein levels (p<0.05 vs. NSC-eGFP AAV-VEH) and [C] higher mPFC 5-HT2AR protein levels (p<0.05 vs. NSC-eGFP AAV-VEH) relative to control rats. The insets are representative electrophoretic bands. Arbitrary units (A.U.) of densitometry are presented. [D] The 5-HT2CR knockdown rats expressed significantly higher premature responses vs. control rats on an IT5 maintenance session (open bars) and an ITI8 challenge session (hatched bars) (*p<0.05 vs. NSC-eGFP AAV). [E] There was no significant difference between 5-HT2CR knockdown and control rats for the number of reinforcers earned. [F] The 5-HT2CR knockdown rats expressed significantly lower percent omissions vs. control rats on an IT5 maintenance session (open bars) and an ITI8 challenge session (hatched bars) (*p<0.05 vs. NSC-eGFP AAV).
Ex vivo analyses indicated that the 5-HT2CR shRNA-eGFP AAV significantly attenuated 5-HT2CR protein expression (Fig. 4B, p<0.05) and augmented 5-HT2AR protein expression (Fig. 4C, p<0.05) relative to control rats. Rats with a knockdown of the 5-HT2CR in the mPFC expressed significantly higher premature responses on both ITI5 maintenance and ITI8 challenge sessions relative to control rats infused with the NSC-eGFP AAV (Fig. 4D, p<0.05). The accuracy in control vs. 5-HT2CR knockdown rats on the ITI5 maintenance (96.3% ± 0.7 vs. 97.5% ± 0.2) and ITI8 challenge sessions (97.6% ± 0.8 vs. 98.3% ± 0.7) did not differ nor did the latency to start the ITI5 (1.3 ± 0.3 sec vs. 1.1 ± 0.3 sec) or ITI8 (0.7 ± 0.3 sec vs. 1.3 ± 0.5 sec) sessions. Control vs. 5-HT2CR knockdown rats also did not differ in the number of reinforcers earned (Fig. 4E, n.s.). There was a significant decrease in percent omissions between control and knockdown rats on both the ITI5 and ITI8 challenge sessions (Fig. 4F, p<0.05). These data indicate a role of the mPFC 5-HT2AR:5-HT2CR balance to selectively govern premature and motivational responding, key facets underlying motor impulsivity in the 1-CSRT task.6
Systemic administration of a selective 5-HT2AR antagonist attenuates motor impulsivity in the 1- or 5-CSRT tasks.11–13,15,24,48 HI rats demonstrated higher levels of mPFC 5-HT2AR protein levels (replicated here; Fig. 2A) and higher pharmacological sensitivity to the selective 5-HT2AR antagonist M100907.9 Thus, we tested the hypothesis that the engineered imbalance in 5-HT2AR:5-HT2CR (Figs. 4B and 4C) would associate with a leftward shift in the potency of M100907 to suppress motor impulsivity. The dose range of M100907 (0.001–0.1 mg/kg) and time of injection (30 min prior) were chosen based upon the protocols employed to demonstrate that high impulsive outbred Sprague-Dawley rats were more sensitive to the behavioral effects of M100907.9 Vehicle-treated 5-HT2CR shRNA-eGFP AAV rats demonstrated elevated premature responses relative to vehicle-treated NSC-eGFP AAV rats (Fig. 5A; p<0.05), as demonstrated previously.6 M100907 dose-dependently decreased premature responses; there was no main effect of pretreatment (NSC-eGFP AAV, 5-HT2CR shRNA-eGFP AAV) on premature responses (F1,68=0.05, n.s.), but a main effect of treatment (M100907 doses) (F3,68=13.82, p<0.001) and a pretreatment X treatment interaction (F3,68=3.07, p<0.05) were observed. In 5-HT2CR knockdown rats, a main effect of M100907 treatment was observed (F3,37=15.56, p<0.001); planned comparisons showed that both the 0.01 and 0.1 mg/kg doses of M100907 decreased premature responses vs. vehicle in knockdown rats. In control rats, a trend towards a main effect of M100907 treatment was observed (F3,30=2.78, p=0.06). Hence, the decrement in 5-HT2CR following infusion of the 5-HT2CR-shRNA-AAV into the mPFC enhances 5-HT2AR control over motor impulsivity.
Figure 5. Knockdown of mPFC 5-HT2CR recapitulates high motor impulsivity and enhances 5-HT2AR regulation of motor impulsivity.
Following stable viral vector expression and 1-CSRT task training, the effects of M100907 (0.001, 0.01, and 0.1 mg/kg) were evaluated under ITI5 conditions. [A] Baseline levels of premature responses in 5-HT2CR knockdown rats administered vehicle (VEH; ▲, dashed line) were significantly higher than the vehicle baseline in control rats (○, dotted line; *p<0.05 vs. NSC-eGFP AAV-VEH). In the 5-HT2CR knockdown rats, M100907 significantly suppressed premature responses at 0.01 and 0.1 mg/kg (#p<0.05 vs. 5-HT2CR shRNA-eGFP AAV-VEH), below the vehicle baseline of control rats. [B] Baseline levels of reinforcers earned in 5-HT2CR knockdown rats administered vehicle (VEH; ▲, dashed line) did not differ from the vehicle baseline in control rats (○,dotted line). M100907 significantly increased the number of reinforcers earned at 0.01 and 0.1 mg/kg (#p<0.05 vs. 5-HT2CR shRNA-eGFP AAV-VEH), above the vehicle baseline of control rats. [C] Baseline levels of omissions in 5-HT2CR knockdown rats administered vehicle (VEH; ▲, dashed line) did not differ from the vehicle baseline in control rats (○, dotted line). M100907 did not alter the number of percent omissions in 5-HT2CR knockdown or control rats.
The number of reinforcers earned increased significantly in 5-HT2CR knockdown, but not control, rats administered M100907 (Fig. 5B), an effect similarly noted in HI rats.9 No main effect of pretreatment (F1,68=0.05, n.s.), a main effect of treatment (F3,68=9.07, n.s.), but no pretreatment X treatment interaction (F3,68=2.08, n.s.) on reinforcers earned was observed. In 5-HT2CR knockdown rats, a main effect of M100907 treatment on reinforcers earned was observed (F3,30=6.56, n.s.); 0.01 and 0.1 mg/kg of M100907 significantly increased reinforcers earned in knockdown rats. In control rats, a main effect of M100907 treatment on reinforcers earned was not observed (F3,30=0.42, n.s.). No main effect of pretreatment (F1,68=0.64, n.s.), no main effect of treatment (F3,68=0.61, n.s.), and no pretreatment X treatment interaction (F3,68=1.20, n.s.) on percent omissions were observed (Fig. 5C). These finding suggests that M100907 may selectively enhance cognition under conditions in which the impulse control system is taxed.9,49,50
Components of motor impulsivity as a cognitive construct include, but are not limited to, inhibitory control, failure to consider the consequences of behavior, a sense of urgency prior to or during task performance, and the attraction to approach and attain rewards (incentive motivation; ‘wanting’).1,51,52 At both molecular and systems levels, the brain utilizes 5-HT within cellular cascades and webs comprised of transcription factors, growth factors and other neurotransmitters, as a mediator of integral physiological and psychological functions, including the processing of incentive-motivational stimuli and impulse control.51,53–56 Acute tryptophan depletion in humans, which temporarily lowers brain 5-HT levels, elevates motor impulsivity57 and disrupts motivated actions selectively in individuals with high inherent impulsivity.58 Depletion of 5-HT in the dorsal raphe nucleus causes a robust increase in impulsive action in rodents,59,60 while elevated 5-HT release in the mPFC positively correlates with motor impulsivity.61,62 The conflicting outcomes from global 5-HT manipulations highlight not only the multidimensionality of impulsivity, but also that the ultimate impact of 5-HT is governed by its actions at 5-HT receptor proteins, including the 5-HT2R family.
The engagement of 5-HT2AR and 5-HT2CR mechanisms in the control of motor impulsivity (for review)31 is further supported by the results of the present study. We discovered that high impulsive rats (both inherent and engineered) displayed enhanced prepotent responding and motivationally-driven behavior (as evidenced by greater premature responses and fewer omissions made, respectively). Further, motor impulsivity (premature responses) and motivation to respond (omissions) associated with the ratio of 5-HT2AR to 5-HT2CR protein expression in the mPFC. The mechanisms that generate a specific receptor protein profile that associates with an expressed behavioral phenotype are currently unknown. Given that the expression of 5-HT receptors in human PFC appears to remain balanced across development into adulthood63 and that early life events are known to trigger plasticity of 5-HT systems,64–66 it is possible that differential developmental experience67 could have durable ramifications for 5-HT receptor control of the mPFC microcircuitry. This hypothesis remains to be tested, however, a functional 5-HT2AR:5-HT2CR rheostat in mPFC may play a pivotal role in spontaneously-occurring individual differences in impulsivity.
Phenotypic variance in motor impulsivity has been linked to several candidate neurotransmitter mechanisms within limbic-corticostriatal circuitry.68 We propose that vulnerability to motor impulsivity associates with expression patterns of 5-HT2AR and 5-HT2CR (present study),6,7,9 while multiple studies implicate dopamine5,7,69, γ-aminobutyric acid (GABA)8,10 and glutamate system involvement.37,70–73 Our observation that knockdown of the mPFC 5-HT2CR does not fully recapitulate the motor impulsivity phenotype characterized in outbred rats suggests an interplay between serotonergic and other neurochemical circuits may be differentially recruited in the expression of distinct components of impulsive behavior. One candidate mechanism is the interaction between 5-HT and glutamate systems in the mPFC. Pretreatment with a glutamate mGlu2/3 receptor agonists has been shown to suppress motor impulsivity generated by excessive 5-HT2AR stimulation locally in the mPFC,37 presumably due to the actions of mGlu2/3 agonists to suppress 5-HT2AR-mediated glutamate release from neurons that terminate onto mPFC pyramidal cells.74 Thus, the differential recruitment of a “functional crosstalk” between the 5-HT2AR and 5-HT2CR (present results), the mGlu2/3 and 5-HT2AR,37 or other combinations of receptors may be critical drivers of the phenotypic profile of impulsivity.
A higher ratio of the 5-HT2AR to 5-HT2CR protein density in frontal cortex has been noted for the mouse, rat and human,75 and here we report that the ratio of 5-HT2AR to 5-HT2CR protein expression in the rat mPFC predicts the inherent level of motor impulsivity in individual rats. That the net consequence of 5-HT2AR and 5-HT2CR function in mPFC may be reciprocal or interactive is supported by the present finding that knockdown of 5-HT2CR in the mPFC increased motor impulsivity and resulted in a compensatory upregulation of 5-HT2AR protein expression and a leftward shift in the potency of M100907 to suppress impulsive behavior. These findings are consistent with a study which suggested that the constitutive knockout of the 5-HT2AR upregulated 5-HT2CR control over the excitability of mPFC pyramidal neurons.39 Further, signal transduction through the 5-HT2AR and 5-HT2CR locally controls the intrinsic microcircuitry of the PFC through postsynaptic modulation of synaptic input to pyramidal neurons and subpopulations of interneurons (for review)76 and potentially via regulation of neurotransmitter release (e.g., acetylcholine, dopamine) by presynaptic 5-HT2AR and/or 5-HT2CR heteroreceptors (for review).77
Our finding that these two GPCRs reside in the same protein complex and that this complex is enriched in the mPFC of low impulsive rats suggests that the behavioral output of the mPFC is dependent in part upon a 5-HT2AR:5-HT2CR protein:protein interaction. Our unpublished data corroborates a recent publication36 which demonstrated that the majority of GABA neurons in the prelimbic mPFC expressed 5-HT2CR immunoreactivity and most co-expressed 5-HT2AR immunoreactivity; a small population of cells with a pyramidal profile also expressed both receptors. The colocalization of the receptors in the same neuron suggests that the 5-HT2AR:5-HT2CR protein complex may occur at the single cell level. Serotonin actions at the 5-HT2AR are excitatory in both interneurons and pyramidal neurons of the neocortex (for review),76 with these actions noted to be activity-dependent78 and projection-specific.79 There is much less known, and some debate, concerning the modulatory actions of 5-HT at the 5-HT2CR in cortical neurons.80 Activation of 5-HT2CR depolarized pyramidal neurons in piriform cortex81 however, the observed 5-HT-induced depolarization of mPFC pyramidal neurons is not blocked by the selective 5-HT2CR antagonist SB242084.39,82 The complexity of the mPFC functional microcircuitry modulated by 5-HT via the 5-HT2R may be founded in the different subpopulations of interneurons which express the receptors (e.g., parvalbumin, calbindin, calretinin).46,83 Further, the impact of a 5-HT2AR:5-HT2CR protein complex on neuronal firing is not known and it is wholly possible that the biochemical, signaling, and pharmacological properties of the protein complex diverge from that of the single receptor, although this is a controversial and active research topic.84–87 Thus, while future research is required to tease apart the relative role of these two GPCRs within the same neurons versus interacting neurons in the mPFC, altered firing of GABA neurons which colocalize the 5-HT2AR and 5-HT2CR may dysregulate pyramidal outflow to drive motor impulsivity. The fact that systemic administration of 5-HT2AR and the 5-HT2CR ligands oppositionally control motor impulsivity9,11–20,22–26 (and other behaviors)88 adds further impetus to disentangling the mechanisms through which these receptors control neuronal output to mediate behaviors that contribute to chronic health disorders (e.g., drug addiction, attention deficit disorder, autism, and obesity/binge eating disorder).
METHODS
General Methods
Animals
Male, outbred Sprague–Dawley rats (n=120); Harlan, Houston, TX) weighing 250–275g upon arrival were housed two/cage under a 12-h light–dark cycle with controlled temperature (21–23°C) and humidity (40–50%). Animals were acclimated for seven days to the colony room prior to the start of handling and experimental procedures. During the 1-CSRT task acquisition and maintenance, rats were food restricted to 90% free-feeding weight; water was available ad libitum except during daily operant sessions. Rats were weighed daily to ensure that their body weights were maintained at 90% of free-feeding levels. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (2011) and with the University of Texas Medical Branch Institutional Animal Care and Use Committee approval.
Drugs
M100907 [R-(1)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol] was synthesized by Kenner Rice (National Institute on Drug Abuse, Bethesda, MD) and dissolved in 1% Tween 80 in 0.9% NaCl.
1-Choice Serial Reaction Time (1-CSRT) task
Procedures occurred in standard five-hole nose-poke operant chambers equipped with a houselight, food tray, and an external pellet dispenser capable of delivering 45 mg pellets (Bio-Serv, Frenchtown, NJ) housed within ventilated and sound-attenuated chambers (MedAssociates, St Albans, VT). The 1-CSRT task methodology has been described in detail previously.6,9,13,15,17 Briefly, rats were habituated to the test chamber; a nose-poke into the singly-illuminated center hole resulted in the delivery of one food pellet into the magazine on the opposite wall of the chamber and simultaneous illumination of the magazine light. During this stage, all responses made in the correctly lit (center) hole resulted in the illumination of the magazine light and presentation of a single food pellet. The training stages thereafter were each comprised of daily sessions of 100 trials to be completed in a maximum of 30 min; each training stage (9 total) involved incrementally lowering the stimulus duration with a 5-sec limited hold and an intertrial interval (ITI) of 5 sec. A maximum of 100 correct responses in a session resulted in a maximum of 100 reinforcers earned; incorrect, premature responses or omissions resulted in a 5-s time-out period and a reduction in reinforcers obtained. Advancement to the next training stage required rats to meet acquisition criteria: ≥50 correct responses, >80% accuracy [correct responses/(correct + incorrect) × 100] and <20% omissions (omitted responses/trials completed × 100).13 Premature responses were employed as the primary indication of motor impulsivity. The number of reinforcers earned provides a measure of task competency and a secondary assessment of motor impulsivity, while percent accuracy was a general indication of attentional capacity. Percent omissions indicated failures of detection of the visual stimuli in the center hole as well as motivation to perform the task.
Identification of motor impulsivity phenotype
After meeting stability criteria for the final training stage over three consecutive ITI5 sessions (with <20% variability, approximately days 25–30), an ITI8 challenge session was conducted in which the ITI was 8-s for the session.6,9,61 High and low impulsive rats were defined as the upper and lower quartile of premature responses assessed on the ITI8 challenge, respectively.
Research Design
Inherent motor impulsivity predicts expression patterns of 5-HT2AR to 5-HT2CR protein in mPFC in individual rats (see experimental timeline; Fig. 2
HI and LI rats were identified from three independent cohorts of outbred rats (Cohort 1: 5-HT2AR and 5-HT2CR expression and ratio; Cohorts 2 and 3: 5-HT2AR:5-HT2CR protein complex). Two to three days following behavioral testing, rats were anesthetized [chloral hydrate solution (400 mg/kg)], decapitated and brains were extracted. The mPFC (containing infralimbic, prelimbic and anterior cingulate cortex) was microdissected immediately over ice, flash frozen in liquid nitrogen and stored at −80°C for subsequent protein extraction.
A crude synaptosomal protein fraction enriched for pre- and postsynaptic proteins (i.e., presynaptic terminals, postsynaptic membranes, postsynaptic density, synaptic protein complexes) was prepared as described previously.40,83 Tissue from the mPFC was homogenized in 10 times w/v ice cold Krebs buffer (125 mM NaCl, 1.2 mM KCl, 1.2 mM MgSO4, 1.2 mM CaCl2, 22 mM Na2CO3, 1 mM NaH2PO4, 10 mM glucose) containing 0.32 M sucrose plus protease inhibitor cocktail and phosphatase inhibitor 2 and 3 cocktails (10 µL/mL; Sigma-Aldrich, St. Louis, MO). The homogenate was centrifuged at 1000 g for 10 min at 4°C to pellet the nuclear fraction. The supernatant was collected and centrifuged at 16,000 g for 20 min at 4°C to pellet the crude synaptosome. The pellet was re-suspended in Krebs buffer with 1% dodecyl maltoside.
Equal amounts of crude synaptosomal protein prepared from the mPFC6,9,40 were separated by SDS-PAGE and transferred to a PVDF membrane for blotting with 5-HT2AR antibody (AB16028, 1:1000; Abcam, Cambridge, MA), 5-HT2CR antibody (D12, 1:100: Santa Cruz Biotechnology, Dallas, TX) or pan-cadherin antibody (AB6528, 1:10000; Abcam). Membranes were incubated with mouse IgG IRDye 800 (1:10000) or rabbit IgG IRDye 680 (1:10000) for detection by Odyssey Imaging System (LI-COR, Lincoln, NE). The integrated intensity of each band was analyzed with the Odyssey Software and 5-HT2AR and 5-HT2CR immunoreactivity normalized to pan-cadherin immunoreactivity.
Co-immunoprecipitation methodology was employed to assess the 5-HT2CR protein complex with 5-HT2AR in the mPFC of HI and LI rats (n=4–5/phenotype). The 5-HT2CR antibody (D12, 45 µg, Santa Cruz) or 5-HT2AR antibody (AB16028, 10 µg, Abcam) was covalently crosslinked onto protein A/G resin as previously described with minor modifications.9,40 Synaptosomal protein was incubated with the antibody-crosslinked resin for 48 hrs at 4°C with constant shaking. The eluted protein was resuspended in resuspension buffer containing 1% SDS and 0.5% NP40 and subjected to the Wes™ automated western blotting system (ProteinSimple, San Jose, CA www.proteinsimple.com/), which utilizes capillary electrophoresis-based immunodetection for higher resolution, sensitivity, and reproducibility (even at low sample concentrations) relative to traditional immunoblotting techniques.9,89 Wes™ reagents (biotinylated molecular weight marker, streptavidin-HRP fluorescent standards, luminol-S, hydrogen peroxide, sample buffer, DTT, stacking matrix, separation matrix, running buffer, wash buffer, matrix removal buffer, secondary antibodies, antibody diluent, and capillaries) were obtained from the manufacturer (ProteinSimple) and used according to the manufacturer’s recommendations. The 5-HT2AR antibody (AB16028, 1:250) or 5-HT2CR antibody (D12, 1:50; Santa Cruz) was diluted with ProteinSimple antibody diluent.
Equal amounts of protein (1 µg) were combined with 0.1X sample buffer and 5X master mix (200 mM DTT, 5X sample buffer, 5X fluorescent standards), gently mixed, and then denatured at 95°C for 5 min. The denatured samples, biotinylated ladder, antibody diluent, primary antibodies, HRP-conjugated secondary antibodies, chemiluminescent substrate, and wash buffer were dispensed to designated wells in a pre-filled microplate (ProteinSimple). Separation electrophoresis (375 V, 31 min, 25°C) and immunodetection in the capillaries were fully automated using the following settings: separation matrix load for 200-s, stacking matrix load for 14-s, sample load for 7-s, antibody diluent for 30 min, primary antibody incubation for 60 min, secondary antibody incubation for 30 min, and chemiluminescent signal exposure for 30-s, 120-s, 240-s, and 480-s. Data analyses were performed using the Compass Software (ProteinSimple).
Genetic loss of mPFC 5-HT2CR confers high motor impulsivity and an imbalance in the 5-HT2R system (see experimental timeline, Fig. 4)
A 24-nucleotide sequence within the coding region of the Htr2c was identified employing methods we have previously reported.90 Two sets of oligonucleotides (Integrated DNA Technology, Coralville, IA) for cloning were synthesized [Htr2c shRNA (top, 5’-TTGAATCCAGACGGGGCACAAATATCCTTCCTGTCAGATATTTGTGCCCCGTCTGGATTATTTTT-3’; bottom, 5’-CTAGAAAAATAATCCAGACGGGGCACAAATATCTGACAGGAAGGATATT TGTGCCCCGTCTGGATTC-3’); Non-silencing control (NSC) shRNA (top, 5’-TTTGTGGAGCCGAGTTTCTAAATTCCGCTTCCTGTCACGGAATTTAGAAACCCGGCTCCAATTTTT-3’; bottom, 5’- CTAGAAAAATTGGAGCCGGGTTTCTAAATTCCGTGACA GGAAGCGGAATTTAGAAACTCGGCTCCAC3’)]. Oligonucleotides were designed with Sap1 and Xbal overhangs to allow ligation downstream of the mU6pro region of a modified pAAV-MCS vector, pAAV-shRNA, which was designed to coexpress hairpin RNAs, under the control of a mU6pro and an SV40 polyadenylation site, as well as eGFP controlled by an independent CMV promoter and hGH polyadenylation sequence. Adeno-associated viral (AAV) serotype type 2 vectors were packaged using a helper-free packaging system (Life Technologies) and purified viral stocks were assayed in camptothecin-treated HT1080 cells to confirm titers of 1–2 × 1011 transducing units/ml.
Rats (n=24) were anesthetized (i.m.) with a cocktail containing xylazine (8.6 mg/kg), acepromazine (1.5 mg/kg), and ketamine (43 mg/kg) in bacteriostatic saline and placed in a stereotaxic apparatus with the upper incisor bar at −3.8 mm below the interaural line. Two microsyringes (28 gauge, Hamilton Company, Reno, NV) were lowered bilaterally at 15° from the midsaggital plane relative to bregma91 to target the mPFC encompassing the ventral prelimbic and dorsal infralimbic subnuclei;6 the coordinates were anteroposterior +3mm, mediolateral +1.8mm, and dorsoventral −5.1mm from the skull. The NSC shRNA-eGFP AAV (“control”; 1.5 µl) or 5-HT2CR shRNA-eGFP (“5-HT2CR knockdown”; 1.5 µl) AAV vectors were infused bilaterally at 0.1 µl/min over 15 min. Rats were allowed three weeks to recover and allow for stable transgene expression prior to behavioral assessment. AAV infection has been well-characterized with stabilization of gene expression in rodent brain at three weeks and with stability for at least 12–18 months post-infection.92, 93
Following intra-mPFC transgene delivery and stable viral vector expression,6 control (n=12) and 5-HT2CR knockdown (n=12) rats were trained to criteria on the 1-CSRT task. Pharmacological test sessions commenced after animals met the stable training criteria >80% accuracy and <20% omissions for five consecutive training sessions on the final training stage with less than 15% variability across sessions;9,15,17 vehicle (1% Tween80; i.p.) or M100907 (0.001, 0.01, 0.1 mg/kg; i.p.) was injected 30 min prior to commencement of 1-CSRT task sessions under ITI5 conditions. Each rat received all doses of M100907 in a balanced, pseudo-randomized order. Rats underwent five daily 1-CSRT task sessions per week; rats were treated with vehicle the day before drug treatments and received only one drug treatment per week.
At the termination of the pharmacological assessments (~1 week), rats were anesthetized (chloral hydrate; 400 mg/kg, i.p.), decapitated and tissue extracted for visualization and 5-HT2R capillary electrophoretic immunoblot analyses.6 A 1-mm coronal section containing the mPFC was placed on a cold glass slide and rapid visualization of eGFP ex vivo was accomplished with a DFP-1 Dual Fluorescent Protein Flashlight by the investigator wearing a pair of VG2 barrier filter glasses (Nightsea, Bedford, MA, USA).6,94 Photomicrographs of coronal sections were taken with a DSLR camera equipped with a macro lens and yellow filter.6,94 Fluorescent and non-fluorescent regions from the mPFC were microdissected and assayed for immunoblotting to assess 5-HT2CR knockdown and 5-HT2AR protein levels ex vivo.6,94 The S2 protein fraction (i.e., soluble protein; see above) was modified by the addition of 0.5% NP40. The 5-HT2AR and 5-HT2CR protein levels were assessed using the Wes™ automated western blotting system (ProteinSimple) as described above. Data analyses were performed using the Compass Software (ProteinSimple).
A subset of rats (n=6) was anesthetized (sodium pentobarbital; 100 mg/kg, i.p.) and perfused transcardially with 3% paraformaldehyde for immunohistochemical analyses.6,95 Brains were removed, post-fixed (2 hrs) and cryoprotected in 30% sucrose solution. Free-floating coronal sections at the level of the mPFC (30 µm) were incubated in 0.5% sodium borohydride to reduce autofluorescence. Sections were blocked (1.5% normal goat serum in 0.4% triton-PBS) prior to incubation with 5-HT2CR antibody (D12; 1:100; Santa Cruz; 2 hrs 25°C, 18 hr 4°C) followed by AlexaFluor 555 to mouse IgG (A21424, 1:2000; Life Technologies; 1 hr 25°C). Slides were coverslipped with Vectashield fluorescent mounting medium with DAPI (Vector Laboratories, Burlingame, CA).
Statistical Analyses
Student’s t-test was employed to analyze outcome measures of 1-CSRT task performance between cohorts, phenotypes or pretreatment groups. The 5-HT2AR and 5-HT2CR protein expression data and the ratio were assessed by Student’s t-test and Pearson’s correlation. The effects of M100907 on 1-CSRT task performance in control and knockdown rats were analyzed by two-way repeated-measures ANOVA for the factors of pretreatment (control or knockdown) and treatment (vehicle or M100907); the effects of treatment were assessed by one-way repeated-measures ANOVA followed by Dunnett’s procedure (for comparisons of treatment means vs. vehicle). The experimenter was blinded to the group allocation (e.g., HI vs. LI; control vs. knockdown) throughout the duration of the study. Analyses were performed in SAS (version 9.4; Cary, NC) with an experiment-wise error rate of α=0.05.
Acknowledgments
This work was supported by NIDA grants K99 DA033374 (N.C.A.), F30 DA034488 (L.H.L.F.), F31 DA035620 (S.E.S-J.), T32 DA07287 (L.H.L.F. and S.E.S-J.), P20 DA024157 (K.A.C.), P50 DA033935 (K.A.C.), K05 DA020087 (K.A.C.), and the Center for Addiction Research at the University of Texas Medical Branch. The research was also supported by the NIH Intramural Research Programs in the Drug Design and Synthesis Section of the Chemical Biology Research Branch within the National Institutes of Health Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA) (K.C.R.). We would like to thank Mr. Siddharth Iyengar and Ms. Justine Saavedra for technical assistance with ex vivo biochemical analyses.
Abbreviations
- 1-CSRT
one-choice serial reaction time task
- 5-HT
serotonin (5-hydroxytryptamine)
- 5-HTXR
5-HTX receptor
- GPCR
G-protein coupled receptor
- AAV
adenoassociated virus
Footnotes
Author Contributions
N.C.A. and K.A.C. conceptualized the experiments, designed and directed the behavioral, biochemical and pharmacological research, interpreted the results, and wrote the manuscript.
S.J.S. carried out behavioral evaluations.
R. M. S. and R. J. D. created the 5-HT2CR shRNA AAV.
L.H.L.F., S.E.S-J. and F.G.M. were involved in the conception and design of the experiments and edited the manuscript.
K.C.R. synthesized M100907 which was provided under a Material Transfer Agreement.
Conflict of Interest
Dr. Cunningham is a consultant for Arena Pharmaceuticals. Dr. Moeller is a consultant for Boehringer-Ingelheim. All other authors declare no conflicts of interest.
REFERENCES
- 1.Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am.J.Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Weafer J, Baggott MJ, de Wit H. Test-retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Exp Clin Psychopharmacol. 2013;21:475–481. doi: 10.1037/a0033659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW. Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology. 2012;37:2057–2066. doi: 10.1038/npp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, O'Neil RT, Fink LH, Li D, Green TA, Moeller FG, Cunningham KA. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology. 2014;39:370–382. doi: 10.1038/npp.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besson M, Pelloux Y, Dilleen R, Theobald DE, Lyon A, Belin-Rauscent A, Robbins TW, Dalley JW, Everitt BJ, Belin D. Cocaine modulation of frontostriatal expression of Zif268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology. 2013;38:1963–1973. doi: 10.1038/npp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprioli D, Sawiak SJ, Merlo E, Theobald DE, Spoelder M, Jupp B, Voon V, Carpenter TA, Everitt BJ, Robbins TW, Dalley JW. Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol Psychiatry. 2014;75:115–123. doi: 10.1016/j.biopsych.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink LHLANC, Fox RG, Rice KC, Moeller FG, Cunningham KA. Individual differences in impulsive action reflect variation in the cortical serotonin 5-HT2A receptor system. Neuropsychopharmacol. 2015 doi: 10.1038/npp.2015.46. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl) 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- 12.Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 13.Anastasio NC, Stoffel EC, Fox RG, Bubar MJ, Rice KC, Moeller FG, Cunningham KA. Serotonin (5-hydroxytryptamine) 5-HT2A receptor: Association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav.Pharmacol. 2011;22:248–261. doi: 10.1097/FBP.0b013e328345f90d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology. 2011;61:468–477. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, Moeller FG. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chemical Neuroscience. 2013;4:110–121. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarra R, Comery TA, Graf R, Rosenzweig-Lipson S, Day M. The 5-HT(2C) receptor agonist WAY-163909 decreases impulsivity in the 5-choice serial reaction time test. Behav.Brain Res. 2008;188:412–415. doi: 10.1016/j.bbr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Anastasio NC, Gilbertson SR, Bubar MJ, Agarkov A, Stutz SJ, Jeng YJ, Bremer NM, Smith TD, Fox RG, Swinford SE, Seitz PK, Charendoff MN, Craft JW, Laezza F, Watson CS, Briggs JM, Cunningham KA. Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior. Journal of Neuroscience. 2013;33:1615–1630. doi: 10.1523/JNEUROSCI.2656-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evenden JL. The pharmacology of impulsive behaviour in rats VII: the effects of serotonergic agonists and antagonists on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology (Berl) 1999;146:422–431. doi: 10.1007/pl00005487. [DOI] [PubMed] [Google Scholar]
- 19.Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- 20.Blokland A, Sik A, Lieben C. Evaluation of DOI, 8-OH-DPAT, eticlopride and amphetamine on impulsive responding in a reaction time task in rats. Behav.Pharmacol. 2005;16:93–100. doi: 10.1097/00008877-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Carli M, Samanin R. The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats' accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology (Berl) 2000;149:259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- 22.Evenden JL. The pharmacology of impulsive behaviour in rats IV: the effects of selective serotonergic agents on a paced fixed consecutive number schedule. Psychopharmacology (Berl) 1998;140:319–330. doi: 10.1007/s002130050773. [DOI] [PubMed] [Google Scholar]
- 23.Hadamitzky M, Koch M. Effects of acute intra-cerebral administration of the 5-HT(2A/C) receptor ligands DOI and ketanserin on impulse control in rats. Behav.Brain Res. 2009;204:88–92. doi: 10.1016/j.bbr.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Koskinen T, Ruotsalainen S, Sirvio J. The 5-HT(2) receptor activation enhances impulsive responding without increasing motor activity in rats. Pharmacol Biochem.Behav. 2000;66:729–738. doi: 10.1016/s0091-3057(00)00241-0. [DOI] [PubMed] [Google Scholar]
- 25.Koskinen T, Ruotsalainen S, Puumala T, Lappalainen R, Koivisto E, Mannisto PT, Sirvio J. Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology. 2000;39:471–481. doi: 10.1016/s0028-3908(99)00159-8. [DOI] [PubMed] [Google Scholar]
- 26.Koskinen T, Haapalinna A, Sirvio J. Alpha-adrenoceptor-mediated modulation of 5-HT2 receptor agonist induced impulsive responding in a 5-choice serial reaction time task. Pharmacol Toxicol. 2003;92:214–225. doi: 10.1034/j.1600-0773.2003.920504.x. [DOI] [PubMed] [Google Scholar]
- 27.Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav.Brain Res. 2011;222:183–192. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav.Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76(Pt B):460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb.Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- 33.Burnet PWJ, Eastwood SL, Lacey K, Harrison PJ. The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Res. 1995;676:157–168. doi: 10.1016/0006-8993(95)00104-x. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Regional distribution and cellular localization of 5-HT2C receptor mRNA in monkey brain: comparison with [3H]mesulergine binding sites and choline acetyltransferase mRNA. Synapse. 2001;42:12–26. doi: 10.1002/syn.1095. [DOI] [PubMed] [Google Scholar]
- 35.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Mol.Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 36.Nocjar C, Alex KD, Sonneborn A, Abbas AI, Roth BL, Pehek EA. Serotonin-2C and −2a receptor co-expression on cells in the rat medial prefrontal cortex. Neuroscience. 2015;297:22–37. doi: 10.1016/j.neuroscience.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wischhof L, Hollensteiner KJ, Koch M. Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav.Pharmacol. 2011;22:805–813. doi: 10.1097/FBP.0b013e32834d6279. [DOI] [PubMed] [Google Scholar]
- 38.Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- 39.Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc.Natl.Acad.Sci.U.S.A. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, Watson CS, Cunningham KA. Serotonin 5-HT2C receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J.Neurochem. 2010;113:1504–1515. doi: 10.1111/j.1471-4159.2010.06694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becamel C, Gavarini S, Chanrion B, Alonso G, Galeotti N, Dumuis A, Bockaert J, Marin P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol.Chem. 2004;279:20257–20266. doi: 10.1074/jbc.M312106200. [DOI] [PubMed] [Google Scholar]
- 42.Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- 43.Gylys KH, Fein JA, Cole GM. Quantitative characterization of crude synaptosomal fraction (P-2) components by flow cytometry. J Neurosci Res. 2000;61:186–192. doi: 10.1002/1097-4547(20000715)61:2<186::AID-JNR9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch.Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- 45.Mengod G, Vilaro MT, Raurich A, Lopez-Gimenez JF, Cortes R, Palacios JM. 5-HT receptors in mammalian brain: receptor autoradiography and in situ hybridization studies of new ligands and newly identified receptors. Histochem.J. 1996;28:747–758. doi: 10.1007/BF02272148. [DOI] [PubMed] [Google Scholar]
- 46.de Almeida J, Mengod G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT(2A) receptors in human and monkey prefrontal cortex. J.Neurochem. 2007;103:475–486. doi: 10.1111/j.1471-4159.2007.04768.x. [DOI] [PubMed] [Google Scholar]
- 47.Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER, Robbins TW. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- 49.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 50.Robbins TW, McAlonan G, Muir JL, Everitt BJ. Cognitive enhancers in theory and practice: studies of the cholinergic hypothesis of cognitive deficits in Alzheimer's disease. Behav Brain Res. 1997;83:15–23. doi: 10.1016/s0166-4328(97)86040-8. [DOI] [PubMed] [Google Scholar]
- 51.Evenden JL. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- 52.Frijda NH. Impulsive action and motivation. Biol.Psychol. 2010;84:570–579. doi: 10.1016/j.biopsycho.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Soubrié P. Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral and Brain Sciences. 1986:319–364. [Google Scholar]
- 54.Van der Kooy D, Fibiger HC, Phillips AG. An analysis of dorsal and median raphe self-stimulation: effects of parachlorophenylalanine. Pharmacol.Biochem.Behav. 1978;8:441–445. doi: 10.1016/0091-3057(78)90083-7. [DOI] [PubMed] [Google Scholar]
- 55.Simon H, Le MM, Cardo B. Intracranial self-stimulation from the dorsal raphe nucleus of the rat: effects of the injection of para-chlorophenylalanine and of alpha-methylparatyrosine. Behav.Biol. 1976;16:353–364. doi: 10.1016/s0091-6773(76)91486-3. [DOI] [PubMed] [Google Scholar]
- 56.Miliaressis E. Serotonergic basis of reward in median raphe of the rat. Pharmacol.Biochem.Behav. 1977;7:177–180. doi: 10.1016/0091-3057(77)90204-0. [DOI] [PubMed] [Google Scholar]
- 57.Worbe Y, Savulich G, Voon V, Fernandez-Egea E, Robbins TW. Serotonin depletion induces 'waiting impulsivity' on the human four-choice serial reaction time task: cross-species translational significance. Neuropsychopharmacology. 2014;39:1519–1526. doi: 10.1038/npp.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark L, Roiser J, Cools R, Rubinsztein D, Sahakian B, Robbins T. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology. 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- 59.Harrison AA, Everitt BJ, Robbins TW. Doubly dissociable effects of median- and dorsal-raphe lesions on the performance of the five-choice serial reaction time test of attention in rats. Behav.Brain Res. 1997;89:135–149. doi: 10.1016/s0166-4328(97)00053-3. [DOI] [PubMed] [Google Scholar]
- 60.Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- 61.Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 62.Puumala T, Sirviö J. Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience. 1998;83:489–499. doi: 10.1016/s0306-4522(97)00392-8. [DOI] [PubMed] [Google Scholar]
- 63.Lambe EK, Fillman SG, Webster MJ, Shannon WC. Serotonin receptor expression in human prefrontal cortex: balancing excitation and inhibition across postnatal development. PLoS.One. 2011;6:e22799. doi: 10.1371/journal.pone.0022799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garoflos E, Stamatakis A, Mantelas A, Philippidis H, Stylianopoulou F. Cellular mechanisms underlying an effect of "early handling" on pCREB and BDNF in the neonatal rat hippocampus. Brain Research. 2005;1052:187–195. doi: 10.1016/j.brainres.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell JB, Iny LJ, Meaney MJ. The role of serotonin in the development and environmental regulation of type II corticosteroid receptor binding in rat hippocampus. Dev.Brain Res. 1990;55:231–235. doi: 10.1016/0165-3806(90)90204-c. [DOI] [PubMed] [Google Scholar]
- 66.Smythe JW, Rowe WB, Meaney MJ. Neonatal handling alters serotonin (5-HT) turnover and 5-HT2 receptor binding in selected brain regions: Relationship to the handling effect on glucocorticoid receptor expression. Dev.Brain Res. 1994;80:183–189. doi: 10.1016/0165-3806(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 67.Benekareddy M, Goodfellow NM, Lambe EK, Vaidya VA. Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J.Neurosci. 2010;30:12138–12150. doi: 10.1523/JNEUROSCI.3245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jupp B, Caprioli D, Dalley JW. Highly impulsive rats: modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction. Disease models & mechanisms. 2013;6:302–311. doi: 10.1242/dmm.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur J Neurosci. 2013;37:1779–1788. doi: 10.1111/ejn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wischhof L, Koch M. Pre-treatment with the mGlu2/3 receptor agonist LY379268 attenuates DOI-induced impulsive responding and regional c-Fos protein expression. Psychopharmacology (Berl) 2012;219:387–400. doi: 10.1007/s00213-011-2441-y. [DOI] [PubMed] [Google Scholar]
- 71.Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and 'impulsive-type' behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- 72.Carli M, Invernizzi RW. Serotoninergic and dopaminergic modulation of cortico-striatal circuit in executive and attention deficits induced by NMDA receptor hypofunction in the 5-choice serial reaction time task. Front Neural Circuits. 2014;8:58. doi: 10.3389/fncir.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behav Brain Res. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 74.Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- 75.Dougherty JP, Aloyo VJ. Pharmacological and behavioral characterization of the 5-HT2A receptor in C57BL/6N mice. Psychopharmacology (Berl) 2011;215:581–593. doi: 10.1007/s00213-011-2207-6. [DOI] [PubMed] [Google Scholar]
- 76.Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 2011;44:449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol.Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- 78.Stephens EK, Avesar D, Gulledge AT. Activity-dependent serotonergic excitation of callosal projection neurons in the mouse prefrontal cortex. Front Neural Circuits. 2014;8:97. doi: 10.3389/fncir.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avesar D, Gulledge AT. Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits. 2012;6:12. doi: 10.3389/fncir.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Frontiers in integrative Neuroscience. 2013;7:25. doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheldon PW, Aghajanian GK. Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: Evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse. 1991;9:208–218. doi: 10.1002/syn.890090307. [DOI] [PubMed] [Google Scholar]
- 82.Beique JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: Implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1667–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasbi A, O'Dowd BF, George SR. Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol.Brain. 2011;4:26. doi: 10.1186/1756-6606-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Meszaros J, Urizar E, Sibley DR, Kellendonk C, Sonntag KC, Graham DL, Colbran RJ, Stanwood GD, Javitch JA. Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry. 2015 doi: 10.1038/mp.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujita W, Gomes I, Devi LA. Revolution in GPCR signalling: opioid receptor heteromers as novel therapeutic targets: IUPHAR review 10. Br J Pharmacol. 2014;171:4155–4176. doi: 10.1111/bph.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog.Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- 89.Liu S-B, Sardi S, Sonom B, Zocco D, McSweeney R, Fraser AD, Halleck AE, Li H, Smeljkal GB, Munevar S, Jin JG, Kawai T, Ghiran I, McGrath JP, Whitman M, Ng S-W, Kuo WP. The application of a novel nanovolume capillary electrophoresis-based protein analysis system in personalized & translational medicine research. J Bioanal Biomed S3. 2013 [Google Scholar]
- 90.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat. Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- 91.Paxinos W, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. San Diego CA: Academic Press; 1998. [Google Scholar]
- 92.Daly TM. Overview of adeno-associated viral vectors. Methods Mol.Biol. 2004;246:157–165. doi: 10.1385/1-59259-650-9:157. [DOI] [PubMed] [Google Scholar]
- 93.Leff SE, Spratt SK, Snyder RO, Mandel RJ. Long-term restoration of striatal L-aromatic amino acid decarboxylase activity using recombinant adeno-associated viral vector gene transfer in a rodent model of Parkinson's disease. Neuroscience. 1999;92:185–196. doi: 10.1016/s0306-4522(98)00741-6. [DOI] [PubMed] [Google Scholar]
- 94.Li X, Wolf ME. Visualization of virus-infected brain regions using a GFP-illuminating flashlight enables accurate and rapid dissection for biochemical analysis. J.Neurosci.Methods. 2011 doi: 10.1016/j.jneumeth.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bubar MJ, Stutz SJ, Cunningham KA. 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS.One. 2011;6:e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]