Abstract
Increases in the prevalence of antibiotic-resistant strains of Staphylococcus aureus have elicited efforts to develop novel antimicrobials to treat these drug-resistant pathogens. One potential treatment repurposes the lytic enzymes produced by bacteriophages as antimicrobials. The phage Twort endolysin (PlyTW) harbors three domains, a cysteine, histidine-dependent amidohydrolases/peptidase domain (CHAP), an amidase-2 domain and a SH3b-5 cell wall binding domain (CBD). Our results indicate that the CHAP domain alone is necessary and sufficient for lysis of live S. aureus, while the amidase-2 domain is insufficient for cell lysis when provided alone. Loss of the CBD results in ∼10X reduction of enzymatic activity in both turbidity reduction and plate lysis assays compared to the full length protein. Deletion of the amidase-2 domain resulted in a protein (PlyTW Δ172-373) with lytic activity that exceeded the activity of the full length construct in both the turbidity reduction and plate lysis assays. Addition of Ca2+ enhanced the turbidity reduction activity of both the full length protein and truncation constructs harboring the CHAP domain. Chelation by addition of EDTA or the addition of zinc inhibited the activity of all PlyTW constructs.
Keywords: peptidoglycan hydrolase, bacteriophage endolysin, autolysin, Staphylococcus aureus
The staphylococcal phage Twort endolysin amidase2 domain shows virtually no exolytic activity and the endopeptidase domain activity is enhanced by the addition of the SH3b cell wall binding domain.
The staphylococcal phage Twort endolysin amidase2 domain shows virtually no exolytic activity and the endopeptidase domain activity is enhanced by the addition of the SH3b cell wall binding domain.
INTRODUCTION
There is a need for novel antimicrobials due to global increases in antibiotic-resistant pathogens. Drug-resistant Staphylococcus aureus impact the health of livestock (bovine, porcine, equine and poultry) (Price et al., 2012), companion animals (dogs, cats and horses) (Davis et al., 2014; Harrison et al., 2014) and humans (Klevens et al., 2007; WHO 2014). Strains resistant to existing alternatives (vancomycin) are becoming more prevalent [vancomycin-resistant S. aureus, 13 cases in the US since 2002 (Centers for Disease Control and Prevention2013)]. With overused antibiotics proving increasingly ineffective, novel antimicrobials are essential.
Bacteriophage endolysins are a source of antimicrobial enzymes for treating multidrug-resistant Gram-positive bacteria (Donovan et al., 2009). These peptidoglycan hydrolase (PGH) enzymes degrade the major structural component of the bacterial cell wall. The enzymes naturally lyse the host cell allowing nascent phage particles to escape (for review see Loessner 2005; Donovan 2007; Fischetti 2008, 2010; Nelson et al., 2012; Shen et al., 2012). Endolysins can harbor any of three unique activities: endopeptidase, amidase (Becker et al., 2009a; Navarre et al., 1999) or glycosidase activity (Pritchard et al., 2007) (for review see Loessner 2005). The near-species specificity of the Gram-positive peptidoglycan structure and the specificity of the endolysin domains distinguish these enzymes as potential narrow-spectrum antimicrobials.
The S. aureus bacteriophage Twort endolysin (PlyTW) is a multidomain endolysin, 467 amino acids in length, containing a cysteine, histidine-dependent amidohydrolase/peptidase (CHAP endopeptidase) domain (12-141aa), an amidase-2 domain (179-318aa) and an SH3b-5 cell wall binding domain (CBD; 382–449 aa) (Finn et al., 2008). The PlyTW lytic activity and gene were first described in 1998 by Loessner et al. (1998). A truncated version of PlyTW harboring the CHAP domain and an incomplete amidase domain was shown to have an N-acetylmuramoyl-L-alanine amidase activity (Loessner et al., 1998). Truncations of PlyTW and the full length Twort phage holin protein, HolTW, were overexpressed in Escherichia coli, and shown to be sufficient to lyse S. aureus cells (Loessner et al., 1998), additionally Daniel et al. (2010) and Pastagia et al. (2011) have described fusions with the PlyTW N-terminal CHAP domain fused to the CBD of phiNM3 endolysin demonstrating its effectiveness as an antimicrobial. This study expands on earlier works by presenting a more complete deletion analysis of PlyTW to determine the contribution of each domain to the lytic activity individually and in combination with the other domains.
MATERIALS AND METHODS
Plasmids, constructs and strains
The Twort genomic DNA clone was kindly provided by Loessener et al. (1998) with the protein sequence available through Genbank (CAA69021.1). Truncations of PlyTW were produced using standard PCR cloning methods as described previously (Becker et al., 2009a). Sequences were PCR amplified using primers indicated in Table 1. Amplified fragments were subsequently purified, digested with XbaI and XhoI and subcloned into pET21a (EMD Biosciences, San Diego, CA), using standard techniques. Engineered restriction enzyme sites were introduced via primer design. All pET21a-derived constructs have an additional eight amino acids at the C-terminus composed of LE (XhoI restriction enzyme cloning site), and a 6xHis tag for nickel chromatography purification. Plasmids, PlyTW Δ146–373 and PlyTW Δ172–373, have an additional two amino acids (LD) introduced by the ligation of XhoI–SalI cut sites at the fusion junction. Construct PlyTW Δ199–325 was generated with an internal XhoI site, generating two additional amino acids (LE) at the fusion junction. All subcloning was performed in E. coli DH5α (Invitrogen, Carlsbad, CA) and construct accuracy verified by DNA sequence analysis. Constructs cloned into pET21a were expressed in E. coli BL21 (DE3) (EMD Biosciences, San Diego, CA) for protein purification.
Table 1.
Primers and plasmids.
| Primer | Sequence | |||
|---|---|---|---|---|
| 1 | TWXBA-F | 5′-CGCGCGTCTAGAAATAATTTTGTTTAACTTTAAGAAG GAGATATACATATGAAAACCCTGAAACAAGCAG-3′ | ||
| 2 | TWXHOI-R | 5′-GTGGTGCTCGAGATATATATCTCCCCATAG-3′ | ||
| 3 | TWNDEI-F | 5′-GGAGATATACATATGAAAACCCTGA-3′ | ||
| 4 | TWXHOI-188R | 5′-ACACCTACCTCGAGATATCCTCGTTTAACC-3′ | ||
| 5 | TWXHOI-199R | 5′-CTGTAATCCTGTCATCTCGAGAGCATC-3′ | ||
| 6 | TWXHOI-245R | 5′-CCA CTG ATT CTC GAG ATG CCA AGC T-3′ | ||
| 7 | TWXHOI-325F | 5′-CTACTAAAACTCTCGAGACTCAGGCTGA-3′ | ||
| 8 | TWXHOI-467R | 5′-GGTGGTGGTGCTCGAGATATATATCTC-3′ | ||
| 9 | TWBGLIIF | 5′-CGTAGAGGATCGAGATCTCGATCC-3′ | ||
| 10 | TWXHOI-392R | 5′-CCTTGACGCTCGAGACACTTAAACGC-3′ | ||
| 11 | TWXBAI-141F | 5′-TCCCCTCTAGAAATAATTTTGTTTAACTTTAAGAAGG AGATATACATATGAGACCTAACTTTGCTACTG-3′ | ||
| 12 | TWSALI-373F | 5′ GCGATCGTCGACTGGAACGTTAATAATTATGG-3′ | ||
| 13 | TWXHOI- 475R | 5′ CTCGTCCTCGAGATATATATCTCCCCATAGCTGACCCA-3′ | ||
| 14 | TWXHOI-146R | 5′ CGCATACTCGAGAGTAGCAAAGTTAGGTC-3′ | ||
| 15 | TWXHOI-172R | 5′ CGCATACTCGAGAATTTTATCTTTATTTATTCC-3′ | ||
| 16 | TWXHOI-323R | 5′CGCATACTCGAGAGTAGGGTCTTTACCTACATGCAAC-3′ | ||
| 17 | TWXHOI-376R | 5′ CGCATACTCGAGAACGTTCCATCCACTTG-3′ | ||
| Construct | Primer 1 | Primer 2 | Cloning vector | |
| PlyTW (5719) | 1 | 2 | pET21a | |
| PlyTW 146′ | 2 | 14 | pET21a | |
| PlyTW 172′ | 1 | 15 | pET21a | |
| PlyTW188 | 3 | 4 | pET21a | |
| PlyTW 199′ | 3 | 5 | pET21a | |
| PlyTW 245′ | 1 | 6 | pET21a | |
| PlyTW 323′ | 1 | 16 | pET21a | |
| PlyTW 376′ | 1 | 17 | pET21a | |
| plyTW 392 | 9 | 10 | pET21a | |
| plyTW 141–392 | 11 | 10 | pET21a | |
| PlyTW Δ146–373 | 12 | 13 | PlyTW 146′ | |
| PlyTW Δ172–373 | 12 | 13 | PlyTW 172′ | |
| plyTW Δ199–325 | 7 | 8 | PlyTW199 | |
Primer names are listed with their sequences. Introduced restriction sites are underlined. Constructs, are listed with the primer pairs, and vectors used in their construction.
Staphylococcus aureus Newman strain was grown at 37°C in either tryptic soy broth for plate lysis assays or brain-heart infusion broth (Becton Dickenson, Sparks, MD) for both turbidity reduction assays and zymogram analysis.
Protein purification
Purification of recombinant PGH constructs for in vitro assays was performed per manufacturer's instructions (Qiagen, Ni-NTA, Germantown, MD) with the following modifications. Protein inductions were performed in modified LB (tryptone, 15 g l−1; yeast extract, 8 g l−1; NaCl, 5 g l−1, pH 7.8) (Schmelcher et al., 2010) at 10°C for 20 h. To avoid solubility issues, 30% glycerol was included in all purification buffers. All samples were filter sterilized through a 0.22 μm after elution and protein concentrations were determined via a nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Sterilized protein preparations were stored at 4°C until time of assay.
SDS-PAGE and zymogram analysis
One μg of the purified fusion proteins and Kaleidoscope protein standards (Invitrogen) was analyzed via 15% SDS-PAGE in Tris-Glycine buffer at 100 v for 1.5 h in the Bio-Rad Mini-PROTEAN 3 gel apparatus (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. Zymogram gels were prepared with 300 ml equivalent of mid-logarithmic phase (OD600nm of 0.4–0.6) S. aureus Newman cells embedded in the gels and electrophoresed simultaneously with the SDS-PAGE gels. The SDS-PAGE gels were stained with Coomassie blue using standard protocols (Becker et al., 2009a). Zymograms were washed twice in excess water for 30 min to remove SDS and incubated for <1 h at room temperature in water until cleared zones developed.
Plate lysis assay
Purified enzymes were serially diluted in saline lysis buffer (SLB; 150 mM NaCl 10 mM Tris buffer, pH 7.5) to yield concentrations of 1000, 100, 10, 1 and 0.1 pmol10 μl–1. 10 μl of each dilution was then spotted onto a freshly plated, air-dried lawn of S. aureus Newman, allowed to air dry and incubated overnight at 37°C.
Turbidity reduction assays
The turbidity assay was modified from the plate reader assay reported previously (Becker et al., 2009a). Enzymes were serially diluted in SLB in wells of a 96-well plate, and reactions were initiated with the addition of thawed S. aureus substrate cells (Becker et al., 2009b) (mid-log phase grown cells, frozen in SLB with 30% glycerol, thawed, washed three times in excess SLB) resuspended in SLB supplemented with varying concentrations of lytic protein, NaCl, CaCl2, MgCl2, MnSO4, ZnSO4 or EDTA at room temperature. Enzymes were equilibrated in the appropriate buffer for 10 min prior to the initiation of each reaction. The maximum rate for each reaction (calculated as a sliding window of 40 s as determined by a plate reader with OD600nm readings taken every 20 s) was reported as turbidity reduction rate (ΔOD600nm min−1) or divided by the μM concentration of each protein in the sample to yield a specific activity (ΔOD600nm μM−1 min−1). Sample raw data is in Fig. S1 (Supporting Information).
RESULTS
SDS and zymogram assays
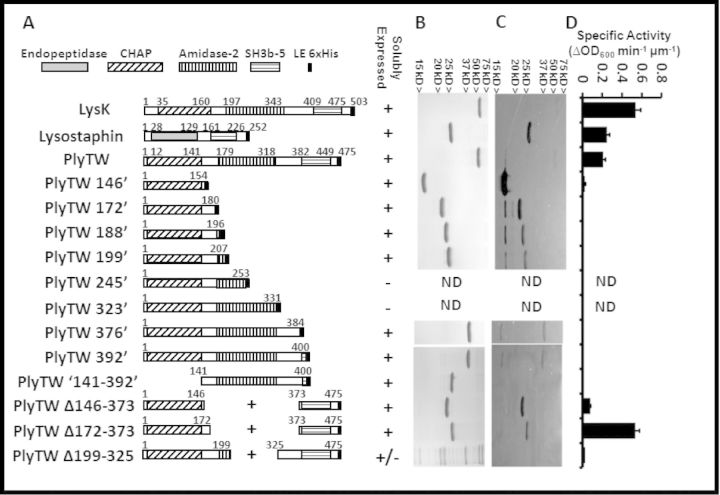
plyTW was subcloned into the pET21a vector, which adds six histidine codons at the C-terminus. This construct (Fig. 1A; PlyTW) retained all three protein domains, CHAP, amidase-2 and the SH3b CBD. Truncations and deletions of the plyTW gene were generated by PCR-subcloning (Becker et al., 2009a) (Fig. 1A) to determine the activities from each domain individually or in combination. SDS-PAGE analysis (Fig. 1B) illustrates that the E. coli-expressed proteins were purified at >95% purity via nickel column chromatography (except constructs PlyTW 245′, PlyTW 323′ and PlyTW Δ199–325). Zymogram analysis indicated that the predicted protein was the only active protein species for most constructs (LysK and lysostaphin, plyTW 146′, PlyTW Δ146–373, PlyTW Δ172–373), and that the CHAP domain alone is sufficient to clear a spot in the zymogram gel while the amidase domain alone (PlyTW141–392) is not. (The SH3b CBD is not expected to have any lytic activity and was not tested independently.) Interestingly, a shadow band (potentially representing a truncated CHAP domain band) approximately the size of the CHAP domain alone (PlyTW 146′) is consistently observed in the zymogram for constructs PlyTW, PlyTW 172′, PlyTW 188′, PlyTW 199′, PlyTW 376′ and PlyTW 392′ (Fig. 1C), while no corresponding band is apparent in the parallel SDS gel. In an attempt to enhance the CHAP domain activity, fusions of the CHAP domain to the SH3b domain were constructed (PlyTW Δ146–373 and PlyTW Δ172–373). Previous studies demonstrated that a deletion of the amidase domain from a similarly structured lysin, LysK, resulted in no loss of activity as compared to the full length protein (Becker 2009a). These constructs did show activity in the zymogram, but the activity not enhanced over the shortest CHAP domain alone construct (PlyTW 146′).
Figure 1.
Schematic representation, SDS-PAGE and zymogram analysis of PlyTW constructs. (A) Full length lysostaphin, LysK, PlyTW endolysin and PlyTW deletion constructs are schematically represented with domains labeled as identified in the Pfam domain database (http://pfam.sanger.ac.uk/protein?entry=O56788). Fusion junction points are noted. Endopeptidase domain (solid gray), CHAP domain (diagonal stripes), amidase domain (vertical stripes), SH3b domain (horizontal stripes) and the 6xHis tag (black box) are represented. At each fusion junction, and 6xHis tag, there is an XhoI restriction enzyme site introduced (corresponding to an LE sequence in the amino acid sequence). Endolysin sequences and SH3b sequences are drawn to scale. His tags are not drawn to scale. (B) SDS-PAGE analysis and (C) Zymogram analysis of 1 μg nickel column purified proteins corresponding to the constructs in panel A. (D) Turbidity reduction analysis of expressed proteins. Specific activities (ΔOD600nm μm−1 min−1) for the PlyTW constructs are presented as the maximal change in OD600nm (during a 40 s interval, i.e. three time points) over the 30 min assay. Each data point [± standard deviation (SD)] represents samples at 0.5 μM protein, in 150 mM NaCl SLB from at least two experiments performed in triplicate (n ≥ 6).
Turbidity reduction assays
To quantify the PlyTW constructs for lytic activity against live S. aureus strain Newman, each purified protein was tested in turbidity reduction assays. The previously characterized LysK phage endolysin (O'Flaherty et al., 2005; Becker et al., 2009a) and the bacteriocin lysostaphin (Browder et al., 1965) were positive control enzymes. When tested in parallel (Fig. 1D), at a concentration of 0.5μM in 150 mM SLB, the activity (ΔOD600nm μm−1 min−1) of LysK (0.54 ± 0.05) was at least 2.2-fold greater than either lysostaphin (0.25 ± 0.02) or PlyTW (0.21 ± 0.02). Deletions, eliminating the SH3b CBD, reduced the activity in the turbidity reduction assay, minimally 8.6-fold (PlyTW vs PlyTW 146′ [0.024 ± 0.006]). Internal deletions of the amidase domain, and the surrounding undefined regions, have varied effects. PlyTW Δ146–373 (0.079 ± 0.003) increased activity 3.3-fold compared to the CHAP domain alone. A second internal deletion, PlyTW Δ172–373, exceeded PlyTW activity, and achieved nearly the same activity as LysK, 0.53 ± 0.04. An additional internal deletion, PlyTW Δ199–325, eliminated the ability to readily purify the construct over background proteins (Fig. 1B). The remaining deletion constructs, while active in the zymogram assay, show no detectable activity at 0.5 μM in the turbidity reduction assay. It is not uncommon for PGH enzymes to give quantitatively different results in a variety of PGH assays, as has been reported for lysostaphin (Kusuma and Kokai-Kun 2005).
Plate lysis assay
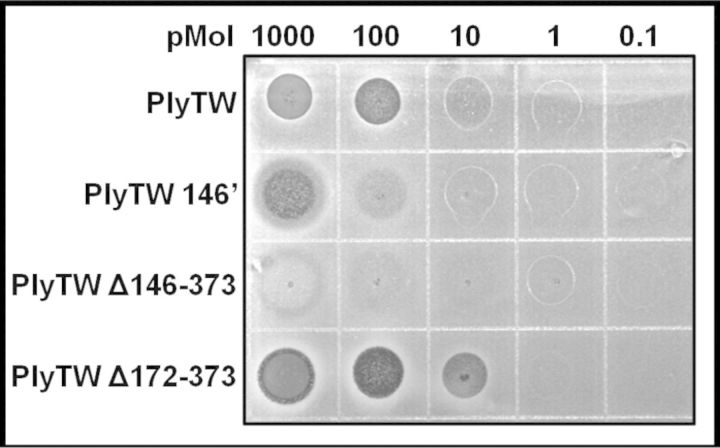
The four highest activity PlyTW constructs were used for further analysis in the plate lysis assay. Each enzyme was tested for its ability to kill untreated, live bacteria (Fig. 2). The results from the plate lysis assay are similar to the turbidity reduction assay results (Fig. 1D). Again the CHAP domain alone (PlyTW 146′) was sufficient to lyse live cells (demonstrating reduced lawn density at 100 pMol), but showed ∼10-fold reduction in activity compared to full length PlyTW (reducing lawn density at 10 pMol). PlyTW Δ146–373 did not show enhanced activity at higher concentrations, but shows some lytic activity at a lower concentration (10 pMol) than the CHAP domain alone (PlyTW 146′). As in the turbidity reduction assay, the CHAP plus SH3b construct (PlyTW Δ172–373) shows enhanced activity over full length PlyTW, with strong lytic activity at 10 pMol.
Figure 2.
Plate lysis assay with PlyTW constructs on S. aureus. 10 μl drops, containing serial 10-fold dilutions from 1 μmol of purified PGH in sterile SLB, were spotted onto fresh lawns of S. aureus strain Newman. Plates were incubated overnight, and then photographed.
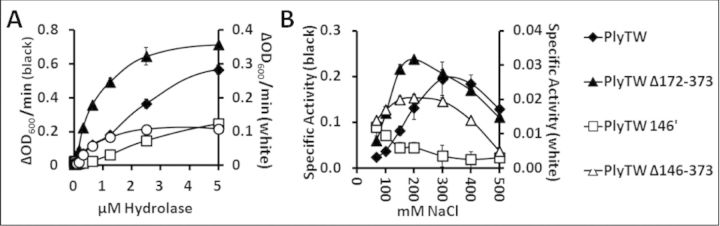
Effect of enzyme concentration and salt concentration
Each of the four constructs was tested at increasing enzyme concentrations to determine the linear range of activity and appropriate molar concentration for further comparisons (Fig. 3A). The lowest equimolar concentration where all four constructs are reliably active was determined to be between 0.6–1.3 μM. Comparative turbidity reduction assays were performed at 1 μM. When each of the enzymes was tested over a range of NaCl concentrations, PlyTW had maximum activity at 300 mM NaCl, whereas both internal deletions of the amidase domain (PlyTWΔ146–373 and PlyTWΔ172–373) had optimal activity at 200 mM NaCl, closer to physiological saline concentrations. PlyTW 146′ had maximal activity at 67.5 mM NaCl, the lowest concentration tested (Fig. 3B).
Figure 3.
The effect of salt concentration on turbidity reduction activity with PlyTW constructs. (A) PlyTW (black diamonds), PlyTW 146′ (white squares), PlyTW Δ146–373 (white triangles) and PlyTW Δ172–373 (black triangles) were added to the turbidity reduction assay to a final concentration of 5 μM in 150 mM SLB, and at 2-fold serial dilutions. The OD600nm was measured in triplicate every 20 s for 10 min. Activities are reported as maximal ΔOD600nm min−1 (± SD). Due to the different levels of activity, the data is represented with two y axes, black markers correspond to the left axis; white markers correspond to the right axis. (B) PlyTW constructs were assayed at 1 μm concentrations in SLB, with NaCl concentrations of 67.5, 100, 150, 200, 300, 400 and 500 mM. Specific activities (maximal ΔOD600nm μm−1 min−1) for the PlyTW constructs are presented. Each data point (± SD) represents triplicate samples at 1μM from at least two experiments performed in triplicate (n ≥ 6).
Effects of divalent cations
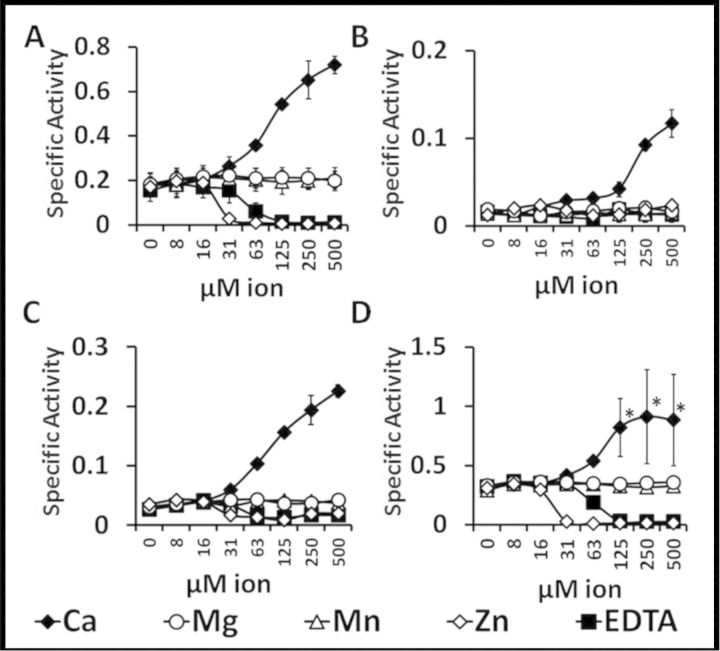
To determine the effect of divalent cations on the activity of PlyTW constructs, each protein was assayed in the presence of 2-fold serial dilutions of 0.5 mM of either Ca2+, Mg2+, Mn2+, Zn2+ or EDTA. Addition of Ca2+ increased the activity of all PlyTW constructs, increasing the PlyTW 4-fold (Fig. 4A), PlyTW 146′ 6.5-fold (Fig. 4B), PlyTWΔ146–373 8.2-fold (Fig. 4C) and PlyTWΔ172–373 increased minimally 2.8-fold, but these results are likely underestimates due to the upper limit of the assay (Fig. 4D). Neither Mg2+ nor Mn2+ had a significant effect on the turbidity assay for any of the constructs tested. Addition of Zn2+ or EDTA inhibited both the full length and the internal deletion constructs (Fig. 4A, C and D), with no detectable effect on PlyTW 146′ potentially due to the inherently low activity of the construct.
Figure 4.
The effects of divalent cations on turbidity reduction activity of PlyTW constructs. Turbidity reduction analysis with PlyTW constructs on S. aureus Newman cells. Live S. aureus were resuspended in SLB (20mM Tris, pH 7.5, 150 mM NaCl) supplemented with 2-fold serial dilutions of each ion, or EDTA, from 0.5mM. 1 μM concentration of each PlyTW (A), PlyTW 146′ (B), PlyTW Δ146–373 (C) and PlyTW Δ172–373 (D) were tested at increasing concentrations of Ca2+ (black diamonds), Mg2+(white circles), Mn2+(white triangles), Zn2+(white diamonds) or EDTA (black squares). The Δ OD600nm was measured in triplicate wells every 20 s for 10 min. Each specific activity (± SD) represents samples performed in at least triplicate (n ≥ 3). Asterisks indicate samples in which the activity was so high, the turbidity was reduced before the initial time point thus increasing the variability of the assay, and under represent the activity of the enzyme under these conditions.
DISCUSSION
The Twort phage endolysin shares a common domain architecture (CHAP-amidase-SH3b) with numerous SH3b containing staphylolytic phage endolysins. The SH3b containing staphylolytic endolysins collated from public data sets with this domain architecture have been cataloged into three conserved groups based on aa identity (97% within group aa identity; <50% between group aa identity) (Becker et al., 2009b). PlyTW is highly divergent from these three groups sharing only 57% identity to its most conserved homolog, PhiWMY endolysin (Yokoi et al., 2005; Becker et al., 2009b). The unique sequences of PlyTW suggesting altered ‘functional specificity’ compared to other SH3b containing endolysins, making it a potential novel antimicrobial. This work demonstrates that the CHAP domain of PlyTW is necessary and sufficient for exolysis of S. aureus cells in three lytic assays. The SH3b domain enhances the activity of the CHAP domain, but is not essential for CHAP lytic activity. CHAP domains that are essential for cell lysis have been identified previously for two staphylococcal endolysins that share a domain organization nearly identical to the Twort endolysin: LysK (Becker et al., 2009a; Horgan et al., 2009) and phi11 endolysin (Donovan, Lardeo and Foster-Frey 2006; Sass and Bierbaum 2007).
The N-terminal CHAP domain of PlyTW 146′ was active in each of the three lytic assays, suggesting that the N-terminus of the protein plays an essential role in cell lysis. These findings are supported by a previous study where Loessner et al. (1998) first isolated the PlyTW gene from the Twort phage genome and identified three phage genomic clones that each expressed a different form of PlyTW in E. coli. The full length 467 aa and two C-terminal truncations (1–271 aa and 1–172 aa) were expressed from phage genomic clones, and these researchers arrived at the same conclusion as we have, namely that the N-terminal region of the protein contains the primary active domain. Interestingly, this early study described an increase of free alanine amino groups (detected as dinitrophenyl-labeled alanine by RP-HPLC) after digestion of S. aureus cell walls with the longest truncation (1–271 aa) indicating an amidase activity. However, the amidase domain alone (PlyTW 141–392) showed no lytic activity in any assay (data not shown). The poor amidase construct activity raises concerns about any truncation where there is potential for non-native constructs to lack activity due to improper folding. It is also possible that the amidase domain has been evolutionarily conserved due to its role in ‘lysis from within’, e.g. the endopeptidase domain might create a substrate that the amidase domain recognizes more readily than intact PG when ‘lysing from within’ (or vice versa). It should also be noted that these results are consistent with findings with several SH3b domain containing staphylococcal phage endolysins [e.g. phill (Donovan et al., 2006; Sass and Bierbaum 2007); LysK (Becker et al., 2009a)] where the ‘amidase alone’ construct is virtually inactive. This is also consistent with several dual lytic domain streptococcal phage endolysins B30 (Donovan et al., 2006), PlyGBS (Cheng and Fischetti 2007) (99% identical to each other) and LambdaSa2 (Donovan and Foster-Frey 2008) which via deletion analysis have each been shown to have an active endopeptidase domain and a nearly silent second lytic domain (glycosidase) in assays that measure ‘lysis from without’. Interestingly, mass spectrophotometric results of purified peptidoglycan digestion products indicate that both lytic domains are active in the full length parental constructs of each of these streptococcal (Donovan et al., 2006; Donovan and Foster-Frey 2008) and staphylococcal constructs (phi11, LysK; staphylococcal mass spectrophotometry data unpublished), suggesting again that these enzymes may respond differently to cell wall peptidoglycan when ‘lysing from within’ vs ‘lysis from without’, as discussed previously (Donovan and Foster-Frey 2008).
The heightened activity levels of the amidase deletion construct PlyTW Δ172–373 are in contrast to the findings with the staphylococcal phage K endolysin, LysK, where deletion of the amidase domain had minimal effect on the exolytic activity of the truncated protein (Becker et al., 2009a). This may be explained simply due to the non-native structure of these constructs, in so far as two additional internal deletion constructs were generated and tested in parallel with each showing reduced activity compared to the full length protein (Fig. 1D). In support of our results with PlyTW Δ172–373, a similar construct, ClyS, has been reported by the Fischetti laboratory as a fusion of PlyTW CHAP domain to the phiNM3 phage endolysin CBD (Daniel et al., 2010).
Consistent with the LysK deletions and fusions (Becker et al., 2009a), and the ClyS fusion (Daniel et al., 2010), when the PlyTW CHAP domain is fused to a CBD, the turbidity reduction assay activity increased ∼10X (Becker et al., 2009a). This is also similar to the work of Sass and Bierbaum (2007) with the phi11 endolysin where the CHAP domain alone was active, but the CHAP-SH3b fusion was much more active on both purified staphylococci cell walls and SDS-treated cells. The PlyTW SH3b domain was fused to the PlyTW CHAP domain in three different constructs (PlyTW Δ146–373, PlyTW Δ172–373, PlyTW Δ199–325). The PlyTW Δ199–325 construct purified poorly and thus showed little to no improved turbidity reduction activity. However, the PlyTW Δ146–373 activity increased the activity of the CHAP domain by ∼4-fold and the construct PlyTW Δ172–373 increased the turbidity reduction activity exceeding the activity of the full length PlyTW activity levels by ∼2-fold, approaching the LysK activity levels (Fig. 1D). Deletion of the SH3b domain from the full length construct (PlyTW 392) reduced the turbidity reduction activity of the full length PlyTW construct to virtually undetectable levels, although at high enough concentrations, activity was detectable in both the plate lysis and turbidity reduction assays (data not shown). This dependence on C-terminal CBD sequences for full lytic activity has been demonstrated previously, for the staphylococcal proteins LysK (Becker et al., 2009a), phi11/LytA endolysin (Donovan et al., 2006; Sass and Bierbaum 2007), lysostaphin (Baba and Schneewind 1996), ALE-1 (Lu et al., 2006) and the Listeria phage proteins Ply118 and Ply500 (Loessner et al., 2002). However, there may be other factors at work because the opposite effect has been observed for the streptococcal B30 endolysin (Donovan et al., 2006) and the nearly identical homologue PlyGBS (Cheng and Fischetti 2007), as well as the bacillus endolysin Ply L (Cheng and Fischetti 2007), where a truncated protein (lacking a CBD) shows higher activity in the absence of the C-terminal CBD. Even more confounding is the work of Hogan et al. which reported that the first 165 aa of LysK (CHAP domain) alone, lacking an SH3b CBD, expressed a lytic activity that was even greater than full length LysK (Horgan et al., 2009). These discrepancies in reported dependence on CBDs may reflect the vagaries of the unique assay conditions (e.g. buffer constituents) employed by different labs to perform PGH activity measurements, for example, in Fig. 3B, the PlyTW 146′ construct lacking a CBD has greater activity in turbidity reduction assays than the full length protein at reduced salt concentrations (50 and 100 mM NaCl).
The differences in activity levels of the many staphylococcal phage endolysin deletion and fusion constructs apparent in the literature demonstrate the need for empirical testing of each novel construct. The fact that numerous labs report differences in antimicrobial or lytic activity from the same protein depending on the PGH assay used (e.g. plate lysis vs turbidity reduction vs zymogram) reminds us that there is still a gap in our understanding as to exactly what each PGH assay is measuring. However, these discrepancies and the lack of uniformity of assay conditions between labs have the added benefit of supporting the argument that these enzymes can likely be tailored to very specific and unique therapeutic applications. For example, the PlyTW Δ172–373 and PlyTW 146′ constructs show much higher activity than full length PlyTW or other CHAP domain constructs in the presence of elevated Ca2+, suggesting that these constructs might be strong candidates for treating bovine mastitis, where the calcium concentration in milk is ~30 mM (https://www.uoguelph.ca/foodscience/book-page/minerals). With continued analysis, there will likely be other unique and unexpected properties of these novel antimicrobials that will lend themselves to very specific and unexpected therapeutic or preventive applications.
SUPPLEMENTARY DATA
Acknowledgments
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
FUNDING
This work was supported in part by funding to DMD from CREES award # 2007-35204-18395, NIH grant 1RO1AI075077-01A1 and US State Department funds supporting US-Pakistani and US-Russian collaborations, as well ARS headquarters via a post-doctoral fellowship.
Conflict of interest statement. None declared.
REFERENCES
- Baba T, Schneewind O. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 1996;15:4789–97. [PMC free article] [PubMed] [Google Scholar]
- Becker SC, Dong S, Baker JR, et al. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol Lett. 2009a;294:52–60. doi: 10.1111/j.1574-6968.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- Becker SC, Foster-Frey J, Stodola AJ, et al. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene. 2009b;443:32–41. doi: 10.1016/j.gene.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Browder HP, Zygmunt WA, Young JR, et al. Lysostaphin enzymatic mode of action. Biochem Biophys Res Co. 1965;19:389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Antibiotic Resistance Threats in the United States. Atlanta: CDC; 2013. [Google Scholar]
- Cheng Q, Fischetti VA. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl Microbiol Biot. 2007;74:1284–91. doi: 10.1007/s00253-006-0771-1. [DOI] [PubMed] [Google Scholar]
- Daniel A, Euler C, Collin M, et al. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Ch. 2010;54:1603–12. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Jackson CR, Fedorka-Cray PJ, et al. Carriage of methicillin-resistant staphylococci by healthy companion animals in the US. Lett Appl Microbiol. 2014;59:1–8. doi: 10.1111/lam.12254. [DOI] [PubMed] [Google Scholar]
- Donovan DM. Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Recent Pat Biotechnol. 2007;1:113–22. doi: 10.2174/187220807780809463. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Becker SC, Dong S, et al. Peptidoglycan hydrolase enzyme fusions for treating multi-drug resistant pathogens. Biotech International. 2009;21:6–10. [Google Scholar]
- Donovan DM, Foster-Frey J. LambdaSa2 prophage endolysin requires Cpl-7-binding domains and amidase-5 domain for antimicrobial lysis of streptococci. FEMS Microbiol Lett. 2008;287:22–33. doi: 10.1111/j.1574-6968.2008.01287.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Foster-Frey J, Dong S, et al. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl Environ Microbiol. 2006;72:5108–12. doi: 10.1128/AEM.03065-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Lardeo M, Foster-Frey J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol Lett. 2006;265:133–9. doi: 10.1111/j.1574-6968.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- Finn RD, Tate J, Mistry J, et al. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–8. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti VA. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. 2008;11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti VA. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol. 2010;300:357–62. doi: 10.1016/j.ijmm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EM, Weinert LA, Holden MT, et al. A shared population of epidemic methicillin-resistant Staphylococcus aureus 15 circulates in humans and companion animals. MBio. 2014;5:e00985–13. doi: 10.1128/mBio.00985-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan M, O'Flynn G, Garry J, et al. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl Environ Microb. 2009;75:872–4. doi: 10.1128/AEM.01831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Kusuma C, Kokai-Kun J. Comparison of four methods for determining lysostaphin susceptibility of various strains of Staphylococcus aureus. Antimicrob Agents Ch. 2005;49:3256–63. doi: 10.1128/AAC.49.8.3256-3263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner MJ. Bacteriophage endolysins—current state of research and applications. Curr Opin Microbiol. 2005;8:480–7. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Loessner MJ, Gaeng S, Wendlinger G, et al. The two-component lysis system of Staphylococcus aureus bacteriophage Twort: a large TTG-start holin and an associated amidase endolysin. FEMS Microbiol Lett. 1998;162:265–74. doi: 10.1111/j.1574-6968.1998.tb13008.x. [DOI] [PubMed] [Google Scholar]
- Loessner MJ, Kramer K, Ebel F, et al. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol Microbiol. 2002;44:335–49. doi: 10.1046/j.1365-2958.2002.02889.x. [DOI] [PubMed] [Google Scholar]
- Lu JZ, Fujiwara T, Komatsuzawa H, et al. Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J Biol Chem. 2006;281:549–58. doi: 10.1074/jbc.M509691200. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Ton-That H, Faull KF, et al. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a D-alanyl-glycine endopeptidase activity. J Biol Chem. 1999;274:15847–56. doi: 10.1074/jbc.274.22.15847. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Schmelcher M, Rodriguez-Rubio L, et al. Endolysins as antimicrobials. Adv Virus Res. 2012;83:299–365. doi: 10.1016/B978-0-12-394438-2.00007-4. [DOI] [PubMed] [Google Scholar]
- O'Flaherty S, Coffey A, Meaney W, et al. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol. 2005;187:7161–4. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastagia M, Euler C, Chahales P, et al. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob Agents Ch. 2011;55:738–44. doi: 10.1128/AAC.00890-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LB, Stegger M, Hasman H, et al. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. MBio. 2012;3:e00305–11. doi: 10.1128/mBio.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard DG, Dong S, Kirk MC, et al. LambdaSa1 and LambdaSa2 Prophage Lysins of Streptococcus agalactiae. Appl Environ Microb. 2007;73:7150–4. doi: 10.1128/AEM.01783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass P, Bierbaum G. Lytic activity of recombinant bacteriophage {phi}11 and {phi}12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microb. 2007;73:347–52. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M, Shabarova T, Eugster M, et al. Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl Environ Microbiol. 2010;76:5745–56. doi: 10.1128/AEM.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Mitchell MS, Donovan DM, et al. Phage-based Enzybiotics, in Bacteriophages in Health and Disease. In: Paul H, Stephen TA, editors. CAB International. Wallingford, UK: 2012. pp. 217–39. [Google Scholar]
- WHO. Antimicrobial Resistance: Global Report on Surveillance. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- Yokoi KJ, Kawahigashi N, Uchida M, et al. The two-component cell lysis genes holWMY and lysWMY of the Staphylococcus warneri M phage varphiWMY: cloning, sequencing, expression, and mutational analysis in Escherichia coli. Gene. 2005;351:97–108. doi: 10.1016/j.gene.2005.03.006. [DOI] [PubMed] [Google Scholar]