Abstract
Silicosis is the most common pneumoconiosis globally, with higher prevalence and incidence in developing countries. To date, there is no effective treatment to halt or reverse the disease progression caused by silica-induced lung injury. Significant advances have to be made in order to reduce morbidity and mortality related to silicosis. In this review, we have highlighted the main mechanisms of action that cause lung damage by silica particles and summarized the data concerning the therapeutic promise of cell-based therapy for silicosis.
1. Introduction
The industrialization process steadily increased the occupational exposition of workers to breathable particles in many work environments. Among these particles, silicon dioxide, or silica, has an important role in respiratory occupational diseases' burden. Inhalation of silica particles causes silicosis: a persistent inflammation with granuloma formation that leads to tissue remodeling and impairment of lung function [1]. Tuberculosis, chronic obstructive pulmonary diseases, and rheumatoid arthritis are some common complications associated to silicosis [2–4]. Long-term silica exposure can also cause lung cancer [5, 6]. To date, the available management of silicosis is focused on controlling associated symptoms and comorbidities and no therapy halts or reverses the disease progression. In the end-stage of lung illness, the patient succumbs to death due to respiratory failure [1].
Over the past decades, many efforts have been made to prevent inhalation of silica dust by workers; however, silicosis is still a public health concern worldwide with higher prevalence in developing countries [7]. In Brazil, Holanda and collaborators showed a prevalence of silicosis of 33% of pit diggers in Ceará state [8] and over 4,500 cases of silicosis cases were related to gold-miners between 1978 and 1988 in Minas Gerais state [9]. Currently, it is expected that over 6 million workers are daily exposed to silica in various labor fields nationwide [4, 10]. China reported more than 500,000 silicosis patients between 1991 and 1995 with about 6,000 new cases and over 24,000 deaths per year [1]. In India, 10 million workers are exposed to silica dust with high risk of developing the disease [11].
Silicosis is also an occupational health issue in developed countries. More than 3 million workers were exposed to silica particles between 1990 and 1993 in Europe, 600,000 of them being in the United Kingdom [12]. In the United States, more than 100,000 workers were exposed to silica dust and over 3,500 new cases were related per year from 1987 to 1996 [13]. The implementation of protective measures has a declined number of new silicosis cases and the mortality rate. However, silicosis is still incurable and new outbreaks happen, such as the exposure of quartz conglomerate workers in Spain, in which the median age of the cohort was 33 years [14]. Furthermore, there is an expansion in the number of work environments with potential silica dust exposure, such as jeans sandblasting and quartz.
An incremental number of articles have shown the efficacy of either systemic or intratracheal administration of stem cells in several animal models of lung injury [15]. Among those, the bone marrow cells are the most studied. They were able to promote lung parenchyma reepithelization, modulate the immune response, and decrease the tissue remodeling [16–19]. Silicosis has a unique pathogenesis process, in which the phagocytosis and release of silica particles in the lung tissue drive the disease progression [20]. This review highlights the main mechanisms of action of silica dust in the alveolar environment and how cell therapy may help patients with silicosis by modulating the inflammation, reducing fibrosis, and, thus, improving lung function.
2. Pathogenesis: The Cycle of Damage in Lung Tissue
Silica particles that overcome the mucociliary defense mechanism in the airways and reach the distal portions in the lung begin the pathogenesis cascade. The most pathogenic particles for humans are those under 10 μm, since they have the aerodynamics required to reach bronchioles and alveoli [4, 21].
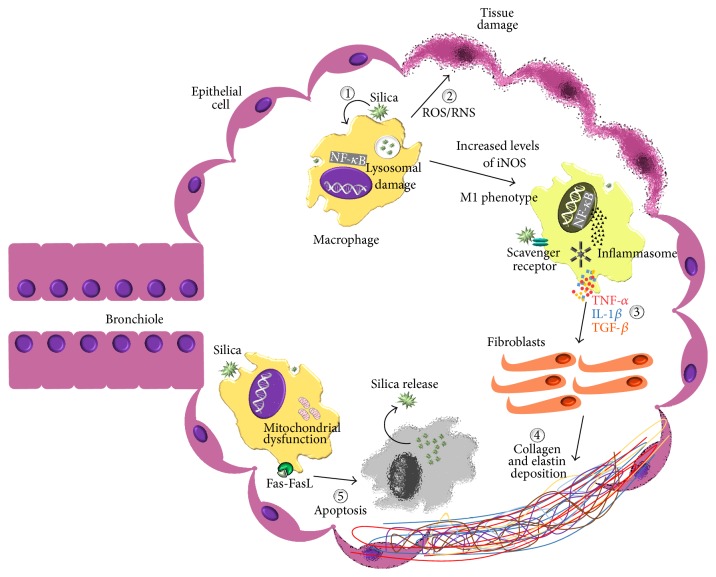
Silicosis is orchestrated by a cycle of phagocytosis and release of these particles that lead to epithelial damage, lung remodeling, and reduction of gas exchange area. Silica-induced lung damage occurs by five main mechanisms (Figure 1): (1) direct cytotoxicity, (2) generation of reactive species, (3) production of cytokines and chemokines, (4) fibrosis, and (5) cell death by apoptosis [22–25].
Figure 1.
Silicosis pathogenesis. The main mechanisms that orchestrate the disease progression caused by silica-induced lung damage are (1) direct cytotoxicity, (2) production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), (3) secretion of inflammatory and fibrotic mediators, (4) lung remodeling through collagen and elastin deposition, and (5) cell death by apoptosis.
Silica particles present a piezoelectric property, which means that under certain applied pressure, the crystals acquire electrical polarity. This provides the ability of silica dust to cause direct cytotoxicity. The particles react with resident cells and cause lipid peroxidation of the membrane in bronchoalveolar cells. Newly fractured particles are the most cytotoxic, since they can generate more free radicals in aqueous medium that disrupt the plasma membrane and release lysosomal enzymes, thus causing tissue damage [22, 26].
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are highly reactive chemicals often produced for the biological defense system against noxious agents. However, the interaction between alveolar macrophages and silica particles causes respiratory burst with high consumption of oxygen, increased levels of inducible nitric oxide synthetize (iNOS), and production of ROS, which is damaging to lung cells [27, 28]. Among ROS/RNS, nitric oxide has a critical role in silicosis pathogenesis. Nitric oxide is formed from the conversion of the amino acid L-arginine to L-citrulline in presence of iNOS, which then interacts with superoxide and forms peroxynitrite that damages mitochondria and DNA, and inactivates several proteins [29–31].
The oxidative stress activates transcriptional factors, such as the nuclear factor kappa B (NF-κB) [31] and activator protein 1 (AP-1) [32]. The interaction of silica with macrophages and epithelial cells translocates NF-κB from the cytoplasm to the nucleus, where it binds to the DNA, starting the transcription and translation of genes involved in inflammatory and fibrogenic processes. The release of cytokines, chemokines, lipid mediators, and growth factors recruits polymorphonuclear and mononuclear cells to the alveolar spaces and around the silica particles, which contribute for the formation of granulomas [22, 31, 33].
Leukotriene B4 is a lipid mediator that mastocytes produce in response to silica stimulation. The leukotriene B4 increases the amount of neutrophils in the inflammation site, and it is also involved in tumorigenesis [34]. Furthermore, the macrophage inflammatory protein- (MIP-) 1 and MIP-2 are chemokines that increase the number of macrophages in response to silica-induced lung injury [29, 35]. Macrophages are the first cells to interact with silica particles and this interaction can activate a range of extracellular signals that lead to polarization of these cells [35]. The M1 macrophages are responsible for the antimicrobial and inflammatory responses, and cells are polarized to this phenotype in the presence of iNOS. Macrophages polarize to M2 phenotype in response to arginase, and in this case they are involved in the inflammation resolution and tissue repairs [36–38].
In alveolar macrophages, the scavenger receptors- (SR-) A and MARCO (macrophage receptor with collagenous structure) recognize and phagocyte the particles to remove them from alveoli [24, 38]. Nevertheless, knockout of MARCO increases inflammation after silica exposure in mice, due to increased lysosomal membrane permeabilization and inflammasome activation [39]. Once silica activates the NLRP3 (NOD-like receptor, pyrin domain containing 3) inflammasome, the cleavage of caspase-1 and production of cytokines occur, such as interleukin- (IL-) 18 and IL-1β [39, 40].
During silica-induced inflammation, epithelial cells and alveolar macrophages secrete IL-1α and IL-1β, respectively [26, 41]. Both cytokines are involved in fibroblasts activation and collagen deposition. IL-1α and IL-1β are agonists, while the IL-1 receptor antagonist (IL-1Ra) occurs naturally in response to inflammation and it can inhibit the effects of IL-1 [42, 43]. Furthermore, IL-1β and tumor necrosis factor- (TNF-) α increase the expression of IL-6, another mediator involved in the disease progression [44, 45].
The increased expression of TNF-α during silicosis leads to fibroblasts recruitment and proliferation [31]. TNF-α also can connect to the cell death receptor and start the apoptosis cascade. The knockout to Fas ligand (FasL) in mice and the use of anti-TNF antibody were able to prevent the silica-induced lung injury [46, 47].
Once fibroblasts were recruited to the damage site, the transforming growth factor- (TGF-) β induces the collagen deposition [48], as well as increased elastin production [49]. The increased expression of metalloproteases- (MMP-) 2 and MMP-9 and inhibition of tissue inhibitor of metalloprotease- (TIMP-) 1 and TIMP-2 cause a lung parenchyma restructuration on the course of the disease [50, 51]. The increased tissue damage caused by silica particles, the degradation of extracellular matrix by MMP, and the exacerbation of concentric deposition of collagen are responsible for granuloma formation and lung remodeling that impairs lung function.
The process of apoptosis is a result of the mitochondrial dysfunction and increased expression of death receptors and their ligands, such as FasL and TNF [46, 47, 52]. Besides the oxidative stress, the silica particles lead to loss of mitochondrial membrane potential, which is followed by activation of caspase-9 and caspase-3 and DNA fragmentation [25]. Cells release chemotactic factors during apoptosis that recruit new inflammatory cells, increasing the inflammation. Importantly, macrophages undergoing apoptosis also release silica particles back to the lung parenchyma, where they are phagocyted again by other macrophages, perpetuating the tissue damage cycle [27].
3. Silicosis Treatment: Still an Unmet Need
Despite the fair amount of outrage over the epidemiologic persistence of silicosis, little has been achieved in terms of treatment development. Research towards a satisfactory therapy for silicosis is happening in a much slower pace than for other chronic lung diseases. Only two registered clinical trials have been concluded in the past 10 years on silicosis treatments [53, 54], leaving a gap to be filled specially by developing countries, such as Brazil, India, and China, in which the prevalence of silicosis facilitates subject availability for clinical tests.
Management of silicosis consists of the use of bronchodilators/cough medication, prevention of exposure to irritants, and close monitoring for respiratory infections. Corticosteroid therapy may be used to reduce bronchitis and ameliorate symptoms as a short-term treatment, but its long-term positive effects have not been proved; it has the disadvantage of increasing risk of infections [55, 56]. Aluminum-based compounds were extensively studied for their ability to coat silica particles, reducing crystals reactivity and therefore protecting the lung tissue. Nevertheless, despite of good results achieved in experimental models [57], clinical studies showed no efficacy or sustained effects of treatment with aluminum in humans [58, 59]. Polyvinyl-pyridine-N-oxide (PVNO), a polymer able to promote cytoprotective effects in in vivo and in vitro models of silica-induced fibrosis, was also widely tested. Prophylactic and therapeutic use of PVNO yielded positive results in animal models [60] but had little efficacy in humans. PVNO delayed the progression of fibrosis in patients in one small clinical study but did not alter the outcome three years after treatment [61] and had limited therapeutic effect depending on factors, such as age and severity of the disease in another [62]. Another drug that has been tested for silicosis treatment is the herbal alkaloid tetrandrine. It has been historically employed for the treatment of pneumoconiosis in Chinese medicine, and its therapeutic use for silicosis has been approved by the State Drugs Administration of China. Tetrandrine alone and in combination with other drugs has been tested in animals and in humans [63]. However, robust clinical trials with objective criteria are still needed to prove its clinical efficacy.
Because of the prominent role of IL-1β in the pathophysiology of silicosis, targeting this cytokine could be an interesting option of treatment for silicosis. In experimental models, inhibition of IL-1 resulted in reduced expression of TGF-β1, collagen I, and fibronectin in the lung [64] and also reduced fibrosis and inflammation in kidneys and heart [65]. Use of an IL-1 receptor antagonist also reduced fibrosis and size of fibrotic nodules in mice [66]. A single report of using the drug anakinra—an IL-1 receptor antagonist—showed amelioration of respiratory symptoms and noticeable improvement in radiology images after six months of treatment in a 37-year-old man [67].
Whole lung lavage seems to have a positive impact in management of silicosis, improving patients prognosis and reducing lung infiltration, though there is no evidence that this approach suffices to prevent disease progression [68–70].
Other approaches have been tested in experimental models: the use of interferon gamma [71], ascorbic acid [72], beta-aminopropionitrile [73], relaxin [74], suppressive oligodeoxynucleotides [75], methyl palmitate [76], N-acetyl-cysteine [77], Dasatinib [78] and Nintedanib [79], and the antagonism/blockage of IL-13 [80] and IL-17A [81], as well as the use of the micro RNA miR-486-5p [82] and gene therapy with a short hairpin RNA to silence β-catenin [83]. All of these exerted positive effects in animal models, especially reducing lung fibrosis, were not translated to clinical trials so far.
As a last resource, lung transplantation is indicated to patients with no more pharmacological options. Nevertheless, the long-term survival after transplantation has a poor prognosis [84].
4. Cell-Based Therapy: From Bench to Bedside
The first bone marrow transplantation was performed in 1962, in order to use the recently discovered hematopoietic stem cells to restore proper physiology to the bone marrow of a patient [85].
In 1981, embryonic stem cells were described by Evans and Kaufman as pluripotent cells derived from the internal mass of a murine embryo, changing the paradigm for stem cells potential [86]. More recently, stem cells and stem-like cells were found in almost all adult tissues [87]. Cell transplantation began to be thought as a possible treatment for multiple diseases, due to the potential of stem cells to differentiate, replacing damaged cells in target tissues. Moreover, cell transplantation exerted beneficial effects in multiple models of diseases independently of cell homing or differentiation, evidencing a paracrine/endocrine effect of the cells [88, 89].
Cell-based therapies have the advantages of modulating inflammation and affecting the remodeling process concomitantly, without presenting toxicity or immunosuppression. These properties make cell therapy an exceptionally advantageous therapeutic approach for fibrotic lung diseases, including silicosis. Several preclinical studies have been exploiting this approach for the treatment of silicosis (Table 1).
Table 1.
Experimental studies using cell therapy in silicosis.
| Model | Cell type | Route | Number of cells | Follow-up | Reference |
|---|---|---|---|---|---|
| C57BL/6 | BMDC | i.t. | 2 × 106 | 30 and 60 days | [96] |
| C57BL/6 | BMMC | i.v. | 2 × 106 | 15 days | [97] |
| Nude mice | HUES-3 | i.t. | 2.5 × 106 | 15 days | [98] |
| Sprague-Dawley | BMSC | i.t. | 3 × 106 | 14 days | [99] |
| C57BL/6 | BMDC | i.t. | 2 doses of 2 × 106 | 60 days | [36] |
| C57BL/6 | BMSC | i.v. | 2 × 105 | 15 and 30 days | [100] |
| C57BL/6 | BMMC | i.v. | 1 × 106 | 70 days | [16] |
BMDC: bone marrow-derived cells; BMMC: bone marrow mononuclear cells; BMSC: bone marrow-mesenchymal stromal cells; HUES: human embryonic stem cells; i.t.: intratracheal; i.v.: intravenous.
The most extensively studied adult cell source for cell therapy is the bone marrow. The bone marrow contains a multitude of cells in different stages of differentiation. Among those cells, hematopoietic stem cells are of particular importance for their capacity to differentiate into immune cells and to modulate immune cell proliferation and activity [90]. Notwithstanding, in the pool of cells present in bone marrow cells that have a primary role in the maintenance, promoting growth and survival of other cells might have even stronger therapeutic potential. These cells are called mesenchymal stromal cells, and beyond their stromal properties they are also multipotent [91]. Both the pool of mononuclear cells present in the bone marrow and the stromal cells only have been shown to cause improvement in a wide range of inflammatory illnesses. Moreover, cell therapy has yielded positive effects in models of lung fibrotic diseases such as asthma, COPD, and bleomycin-induced lung fibrosis [92–94]. Clinical trials on cell therapy for lung diseases are advancing, and studies have been registered in Europe, Brazil, Australia, Canada, and the United States [95].
In 2009, our group published the first work using cell-based therapy in murine model of silicosis. Lassance et al. used a local infusion of a population of adherent mononuclear cells (BMDC) and evaluated the effects in two time points. The results showed reduction in the inflammation process thirty days after treatment, thus improving lung function; but these beneficial effects seemed to fade within sixty days [96]. A follow-up on this study was published in 2013, showing that multiple doses of BMDC prevent the disease progression. Two infusions of bone marrow cells reduced inflammation (fractional area of granuloma and number of total and M1 macrophages), lung remodeling (TGF-β level, deposition of collagen, and elastic fibers), and apoptosis (caspase-3 level and number of apoptotic cells). All these effects result in improvement of lung mechanics parameters. An increased level of IL-1Ra seems to have a role in the sustained cell therapy effects for a longer time in the same model of silicosis [36].
In 2011, a study using the whole pool of bone marrow-derived mononuclear cells (BMMC) in mice showed prophylactic properties on silica-induced lung damage. The systemic infusion of BMMC reduced the mRNA expression of caspase-3, IL-1β, IL-1α, and TGF-β [97]. Furthermore, therapeutic treatment with BMMC in late stages of silicosis was able to reduce lung fibrosis and improve lung function but did not succeed in reversing the inflammation. A reduction in the number of macrophages was accompanied by an expansion of T regulatory cells, which maintained the cellular infiltration, although switching to a different inflammatory profile [16]. BMMC offer the advantages of autologous transplantation, which minimize the possibility of rejection and harvesting and infusion at the same day, without in vitro culture expansion.
These results encouraged us to advance to a phase I clinical trial to test the safety of the autologous transplantation of bone marrow mononuclear cells in silicotic patients. Five patients underwent BMMC instillation through bronchoscopy. No adverse effects were observed in these patients up to a year after treatment. Additionally, an early increase in lung perfusion was observed after treatment [54].
The disadvantages of the use of bone marrow mononuclear cells are the invasiveness of the procedure—especially considering that multiple doses may be required for sustained effects—and the variability of the constitution of the bone marrow. Chronic inflammatory diseases can affect the proportions of bone marrow cell populations [101, 102], making autologous transplantation possibly heterogenic over different patients and stages of the disease.
As an alternative to that, mesenchymal cells could constitute an interesting option for cell therapy in silicosis. Zhao and collaborators showed that bone marrow-derived mesenchymal cells (BMSC) promote amelioration of fibrosis and inflammation in a rat model of silicosis, with possible similar mechanisms to those associated with BMMC therapy: suppression of IL1 signaling through IL1-Ra and decrease in TNF-α expression [99].
Results from a clinical trial using mesenchymal stromal cells and a genetically modified culture of mesenchymal cells were recently published [53]. In this study, the administration of MSC did not cause any adverse reactions, and after six months oxyhemoglobin saturation in the blood of the patients suggests improvement of gas exchange in the lungs. In some of the patients, a decrease in silica nodules' numbers was also observed.
Another view on the mechanisms of action of mesenchymal cell therapy in silica-induced lung fibrosis has been proposed by Choi et al. in 2014. The authors suggest that extracellular vesicles released by BMSC are vectors of the therapeutic effect and could be used as a treatment by themselves [100].
Additionally, cell therapy with embryonic cells, as a bioengineering approach for the reconstitution of injured epithelium has been investigated in a model of silica-induced injury and was able to prevent fibrosis and decrease mortality [98].
5. Conclusion
In view of the lack of effective therapeutic interventions for silicosis, cell-based therapy constitutes a promising treatment for silicosis. The key effects of this therapy include the decrease of deleterious proinflammatory and profibrotic processes, reduction of apoptosis, and enhancement of repair following lung damage. These beneficial effects appear to be independent of cell engraftment, but due to paracrine/endocrine action (e.g., secretion of anti-inflammatory, antifibrotic mediators, and extracellular vesicles). Considering the wide range of cell therapy options, optimization of cell therapy protocols and advancements in clinical trials can lead to an important breakthrough for the management of the patients affected by this persistent disease.
Acknowledgments
The Brazilian Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Level Personnel (CAPES), and the Carlos Chagas Filho Rio de Janeiro State Research Foundation (FAPERJ) supported this work.
Disclosure
The funders have no role in the decision to publish or prepare the paper.
Conflict of Interests
The authors declare no conflict of interests.
Authors' Contribution
Miquéias Lopes-Pacheco and Elga Bandeira have equal contribution to this work.
References
- 1.Leung C. C., Yu I. T. S., Chen W. Silicosis. The Lancet. 2012;379(9830):2008–2018. doi: 10.1016/s0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 2.Hnizdo E., Murray J. Risk of pulmonary tuberculosis relative to silicosis and exposure to silica dust in South African gold miners. Occupational and Environmental Medicine. 1998;55(7):496–502. doi: 10.1136/oem.55.7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hnizdo E., Vallyathan V. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence. Occupational and Environmental Medicine. 2003;60(4):237–243. doi: 10.1136/oem.60.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filho M. T., Santos U. D. E. P. Silicose. Jornal Brasileiro de Pneumologia. 2006;32:41–47. doi: 10.1590/s1806-37132006000800008. [DOI] [PubMed] [Google Scholar]
- 5.Brown T. Silica exposure, smoking, silicosis and lung cancer-complex interactions. Occupational Medicine. 2009;59(2):89–95. doi: 10.1093/occmed/kqn171. [DOI] [PubMed] [Google Scholar]
- 6.Lo Re S., Yakoub Y., Devosse R., et al. Uncoupling between inflammatory and fibrotic responses to silica: evidence from MyD88 knockout mice. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0099383.e99383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W., Liu Y., Wang H., et al. Long-term exposure to silica dust and risk of total and cause-specific mortality in Chinese workers: a cohort study. PLoS Medicine. 2012;9(4) doi: 10.1371/journal.pmed.1001206.e1001206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holanda M. A., Holanda M. A., Martins M. P. S., Felismino P. H., Pinheiro V. G. F. Silicosis in Brazilian pit diggers: relationship between dust exposure and radiologic findings. American Journal of Industrial Medicine. 1995;27(3):367–378. doi: 10.1002/ajim.4700270306. [DOI] [PubMed] [Google Scholar]
- 9.Carneiro A. P. S., Barreto S. M., Siqueira A. L., Cavariani F., Forastiere F. Continued exposure to silica after diagnosis of silicosis in Brazilian gold miners. American Journal of Industrial Medicine. 2006;49(10):811–818. doi: 10.1002/ajim.20379. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro F. S. N., De Camargo E. A., Algranti E., Wunsch Filho V. Occupational exposure to silica in Brazil in 2001. Revista Brasileira de Epidemiologia. 2008;11(1):89–96. doi: 10.1590/S1415-790X2008000100008. [DOI] [Google Scholar]
- 11.Jindal S. K. Silicosis in India: past and present. Current Opinion in Pulmonary Medicine. 2013;19(2):163–168. doi: 10.1097/MCP.0b013e32835bb19e. [DOI] [PubMed] [Google Scholar]
- 12.Kauppinen T. Occupational exposure to carcinogens in the European Union. Occupational and Environmental Medicine. 2000;57(1):10–18. doi: 10.1136/oem.57.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linch K. D., Miller W. E., Althouse R. B., Groce D. W., Hale J. M. Surveillance of respirable crystalline silica dust using OSHA compliance data (1979–1995) American Journal of Industrial Medicine. 1998;34(6):547–558. doi: 10.1002/(sici)1097-0274(199812)34:6lt;547::aid-ajim2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Alonso A., Córdoba-Doña J. A., Millares-Lorenzo J. L., Figueroa-Murillo E., García-Vadillo C., Romero-Morillo J. Outbreak of silicosis in Spanish quartz conglomerate workers. International Journal of Occupational and Environmental Health. 2014;20(1):26–32. doi: 10.1179/2049396713Y.0000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss D. J. Stem cells, cell therapies, and bioengineering in lung biology and diseases. Comprehensive review of the recent literature 2010–2012. Annals of the American Thoracic Society. 2013;10(5):S45–S97. doi: 10.1513/annalsats.201304-090aw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes-Pacheco M., Ventura T. G., de Oliveira H. D., et al. Infusion of bone marrow mononuclear cells reduces lung fibrosis but not inflammation in the late stages of murine silicosis. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0109982.e109982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz L. A., Gambelli F., McBride C., et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuller-Levis G., Gordon R. E., Wang C., Park S. Y., Park E. Protection of bleomycin-induced fibrosis and inflammation by taurine. International Immunopharmacology. 2009;9(7-8):971–977. doi: 10.1016/j.intimp.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Cruz F. F., Antunes M. A., Abreu S. C., et al. Protective effects of bone marrow mononuclear cell therapy on lung and heart in an elastase-induced emphysema model. Respiratory Physiology and Neurobiology. 2012;182(1):26–36. doi: 10.1016/j.resp.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton R. F., Jr., Thakur S. A., Holian A. Silica binding and toxicity in alveolar macrophages. Free Radical Biology and Medicine. 2008;44(7):1246–1258. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuda A., Henry F. S., Butler J. P. Particle transport and deposition: basic physics of particle kinetics. Comprehensive Physiology. 2013;3(4):1437–1471. doi: 10.1002/cphy.c100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castranova V. From coal mine dust to quartz: mechanisms of pulmonary pathogenicity. Inhalation Toxicology. 2000;12(3):7–14. doi: 10.1080/08958378.2000.11463226. [DOI] [PubMed] [Google Scholar]
- 23.Castranova V., Vallyathan V. Silicosis and coal workers' pneumoconiosis. Environmental Health Perspectives. 2000;108(4):675–684. doi: 10.1289/ehp.00108s4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huaux F. New developments in the understanding of immunology in silicosis. Current Opinion in Allergy and Clinical Immunology. 2007;7(2):168–173. doi: 10.1097/aci.0b013e32802bf8a5. [DOI] [PubMed] [Google Scholar]
- 25.Joshi G. N., Knecht D. A. Silica phagocytosis causes apoptosis and necrosis by different temporal and molecular pathways in alveolar macrophages. Apoptosis. 2013;18(3):271–285. doi: 10.1007/s10495-012-0798-y. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg M. I., Waksman J., Curtis J. Silicosis: a review. Disease-a-Month. 2007;53(8):394–416. doi: 10.1016/j.disamonth.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Fubini B., Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radical Biology and Medicine. 2003;34(12):1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 28.Blackford J. A., Jr., Antonini J. M., Castranova V., Dey R. D. Intratracheal instillation of silica up-regulates inducible nitric oxide synthase gene expression and increases nitric oxide production in alveolar macrophages and neutrophils. American Journal of Respiratory Cell and Molecular Biology. 1994;11(4):426–431. doi: 10.1165/ajrcmb.11.4.7522485. [DOI] [PubMed] [Google Scholar]
- 29.Zeidler P. C., Hubbs A., Battelli L., Castranova V. Role of inducible nitric oxide synthase-derived nitric oxide in silica-induced pulmonary inflammation and fibrosis. Journal of Toxicology and Environmental Health—Part A. 2004;67(13):1001–1026. doi: 10.1080/15287390490447296. [DOI] [PubMed] [Google Scholar]
- 30.Porter D. W., Millecchia L., Robinson V. A., et al. Enhanced nitric oxide and reactive oxygen species production and damage after inhalation of silica. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2002;283(2):L485–L493. doi: 10.1152/ajplung.00427.2001. [DOI] [PubMed] [Google Scholar]
- 31.Ding M., Chen F., Shi X., Yucesoy B., Mossman B., Vallyathan V. Diseases caused by silica: mechanisms of injury and disease development. International Immunopharmacology. 2002;2(2-3):173–182. doi: 10.1016/s1567-5769(01)00170-9. [DOI] [PubMed] [Google Scholar]
- 32.Ding M., Shi X., Dong Z., et al. Freshly fractured crystalline silica induces activator protein-1 activation through ERKs and p38 MAPK. The Journal of Biological Chemistry. 1999;274(43):30611–30616. doi: 10.1074/jbc.274.43.30611. [DOI] [PubMed] [Google Scholar]
- 33.Di Giuseppe M., Gambelli F., Hoyle G. W., et al. Systemic inhibition of NF-κB activation protects from silicosis. PLoS ONE. 2009;4(5) doi: 10.1371/journal.pone.0005689.e5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satpathy S. R., Jala V. R., Bodduluri S. R., et al. Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth. Nature Communications. 2015;6, article 7064 doi: 10.1038/ncomms8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misson P., van den Brûle S., Barbarin V., Lison D., Huaux F. Markers of macrophage differentiation in experimental silicosis. Journal of Leukocyte Biology. 2004;76(5):926–932. doi: 10.1189/jlb.0104019. [DOI] [PubMed] [Google Scholar]
- 36.Lopes-Pacheco M., Xisto D. G., Ornellas F. M., et al. Repeated administration of bone marrow-derived cells prevents disease progression in experimental silicosis. Cellular Physiology and Biochemistry. 2013;32(6):1681–1694. doi: 10.1159/000356603. [DOI] [PubMed] [Google Scholar]
- 37.Poljakovic M., Porter D. W., Millecchia L., et al. Cell- and isoform-specific increases in arginase expression in acute silica-induced pulmonary inflammation. Journal of Toxicology and Environmental Health A. 2007;70(2):118–127. doi: 10.1080/15287390600755075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murthy S., Larson-Casey J. L., Ryan A. J., He C., Kobzik L., Carter A. B. Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. The FASEB Journal. 2015;29(8):3527–3536. doi: 10.1096/fj.15-271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biswas R., Hamilton R. F., Holian A. Role of lysosomes in silica-induced inflammasome activation and inflammation in absence of MARCO. Journal of Immunology Research. 2014;2014:10. doi: 10.1155/2014/304180.304180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peeters P. M., Perkins T. N., Wouters E. F. M., Mossman B. T., Reynaert N. L. Silica induces NLRP3 inflammasome activation in human lung epithelial cells. Particle and Fibre Toxicology. 2013;10, article 3 doi: 10.1186/1743-8977-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava K. D., Rom W. N., Jagirdar J., Ting-An Y. I. E., Gordon T., Tchou-Wong K.-M. Crucial role of interleukin-1β and nitric oxide synthase in silica-induced inflammation and apoptosis in mice. American Journal of Respiratory and Critical Care Medicine. 2002;165(4):527–533. doi: 10.1164/ajrccm.165.4.2106009. [DOI] [PubMed] [Google Scholar]
- 42.Arend W. P., Gabay C. Physiologic role of interleukin-1 receptor antagonist. Arthritis Research. 2000;2(4):245–248. doi: 10.1186/ar94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yucesoy B., Vallyathan V., Landsittel D. P., et al. Polymorphisms of the IL-1 gene complex in coal miners with silicosis. American Journal of Industrial Medicine. 2001;39(3):286–291. doi: 10.1002/1097-0274(200103)39:3<286::AID-AJIM1016>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Vanhee D., Gosset P., Boitelle A., Wallaert B., Tonnel A. B. Cytokines and cytokine network in silicosis and coal workers' pneumoconiosis. European Respiratory Journal. 1995;8(5):834–842. [PubMed] [Google Scholar]
- 45.Wynn T. A., Ramalingam T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Medicine. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borges V. M., Falcão H., Leite-Júnior J. H., et al. Fas ligand triggers pulmonary silicosis. Journal of Experimental Medicine. 2001;194(2):155–164. doi: 10.1084/jem.194.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borges V. M., Lopes M. F., Falcão H., et al. Apoptosis underlies immunopathogenic mechanisms in acute silicosis. American Journal of Respiratory Cell and Molecular Biology. 2002;27(1):78–84. doi: 10.1165/ajrcmb.27.1.4717. [DOI] [PubMed] [Google Scholar]
- 48.Jagirdar J., Begin R., Dufresne A., Goswami S., Lee T. C., Rom W. N. Transforming growth factor-beta (TGF-beta) in silicosis. American Journal of Respiratory and Critical Care Medicine. 1996;154(4):1076–1081. doi: 10.1164/ajrccm.154.4.8887610. [DOI] [PubMed] [Google Scholar]
- 49.Mariani T. J., Crouch E., Roby J. D., Starcher B., Pierce R. A. Increased elastin production in experimental granulomatous lung disease. The American Journal of Pathology. 1995;147(4):988–1000. [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Ramos J., Segura-Valdez M. D. L., Vanda B., Selman M., Pardo A. Matrix metalloproteinases 2, 9, and 13, and tissue inhibitors of metalloproteinases 1 and 2 in experimental lung silicosis. American Journal of Respiratory and Critical Care Medicine. 1999;160(4):1274–1282. doi: 10.1164/ajrccm.160.4.9808006. [DOI] [PubMed] [Google Scholar]
- 51.Scabilloni J. F., Wang L., Antonini J. M., Roberts J. R., Castranova V., Mercer R. R. Matrix metalloproteinase induction in fibrosis and fibrotic nodule formation due to silica inhalation. American Journal of Physiology: Lung Cellular and Molecular Physiology. 2005;288(4):L709–L717. doi: 10.1152/ajplung.00034.2004. [DOI] [PubMed] [Google Scholar]
- 52.Yao S.-Q., Rojanasakul L. W., Chen Z.-Y., et al. Fas/FasL pathway-mediated alveolar macrophage apoptosis involved in human silicosis. Apoptosis. 2011;16(12):1195–1204. doi: 10.1007/s10495-011-0647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W. W., Wang H. X., Yu W., et al. Treatment of silicosis with hepatocyte growth factor-modified autologous bone marrow stromal cells: a non-randomized study with follow-up. Genetics and Molecular Research. 2015;14(3):10672–10681. doi: 10.4238/2015.september.9.7. [DOI] [PubMed] [Google Scholar]
- 54.Morales M. M., Souza S. A., Loivos L. P., et al. Pilot safety study of intrabronchial instillation of bone marrow-derived mononuclear cells in patients with silicosis. BMC Pulmonary Medicine. 2015;15, article 66 doi: 10.1186/s12890-015-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma S. K., Pande J. N., Verma K. Effect of prednisolone treatment in chronic silicosis. American Review of Respiratory Disease. 1991;143(4):814–821. doi: 10.1164/ajrccm/143.4_pt_1.814. [DOI] [PubMed] [Google Scholar]
- 56.Goodman G. B., Kaplan P. D., Stachura I., Castranova V., Pailes W. H., Lapp N. L. Acute silicosis responding to corticosteroid therapy. Chest. 1992;101(2):366–370. doi: 10.1378/chest.101.2.366. [DOI] [PubMed] [Google Scholar]
- 57.Dubois F., Bégin R., Cantin A., et al. Aluminum inhalation reduces silicosis in a sheep model. American Review of Respiratory Disease. 1988;137(5):1172–1179. doi: 10.1164/ajrccm/137.5.1172. [DOI] [PubMed] [Google Scholar]
- 58.Mccartney E. Aluminium therapy of silicosis. British Medical Journal. 1946;1:839–840. [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy M. C. Aluminium powder inhalations in the treatment of silicosis of pottery workers and pneumoconiosis of coal-miners. British Journal of Industrial Medicine. 1956;13(2):85–101. doi: 10.1136/oem.13.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlipköter H. W. Possibilities of causal prophylaxis and therapy of pneumoconiosis. Archives of Environmental Health. 1970;21(2):181–191. doi: 10.1080/00039896.1970.10667218. [DOI] [PubMed] [Google Scholar]
- 61.Zhao J., Liu J., Li G. Long-term follow-up observations of the therapeutic effect of PVNO on human silicosis. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene Serie B. 1983;178:259–262. [PubMed] [Google Scholar]
- 62.Idec-Sadkowska I., Andrzejak R., Antonowicz-Juchniewicz J., Kaczmarek-Wdowiak B. Trials of casual treatment of silicosis. Medycyna Pracy. 2006;57(3):271–280. [PubMed] [Google Scholar]
- 63.Xie Q.-M., Tang H.-F., Chen J.-Q., Bian R.-L. Pharmacological actions of tetrandrine in inflammatory pulmonary diseases. Acta Pharmacologica Sinica. 2002;23(12):1107–1113. [PubMed] [Google Scholar]
- 64.Guo J., Gu N., Chen J., et al. Neutralization of interleukin-1 beta attenuates silica-induced lung inflammation and fibrosis in C57BL/6 mice. Archives of Toxicology. 2013;87(11):1963–1973. doi: 10.1007/s00204-013-1063-z. [DOI] [PubMed] [Google Scholar]
- 65.Guo J., Shi T., Cui X., et al. Effects of silica exposure on the cardiac and renal inflammatory and fibrotic response and the antagonistic role of interleukin-1 β in C57BL/6 mice. Archives of Toxicology. 2016;90(2):247–258. doi: 10.1007/s00204-014-1405-5. [DOI] [PubMed] [Google Scholar]
- 66.Piguet P. F., Vesin C., Grau G. E., Thompson R. C. Interleukin 1 receptor antagonist (IL-1ra) prevents or cures pulmonary fibrosis elicited in mice by bleomycin or silica. Cytokine. 1993;5(1):57–61. doi: 10.1016/1043-4666(93)90024-Y. [DOI] [PubMed] [Google Scholar]
- 67.Cavalli G., Fallanca F., Dinarello C. A., Dagna L. Treating pulmonary silicosis by blocking Interleukin 1. American Journal of Respiratory and Critical Care Medicine. 2015;191(5):596–598. doi: 10.1164/rccm.201412-2150le. [DOI] [PubMed] [Google Scholar]
- 68.Stafford M., Cappa A., Weyant M., et al. Treatment of acute silicoproteinosis by whole-lung lavage. Seminars in Cardiothoracic and Vascular Anesthesia. 2013;17(2):152–159. doi: 10.1177/1089253213486524. [DOI] [PubMed] [Google Scholar]
- 69.Mason G. R., Abraham J. L., Hoffman L., Cole S., Lippmann M., Wasserman K. Treatment of mixed-dust pneumoconiosis with whole lung lavage. American Review of Respiratory Disease. 1982;126(6):1102–1107. doi: 10.1164/arrd.1982.126.6.1102. [DOI] [PubMed] [Google Scholar]
- 70.Schuyler M. R., Gaumer H. R., Stankus R. P., Kaimal J., Hoffmann E., Salvaggio J. E. Bronchoalveolar lavage in silicosis: evidence of type II cell hyperplasia. Lung. 1979;157(1):95–102. doi: 10.1007/BF02713602. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y., Chen J., Dong J., Liu W. Antifibrotic effect of interferon gamma in silicosis model of rat. Toxicology Letters. 2005;155(3):353–360. doi: 10.1016/j.toxlet.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Kaw J. L., Zaidi S. H. Effect of ascorbic acid on pulmonary silicosis of guinea pigs. Archives of Environmental Health. 1969;19(1):74–82. doi: 10.1080/00039896.1969.10666807. [DOI] [PubMed] [Google Scholar]
- 73.Levene C. I., Bye I., Saffiotti U. The effect of beta-aminopropionitrile on silicotic pulmonary fibrosis in the rat. British Journal of Experimental Pathology. 1968;49(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- 74.Li X.-F., Liao J., Xin Z.-Q., Lu W.-Q., Liu A.-L. Relaxin attenuates silica-induced pulmonary fibrosis by regulating collagen type I and MMP-2. International Immunopharmacology. 2013;17(3):537–542. doi: 10.1016/j.intimp.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 75.Sato T., Shimosato T., Alvord W. G., Klinman D. M. Suppressive oligodeoxynucleotides inhibit silica-induced pulmonary inflammation. Journal of Immunology. 2008;180(11):7648–7654. doi: 10.4049/jimmunol.180.11.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharawy M. H., El-Agamy D. S., Shalaby A. A., Ammar E.-S. M. Protective effects of methyl palmitate against silica-induced pulmonary fibrosis in rats. International Immunopharmacology. 2013;16(2):191–198. doi: 10.1016/j.intimp.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L., He Y., Li Q., et al. N-acetylcysteine alleviated silica-induced lung fibrosis in rats by down-regulation of ROS and mitochondrial apoptosis signaling. Toxicology Mechanisms and Methods. 2013;24(3):212–219. doi: 10.3109/15376516.2013.879974. [DOI] [PubMed] [Google Scholar]
- 78.Cruz F. F., Horta L. F. B., Maia L. D. A., et al. Dasatinib reduces lung inflammation and fibrosis in acute experimental silicosis. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147005.e0147005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wollin L., Maillet I., Quesniaux V., Holweg A., Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. The Journal of Pharmacology and Experimental Therapeutics. 2014;349(2):209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 80.Ferreira T. P. T., De Arantes A. C. S., Do Nascimento C. V. M. F., et al. IL-13 immunotoxin accelerates resolution of lung pathological changes triggered by silica particles in mice. Journal of Immunology. 2013;191(10):5220–5229. doi: 10.4049/jimmunol.1203551. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y., Li C., Weng D., et al. Neutralization of interleukin-17A delays progression of silica-induced lung inflammation and fibrosis in C57BL/6 mice. Toxicology and Applied Pharmacology. 2014;275(1):62–72. doi: 10.1016/j.taap.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 82.Ji X., Wu B., Fan J., et al. The anti-fibrotic effects and mechanisms of microRNA-486-5p in pulmonary fibrosis. Scientific Reports. 2015;5 doi: 10.1038/srep14131.14131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Dai W., Wang Y., Gu Q., Yang D., Zhang M. Blocking the Wnt/β-catenin pathway by lentivirus-mediated short hairpin RNA targeting β-catenin gene suppresses silica-induced lung fibrosis in mice. International Journal of Environmental Research and Public Health. 2015;12(9):10739–10754. doi: 10.3390/ijerph120910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartert M., Senbaklavacin O., Gohrbandt B., Fischer B. M., Buhl R., Vahld C.-F. Lung transplantation: a treatment option in end-stage lung disease. Deutsches Ärzteblatt International. 2014;111(7):107–116. doi: 10.3238/arztebl.2014.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas E. D., Phillips J. H., Finch C. A. Recovery from marrow failure following isogenic marrow infusion. The Journal of the American Medical Association. 1964;188:1041–1043. doi: 10.1001/jama.1964.03060380009002. [DOI] [PubMed] [Google Scholar]
- 86.Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 87.da Silva Meirelles L., Chagastelles P. C., Nardi N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 88.da Silva Meirelles L., Fontes A. M., Covas D. T., Caplan A. I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine and Growth Factor Reviews. 2009;20(5-6):419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Krause D. S. Bone marrow-derived cells and stem cells in lung repair. Proceedings of the American Thoracic Society. 2008;5(3):323–327. doi: 10.1513/pats.200712-169DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ratajczak M. Z. Phenotypic and functional characterization of hematopoietic stem cells. Current Opinion in Hematology. 2008;15(4):293–300. doi: 10.1097/moh.0b013e328302c7ca. [DOI] [PubMed] [Google Scholar]
- 91.Caplan A. I. Mesenchymal stem cells. Journal of Orthopaedic Research. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 92.Abreu S. C., Antunes M. A., de Castro J. C., et al. Bone marrow-derived mononuclear cells vs. Mesenchymal stromal cells in experimental allergic asthma. Respiratory Physiology and Neurobiology. 2013;187(2):190–198. doi: 10.1016/j.resp.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 93.Cruz F. F., Borg Z. D., Goodwin M., et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Translational Medicine. 2015;4(11):1302–1316. doi: 10.5966/sctm.2014-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moodley Y., Atienza D., Manuelpillai U., et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. The American Journal of Pathology. 2009;175(1):303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weiss D. J. Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2014;32(1):16–25. doi: 10.1002/stem.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lassance R. M. R., Prota L. F. M., Maron-Gutierrez T., et al. Intratracheal instillation of bone marrow-derived cell in an experimental model of silicosis. Respiratory Physiology & Neurobiology. 2009;169(3):227–233. doi: 10.1016/j.resp.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Maron-Gutierrez T., Castiglione R. C., Xisto D. G., et al. Bone marrow-derived mononuclear cell therapy attenuates silica-induced lung fibrosis. European Respiratory Journal. 2011;37(5):1217–1225. doi: 10.1183/09031936.00205009. [DOI] [PubMed] [Google Scholar]
- 98.Spitalieri P., Quitadamo M. C., Orlandi A., et al. Rescue of murine silica-induced lung injury and fibrosis by human embryonic stem cells. European Respiratory Journal. 2012;39(2):446–457. doi: 10.1183/09031936.00005511. [DOI] [PubMed] [Google Scholar]
- 99.Zhao M.-M., Cui J.-Z., Cui Y., et al. Therapeutic effect of exogenous bone marrow-derived mesenchymal stem cell transplantation on silicosis via paracrine mechanisms in rats. Molecular Medicine Reports. 2013;8(3):741–746. doi: 10.3892/mmr.2013.1580. [DOI] [PubMed] [Google Scholar]
- 100.Choi M., Ban T., Rhim T. Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Molecules and Cells. 2014;37(2):133–139. doi: 10.14348/molcells.2014.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abreu S. C., Antunes M. A., Mendonça L., et al. Effects of bone marrow mononuclear cells from healthy or ovalbumin-induced lung inflammation donors on recipient allergic asthma mice. Stem Cell Research and Therapy. 2014;5, article 108 doi: 10.1186/scrt496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mannheimer E. G., Carvalho A. B., Quintanilha L. F., et al. Bone marrow cells obtained from cirrhotic rats do not improve function or reduce fibrosis in a chronic liver disease model. Clinical Transplantation. 2011;25(1):54–60. doi: 10.1111/j.1399-0012.2009.01191.x. [DOI] [PubMed] [Google Scholar]