Abstract
The introduction of trastuzumab therapy markedly improved the poor prognosis associated with HER2-amplified breast cancers. Despite this, the presence of primary and acquired resistance to trastuzumab treatment remains a significant common challenge. The identification of resistance mechanisms and the incorporation of new drugs that achieve a better blockade of HER family receptors signaling have resulted in improved outcomes. The phosphatidylinositol 3′-kinase/protein kinase B/mammalian target of rapamycin pathway, cross-talk with estrogen receptors, immune response, cell cycle control mechanisms, and other tyrosine kinase receptors such as insulin-like growth factor I receptor are potential pathways involved in trastuzumab resistance. Different therapeutic interventions targeting these pathways are currently under evaluation.
Keywords: HER2 overexpression, breast cancer, trastuzumab, resistance, biomarker
Introduction
Overexpression of HER2 (ErbB2) proto-oncogene is present in about 20%–30% of breast carcinomas (BCs). It confers a more aggressive phenotype and, historically, it was associated with a poor prognosis.1 HER2 belongs to the human epidermal growth factor receptor family, consisting of four transmembrane tyrosine kinase receptors (TKRs), namely, HER1–HER4.2 Each of these receptors has a similar structure, with an extracellular binding domain, a transmembrane segment, an intracellular tyrosine-kinase domain (except for HER3), and an intracellular C-terminal tail with multiple tyrosine residues. Ligand binding to the extracellular domain (ECD) induces dimerization of two receptors (homodimerization if two identical receptors, heterodimerization if not), which activates the tyrosine-kinase domains, phosphorylating the tyrosine residues of its binding partner.3 HER2 is distinct in having no known ligand, but it is the preferred dimerization partner of the remaining members of the HER family because it displays a high catalytic activity. Furthermore, its ECD can adopt an open conformation resembling a ligand-activated state. If HER2 is amplified, it can activate other HER family members in the absence of ligands. This reaction activates downstream signaling cascades that induce cell proliferation through the Ras-mitogen-activated protein kinases (MAPK) pathway and inhibits cell death through the phosphatidylinositol 3′-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway (Fig. 1).4 The incorporation of trastuzumab and, more recently, new drugs against HER2 to treatment of this disease has changed the natural course of HER2-positive BC.5,6 Trastuzumab acts by different mechanisms to inhibit cell growth as follows: prevention of HER2 dimerization, downregulation of the HER2 receptor by endocytic destruction of the receptor, accumulation of the cyclin-dependent kinase (CDK) inhibitor p27 and cell cycle arrest, induction of antibody-dependent cellular cytotoxicity, and inhibition of constitutive HER2 cleavage/shedding mediated by metalloproteases.7 In combination with chemotherapy, trastuzumab has been shown to increase overall survival (OS) in early8,9 and advanced5 BC with HER2 overexpression. Regardless, some patients experience tumor recurrence after an adjuvant treatment and, in the metastatic setting, most patients eventually experience disease progression. This fact reflects the existence of mechanisms of resistance to trastuzumab that will be reviewed below.
Figure 1.
Signal transduction by HER2 dimerization.
Methods
We identified studies of interest by conducting an electronic literature search in PubMed and conference proceedings of the American Society of Clinical Oncology, San Antonio Breast Cancer Conference, and the European Society for Medical Oncology. The following search terms were included: breast cancer, trastuzumab, resistance, pertuzumab, lapatinib, and trastuzumab-emtansine. The search was performed without filters and all years were included. We focused on summarizing those resistance mechanisms that have been evaluated in the clinical setting.
Mechanisms of Resistance to Trastuzumab
In the preclinical setting, several mechanisms of resistance to trastuzumab have been described. Some of them have been evaluated as prognostic factors and others as predictors associated with treatment benefit in prespecified studies in clinical trials performed in early and advanced disease. These studies have some limitations, such as the limited statistical power to allow multiple comparisons, the difficulty of obtaining adequate tumor samples from all patients, and the possible changes in expression and mutational profile, which a tumor can experience throughout its evolution.10 The last circumstance could be relevant in those trials performed in the relapse time with tumor samples available only from the primary tumor.
Drug resistance can be evidenced as a lack of positive response to therapy (intrinsic resistance) or as disease progression after an initial clinical benefit (acquired response). The mechanisms of intrinsic resistance to trastuzumab develop before therapy application. Most of them are related to an inactive target receptor (like truncated HER2 receptors lacking extracellular trastuzumab-binding domain) or alterations of target downstream components in the PI3K/Akt/mTOR signaling pathway. Acquired resistance mostly occurs as a consequence of alterations located on the target signaling level and involves an active target receptor. Upregulation of other TKRs or their ligands belongs to this group. However, some mechanisms have been described in both the groups.11
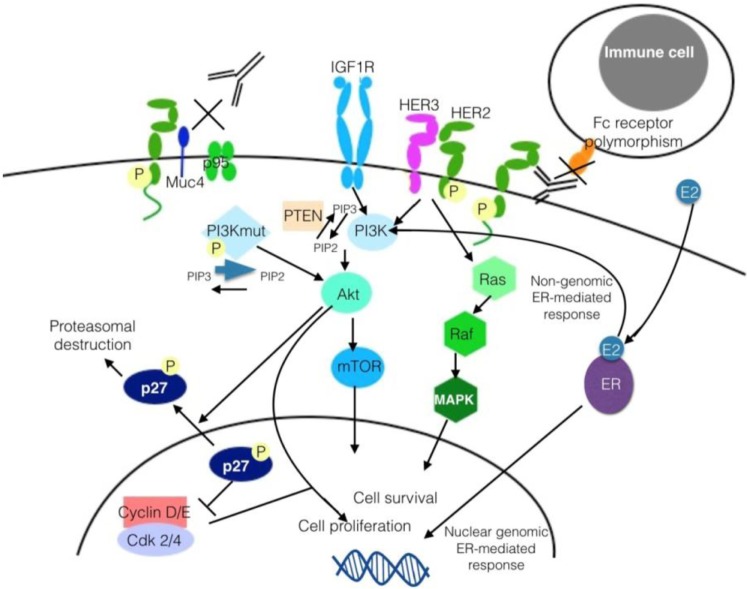
The different mechanisms have been grouped into the following categories (Fig. 2).
Figure 2.
Mechanisms of resistance to trastuzumab.
Abbreviations: Akt, protein kinase B; Cdk 2/4, cyclin-dependent kinase 2/4; E2, estradiol; ER, estrogen receptor; IGF1R, insulin-like growth factor I receptor; HER, human epidermal growth factor receptor; MAPK, Mitogen-activated protein kinases; mTOR, mammalian target of rapamycin; P, phosphorylation; PI3K, phosphatidylinositol 3′-kinase; PI3Kmut, mutated phosphatidylinositol 3′-kinase; PTEN, phosphatase and tensin homolog.
Escape from antibody-dependent cell-mediated cyto-toxicity
In 1992, Aaltomaa et al showed the relationship between lymphocytic infiltrate and increased survival in breast tumors of 489 patients with early disease.12 More recently, the percentage of tumor-associated lymphocytes was positively associated with a higher pathological complete response (pCR) rate to neoadjuvant chemotherapy based on anthracyclines and taxanes.13 The same was observed in a subanalysis of the GeparQuattro trial that added trastuzumab to neoadjuvant chemotherapy. A strong lymphocyte infiltrate was associated with a higher pCR rate in this trial.14 These observations reflect the relevance of immune response on cancer evolution. Immune response also plays a key role in the therapeutic activity of monoclonal antibodies (mAbs). Trastuzumab covers HER2 and, by binding to Fc receptors expressed on natural killer (NK) cells, antigen-presenting cells, or immune effector cells, it causes them to become active and lyse the antibody-coated tumor cell.15 This response is modulated by mAb binding, expression of different polymorphic receptors on immune cells, level of tumor antigen expression by tumor cells, concentration of mAb used, and the frequency and reactivity of immune cells in the tumor microenvironment.16 Three Fc -receptor polymorphisms were studied as predictive factors of trastuzumab response in 54 patients with advanced HER2-amplified BC. This retrospective study showed an association between a higher response rate (RR) and progression-free survival (PFS) and the FcRIIIa-158 valine (V)/phenylalanine (F), FcRIIa-131 histidine (H)/arginine (R), and FcRIIb-232 isoleucine (I)/threonine (T) polymorphisms.17 In contrast, other studies in the adjuvant setting did not show a correlation between these polymorphisms and disease-free survival (DFS) in patients treated with trastuzumab.14,18,19
In the neoadjuvant trial NeoSphere, expression of programmed death-1 receptor (PD-1) and its ligand, PD-L1, that negatively regulates T-cell activation, was associated with a lower pCR rate.20 A model to identify immune gene-enriched tumors was developed in a study with tumor samples of 1,282 patients enrolled in the trastuzumab adjuvant trial N9831. Those cases with a high expression of nine or more genes of the model had a longer relapse-free survival, but this association was seen only in patients who received trastuzumab, whereas there were no differences in the arm of chemo therapy alone.21
This mechanism suggests the therapeutic strategy of combining anti-HER2 therapies and an agent that can enhance immune response, such as anti-PD-1/-PD-L1 or anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) mAbs. Table 1 summarizes the ongoing clinical trials with immunotherapy and anti-HER2 agents.
Table 1.
Ongoing clinical trials with combinations of immunotherapy and anti-HER2 agents.
| STUDY | PHASE | SETTING | TREATMENT |
|---|---|---|---|
| PANACEA NCT02129556 | Phase Ib/II | Advanced disease | MK-3475 (mAb against PD-1) + Trastuzumab |
| NCT02605915 | Ib | locally advanced and metastatic disease | Atezolizumab + trastuzumab + pertuzumab or Atezolizumab + T-DM1 |
| PembroMab NCT02318901 | II | Metastatic disease | Pembrolizumab + T-DM1 |
Source: www.clinicaltrials.gov. Accessed November 20, 2015.
Expression of other TKRs and proteins in the cellular membrane
Expression of other members of the HER family
Trastuzumab may not be able to completely inhibit the signaling pathway because of redundant ligands and receptors that enable alternative dimerization patterns. In this category, epidermal growth factor receptor (EGFR/HER1) and HER3 are the receptors with a more significant role in trastuzumab resistance.
Coexpression of EGFR in HER2-overexpressed BC has been associated with worse survival in several retrospective series22–24 and in a subanalysis of the trastuzumab adjuvant trial N9831.25 Inhibition of both TKRs showed synergistic effects in vitro.26 Lapatinib is an orally active small molecule that reversibly inhibits ErbB1 and ErbB2 tyrosine kinases, leading to inhibition of MAPK and PI3K signaling.27 It showed in vitro activity and efficacy in HER2+ tumors that had progressed to trastuzumab.27,28 A phase III clinical trial comparing lapatinib and capecitabine or capecitabine alone for advanced HER2+ BC that had progressed to trastuzumab and a taxane showed a significant improvement in time to progression with the combination (hazard ratio [HR]) of 0.57 (95% confidence interval [CI], 0.43–0.77; P < 0.001).29 Combining lapatinib and trastuzumab improved results compared to lapatinib alone in trastuzumab-refractory metastatic patients, suggesting the potential of dual HER receptor blockade to inhibit the pathway activation.30 In the neoadjuvant setting, chemotherapy and dual blockade with trastuzumab and lapatinib significantly increased the pCR rate in the NeoALTTO trial (lapatinib and trastuzumab: 51.3%; 95% CI: 43.1–59.5 vs trastuzumab alone: 29.5%; 95% CI: 22.4–37.5; P = 0.0001; no significant differences between trastuzumab and lapatinib alone).31 This trial did not show differences in DFS or OS,32 but it was not powered to detect significant differences in survival outcomes. The ALTTO trial evaluated the efficacy of this dual blockade in the adjuvant setting. First results were reported at 4.5 years median follow-up with a lower than expected number of events. Despite a lower risk of a DFS event with the combination, differences were not statistically significant.33 A resistance mechanism to lapatinib belongs to this same category, since lapatinib-induced HER2 inhibition causes a compensatory PI3K/Akt- and FoxO3A-dependent upregulation of HER3.34
Although HER3 lacks a functioning kinase domain, it has six phosphotyrosine sites on its C-terminal tail, so that HER2–HER3 is the most potent dimer, in part, because phosphorylated HER3 augments signaling through PI3K/Akt/mTOR pathway.35 When HER2 is overexpressed, these heterodimers can be formed even in the absence of a ligand, in a distinct conformation.36 Anyway, HER3 ligands such as heregulin/neuregulin β1 play an important role in trastuzumab resistance. They can be produced by breast tumor cells, and its paracrine or autocrine secretion can trigger the formation of heterodimers HER2:HER3, which are incompletely blocked with trastuzumab.37,38 Pertuzumab is a mAb that binds to subdomain II (also known as dimerization site) of the ECD of HER2, preventing HER2 heterodimerization with HER1, HER3, or HER4.39 In the clinical setting, pertuzumab combined with trastuzumab showed an RR of 24.2% and a median PFS of 5.5 months in 66 patients with trastuzumab-resistant HER2+ BC.40 A second cohort of 29 patients who received pertuzumab monotherapy obtained an RR of only 3.4%. With the addition of trastuzumab, it increased to 17.6%, suggesting that the effect of combining two anti-HER receptor mAb results in an improved receptor blockade.41 Finally, this hypothesis has been confirmed in the CLEOPATRA study, a phase III trial that compared first-line treatment with docetaxel and trastuzumab versus both drugs plus pertuzumab in advanced HER2+ BC.42 The experimental arm showed a six-month improvement in PFS (18.5 vs 12.4 months, HR: 0.62; 95% CI, 0.51–0.75; P < 0.001)35 and OS increased to 15 months with pertuzumab (HR: 0.68; 95% CI, 0.56–0.84; P < 0.001), becoming the standard option for these patients.37,43
Expression of other membrane-associated receptors
The most studied receptor in this group is the insulin-like growth factor I receptor (IGF1R), a heterotetrameric transmembrane TKR widely expressed in normal tissues. Ligand binding to IGF1R activates the same pathways than HER family receptors, such as PI3K/Akt/mTOR and MAPK.44 Deregulation of IGF1R signaling appears in many solid tumors and it has been related to malignant transformation, making it a therapeutic target of interest.45,46 In HER2+ BC, overexpressed IGF1R can be recruited into signaling complexes with HER2 and HER3, which activate PI3K.47 It was associated with trastuzumab resistance in a subset of 155 patients with HER2+ metastatic BCs.24 Studies in vitro have shown synergistic effects of met-formin48 and also of figitumumab,49 a human mAb that blocks IGF1R ligand binding, with anti-HER2 drugs.
The Met TKR and its ligand, hepatocyte growth factor, are overexpressed in some HER2+ tumors. In vitro, its coexpression with HER2 was associated with trastuzumab resistance through sustained Akt activation.50 Another TKR, EphA2, has shown, when overexpressed, relationship with a worse prognosis. Treatment with trastuzumab seems to promote EphA2 phosphorylation by activating Src kinase, which increases signaling through PI3K/Akt and MAPK pathways, leading to trastuzumab resistance.51 Finally, use of recombinant human erythropoietin might be associated with trastuzumab resistance. Its binding to the receptor for erythropoietin can activate Src mediated by Jak2 and inactivate phosphatase and tensin homolog (PTEN).52
High level of cathecolamines has been reported in tumor microenvironment in breast cancer.53 Their signaling affects the expression of genes in tumor as well as mesenchymal and immune cells, and it is involved in cancer invasion and metastasis.54,55 The β2-adrenergic receptor (β2-AR) signaling pathway induces upregulation of the HER2 expression, and HER2 signaling, through activation or extracellular signal- regulated kinase, can enhance the synthesis of catheco-lamines.56 The positive feedback mechanism may promote the expression of both receptors and generate enhanced growth signaling. The expression of β2-AR, which mediates most catecholamine-induced effects, negatively correlates with trastuzumab response. This action seems to be mediated by the activation of PI3K/Akt/mTOR pathway.57 This mechanism suggests the evaluation of combination therapy with trastuzumab plus β-blocker in HER2-overexpressing BC.
Crosstalk between estrogen receptor and HER2 pathways
HER2 overexpression has been linked with resistance to tamoxifen in vitro58 and in vivo. Patients with estrogen receptor (ER) and HER2-positive advanced BC treated with endocrine therapy showed a lower RR compared with those without HER2 overexpression.46,47 Conversely, preclinical data showed that ER activity can function as an escape pathway in ER-positive/HER2-positive cells exposed to trastuzumab and lapatinib.59 In addition, those ER+ HER2+ tumors included in the neoadjuvant clinical trials with chemotherapy and anti-HER2 drugs obtained lower PCR rates as compared with those ER negative.31,60–62 These observations suggest the existence of a bidirectional cross-talk between both pathways, so that targeted therapy against a signaling pathway can be followed by tumor growth through the others. ER is mainly a nuclear receptor and functions as a ligand-dependent transcription factor that regulates expression of different genes, such as IGF1R, cyclin D1, bcl-2, VEGF-R, receptors of HER family, or ligands such as amphiregulin or TGF-α (estrogen genomic-signaling pathway).63
There is also a small pool of ER located in the cytoplasm and non-nuclear subcellular fractions. Activation of these ER increases the levels of cyclic adenosine monophosphate and other second messengers. This reaction can activate various TKRs such as IGF-IR, EGFR, and HER2 (estrogen non-genomic signaling pathway).64 This pool of ER can also interact with protein kinases, such as PI3K, and adaptor molecules, such as Src.65 Furthermore, different growth factor-dependent kinases can phosphorylate the ER and coregulators of the ER pathway, so the inhibitory action of endocrine therapies, mainly selective estrogen receptor modulators, can be weakened in the case of HER2 overexpression.66,67
Simultaneously inhibiting both HER2 and ER pathways has shown to be more effective than ER inhibition alone in the metastatic setting (Table 2), although neither trial has demonstrated an increase in OS.
Table 2.
Phase III clinical trials of hormone treatment and anti-HER2 agents.
| STUDY | TREATMENT ARMS | N | RR (CBR) | PFS (MONTHS) | OS (MONTHS) | COMMENTATION |
|---|---|---|---|---|---|---|
| TanDEM68 | Anastrozole Anastrozole + trastuzumab |
104 103 |
6,8% 20,3% P = 0.018 (27,9% vs 42,7%; P = 0.026) |
2,4 4,8 P = 0.016 |
23,9 28,5 P = 0.325 |
23–29, 8% negative hormone receptors in central review. Cross over between arms. 15% patients without progression after 2 years. |
| EGF3000869 | Letrozole + placebo Letrozole + lapatinib |
108 111 |
15% 28% P = 0.021 (29% vs 48%; P = 0.003) |
3 8,2 P = 0.019 |
33,3 32,3 |
10–15% patients without progression after 2 years. |
| eLEcTRA70 | Letrozole Letrozole + trastuzumab |
31 26 |
13% 27% P = 0.3124 (39% vs 65%; P = 0.0636) |
3,3 14,1 P = 0.23 |
Early closure because of slow recruitment. Differences in basal characteristics between arms. |
Intrinsic alterations in HER2
HER2 carboxy-terminal fragments, also known as p95HER2 fragments, are a subtype of HER2 receptors that are characterized by the lack of ECD, where the binding point of trastuzumab is located. These fragments can arise by the shedding of ECD by a metalloprotease (ADAM10) or by alternative initiation or translation of the mRNA-encoding HER2.71 Their expression has been associated with trastuzumab resistance in some retrospective studies72,73 but not in others,74,75 whereas it had no influence on lapatinib efficacy in a retrospective analysis of two clinical trials with lapatinib, such as EGF2000929 and EGF100151.76 The clinical trial CHER-LOB randomized 121 patients to receive neoadjuvant chemotherapy with trastuzumab, lapatinib, or their combination. In this study, expression of p95 was not associated with pCR overall and in each arm, and it did not predict for sensitivity to any treatment, although the authors recognized that the small size and the lack of a standardized p95-HER2 assay could be potential limitations of the analysis.77
A HER2 splice variant with enhanced transforming activity, HER2Δ16, has been described in BC cell lines and tumors. It is characterized by an imbalance in the number of cysteines in the ECD portion and by the constitutive generation of stable HER2 homodimers.78 Its appearance is a tumor-specific event, and it is associated with trastuzumab resistance. This role seems to be mediated by Src kinase, which can stabilize HER2Δ16 expression and couple it to multiple mitogenic and cell motility pathways, and can inactivate PTEN through phosphorylation.79 Then, dasatinib, a Src kinase family inhibitor, could be useful in this setting, although a phase II trial of 70 patients, 24 of them with HER2+ tumors previously treated with anti-HER2 agents, showed only 1 partial response in that subgroup.80
The Hsp90 chaperone complex is involved in the conformational maturation, stability, and activation of several oncoproteins,81 including HER2. Its inhibition induces pro-teasomal degradation of HER2, and this action is enhanced by the addition of trastuzumab.82 Hsp90 inhibition has shown antitumor activity in trastuzumab-sensitive and trastuzumab-resistant xenografts, so it may represent a novel therapeutic approach.83,84 This strategy was also effective in an in vivo tumor model that overexpressed p95HER2 and was resistant to trastuzumab.85
HER2 testing and quantification of HER2 expression
HER2 overexpression/amplification is a necessary condition for trastuzumab activity. Two diagnostic techniques are currently approved for assigning HER2 status in clinical practice as follows: immunohistochemistry (IHC) and in situ hybridization (ISH). Whereas IHC uses an antibody to evaluate HER2 protein expression, ISH determines the number of HER2 copies per nucleus only or as a dual-probe technique, where hybridization of a chromosome 17 centromere probe (chromosome enumeration probe 17, CEP17) allows determination of the HER2:CEP17 ratio.86 Some limitations for these methods are the identification of unusual HER2 geno-typic abnormalities, such as aneusomy of chromosome 17 (polysomy and monosomy), colocalization of HER2 and CEP17 signals that affect HER2/CEP17 ratio in dual signal ISH assays, and genomic heterogeneity. These situations are not considered resistance mechanisms, although they may influence treatment decisions. Retrospective data showed that elevated CEP17 (polysomy) count might account for trastuzumab response in tumors with normal HER2:CEP17 ratios.87,88 In these cases, an experts’ consensus suggests that confirmatory IHC can provide useful supporting information. Furthermore, when the mean HER2 copy number is ≥6, and the IHC score is 2+, tumors should be assessed as HER2+, irrespective of HER2:CEP17 ratio.89 Genomic heterogeneity has been defined by the College of American Pathologists according to the presence of >5% but <50% of infiltrating tumor cells with HER2 amplification by ISH.90 Its prevalence is around 11%–40% of HER2-amplified BCs, and two retrospective studies have suggested an association with worse prognosis.91,92 Clinical studies are still necessary to evaluate the potential benefit from trastuzumab.
In HER2-amplified BC, levels of HER2 expression, evaluated by quantification of mRNA or protein expression, have been associated with treatment benefit in the neoadjuvant studies TRYPHAENA93 and NeoSphere74 (in this study, only for ER-negative tumors), whereas a nonsignificant difference was found in the adjuvant trial N9831.94 In the CLEOPA-TRA trial, patients with high levels of HER2 protein, HER2 nRNA, and HER3 mRNA had a better prognosis than those with low levels in both treatment arms. A consistent PFS benefit from pertuzumab was shown, independent of expression levels of any of the markers, so they were not useful as predictive factors in this trial.95
Aberrant activation of PI3K/Akt/mTOR pathway
The PI3K/Akt pathway is a downstream signaling pathway than can be activated by HER2 and other TKR signaling. It can be constitutively activated by amplification or mutation of the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PI3KCA)96 or Akt1,97 or by mutation or loss of expression of tumor suppressors that inhibit the pathway, such as PTEN95 and inositol polyphosphate-4-phosphatase, type II (INPP4B).98 These events seem to be common, so that PI3KCA mutations, PTEN mutations/loss of expression, and INPP4B loss of expression were present, respectively, in 42%, 19%, and 30% of HER2-enriched tumors of the Cancer Genome Atlas Network report.99 Constitutive activation by one of these mechanisms has been associated with trastuzumab resistance nonuniformly. A recent meta-analysis that analyzed the predictive role of PI3KCA mutations, PTEN loss, and PI3K pathway activation only found a significant association between PTEN loss and trastuzumab resistance for advanced disease, although different assays and cutoffs used could explain the conflicting results.100
Data are available from studies with dual HER2 blockade: various neoadjuvant trials with a combination of trastuzumab and pertuzumab or trastuzumab and lapatinib showed better pCR rates in those patients without PI3KCA mutations.77,101 In the CLEOPATRA trial, tumors with PI3KCA mutations had a worse prognosis independent of the treatment arm.102
Trastuzumab-emtansine (T-DM1) is an antibody–drug conjugate with a complex compound obtained by the conjugation of trastuzumab, a stable thioether linker, and the potent cytotoxic drug maytansine derivate (DM1), which inhibits cell division and induces cell death.103 This new agent was compared with the combination of lapatinib and capecitabine for the treatment of metastatic patients who had progressed to trastuzumab and a taxane in the phase III trial EMILIA. Median PFS and OS were superior to T-DM1104 and, whereas patients with PI3KCA mutations had a worse outcome compared with wild-type tumors in the capecitabine–lapatinib arm, there were no differences in patients who received T-DM1.105 The effect of PI3KCA mutations on lapatinib response is concordant with the NeoALTTO results,101 but not with other small neoadjuvant studies, where no differences were observed and PTEN loss was even associated with higher pCR with lapatinib.106
An attempt to reverse this resistance mechanism was done with everolimus, an mTOR inhibitor, in the BOLERO-1 and BOLERO-3 trials. BOLERO-1 is a phase III trial in which first-line treatment for advanced disease was administered with trastuzumab plus weekly paclitaxel plus everolimus 10 mg once a day orally or placebo.107 BOLERO-3 is a phase III trial in which patients with HER2+ metastatic BC who had progressed to trastuzumab and a taxane were randomized to receive vinorelbine, trastuzumab, and everolimus 5 mg daily or placebo.108 In the full population, the BOLERO-1 trial did not show significant differences between both groups (median PFS 14.95 months in the everolimus group vs 14.49 months in the placebo group; HR 0.89 [95% CI, 0.73–1.08]; P = 0.1166). In contrast, median PFS was significantly longer in the everolimus group in the BOLERO-3 trial, although the difference was small (7 vs 5.78 months (HR 0.78 [95% CI, 0.65–0.95]; P = 0.0067). The BOLERO-1 population was treated in the first-line setting, unlike the BOLERO-3 population, consisting of patients with trastuzumab resistance disease. Everolimus dose was also different between trials, although the median relative dose intensity of everolimus was 0.54 in BOLERO-1. PI3K/Akt/mTOR pathway activation is a known resistance mechanism to trastuzumab, and it was related with more benefit from everolimus than those with lower PI3K/mTOR pathway activity in BOLERO-3. The proportion of patients with activation of this pathway is not reported in BOLERO-1. PFS in the HR negative population was a second primary efficacy endpoint in the BOLERO-1 trial. In this subgroup, a PFS benefit was seen for everolimus (20.27 vs 13.08 months; HR 0.66 [95% CI, 0.48–0.91]; P = 0.0049), although this did not cross the protocol specified threshold of significance (P = 0.0044). Although it was not an endpoint of the BOLERO-3 trial, analyses of specific subgroups were preplanned and the everolimus benefit was more pronounced in the HR negative subpopulation. The cross-talk between the HER2 and estrogen receptor pathways acts as an escape mechanism for trastuzumab in HR positive tumors, so it is unknown if the efficacy might be enhanced if the estrogen pathway is inhibited concomitantly.
Clinical trials combining PI3K/Akt pathway inhibitors and anti-HER2 agents are ongoing and they are summarized in Table 3.
Table 3.
Ongoing studies evaluating inhibitors of PI3K/Akt pathway in HER2 overexpressed breast cancer.
| STUDY | SETTING | PHASE | TARGET | TREATMENT ARMS |
|---|---|---|---|---|
| NeoPHOEBE NCT01816594 |
Neoadjuvant | II randomized | PI3K | Trastuzumab + paclitaxel + BKM120 Trastuzumab + paclitaxel + placebo |
| NCT02038010 | Advanced disease | I | T-DM1 + BYL719 | |
| NCT01471847 | Advanced disease | Ib/II | BEZ235 + paclitaxel (in phase II, the combination will be compared to capecitabine and lapatinib) | |
| NCT01132664 | Advanced disease | Ib/II | BKM120 + trastuzumab + capecitabine | |
| NCT01042925 | Advanced disease | I/II | XL147 (SAR245408) + trastuzumab + paclitaxel | |
| NCT01245205 | Advanced disease | I | Akt | MK2206 + lapatinib |
Source: www.clinicaltrials.gov. Accessed November 20, 2015.
Alterations in apoptosis and cell cycle control
One of the ultimate effects of HER2 signal activation is cell death inhibition, so it is reasonable that alterations in the apoptotic machinery can induce resistance to trastuzumab. For example, high levels of Bcl-2-like protein 11 (BIM), a member of the proapoptotic BH-only BCL2 family, have been associated to sensitivity to lapatinib.109 In vitro, the down-regulation of PTK6, a nonreceptor tyrosine kinase, in lapatinib-resistant HER2+ BC cells, can enhance the expression of BIM, inducing apoptosis.110 Overexpression of t-Darpp, a truncated form of the dual kinase/phosphatase inhibitor Darpp-32, has been linked to acquired resistance to trastu-zumab111 and to lapatinib, in this case related to impaired BIM accumulation.112
P27Kip1 is a CDK inhibitor that blocks cyclin E/CDK2 complexes, which induce cell cycle arrest. It can be phosphorylated by Akt and then targeted for proteasomal destruction, so cell cycle can progress. Amplification/overexpression of cyclin E has been associated with lower RR and PFS in a small study with 34 patients treated with trastuzumab.113
Conclusion
Breast cancer researchers have reached a deep knowledge of the HER2 pathway and the mechanism of action of trastuzumab, which have allowed developing new drugs with activity in resistance circumstances. Anyway, data suggest that continuing trastuzumab in combination with different chemotherapy agents following progression may be of additional clinical benefits.25,33 This fact suggests that, at least in some cases, resistance to trastuzumab is not complete and can be reversed by acting on the escape route. The known resistance mechanisms come mainly from in vitro studies and their usefulness as predictive or prognostic factors in different clinical studies is often discordant. In most of the studies, each bio-marker is evaluated individually, when it is likely that different mechanisms coexist, but controlling study results for all of them is a difficult challenge. Currently, no biomarker is ready to be used in daily practice to choose a particular anti-HER2 drug. Even so, this strategy has provided significant improvements in treatment outcomes of HER2 overexpressed BC, as demonstrated by the development of pertuzumab.
Footnotes
ACADEMIC EDITOR: William Chi-shing Cho, Editor in Chief
PEER REVIEW: Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1496 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Analyzed the data: ML, PG, YF. Wrote the first draft of the manuscript: ML. Contributed to the writing of the manuscript: PG, YF, IP, LS. Agree with manuscript results and conclusions: ML, PG, YF, IP, LS. Jointly developed the structure and arguments for the paper: ML, PG. Made critical revisions and approved final version: ML, PG, LS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Yarden Y. The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(suppl 4):S3–8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 3.Brennan PJ, Kumogai T, Berezov A, Murali R, Greene MI. HER2/Neu: mechanisms of dimerization/oligomerization. Oncogene. 2000;19:6093–101. doi: 10.1038/sj.onc.1203967. [DOI] [PubMed] [Google Scholar]
- 4.Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001;28(5 suppl 16):4–11. doi: 10.1016/s0093-7754(01)90276-3. [DOI] [PubMed] [Google Scholar]
- 8.Dahabreh IJ, Linardou H, Siannis F, Fountzilas G, Murray S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008;13(6):620–30. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 9.Yin W, Jiang Y, Shen Z, Shao Z, Lu J. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS One. 2011;6(6):e21030. doi: 10.1371/journal.pone.0021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato F, Saji S, Toi M. Genomic tumor evolution of breast cancer. Breast Cancer. 2015;23(1):4–11. doi: 10.1007/s12282-015-0617-8. [DOI] [PubMed] [Google Scholar]
- 11.Asić K. Dominant mechanisms of primary resistance differ from dominant mechanisms of secondary resistance to targeted therapies. Crit Rev Oncol Hematol. 2016;97:178–96. doi: 10.1016/j.critrevonc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A(4–5):859–64. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 14.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes (TILs) indicate trastuzumab benefit in early-stage HER2-positive breast cancer (HER2+ BC) Cancer Res. 2013;73(24 suppl):S1–05. [Google Scholar]
- 15.Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol. 2011;2011:379123. doi: 10.1155/2011/379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28(28):4390–9. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 18.Hurvitz SA, Betting DJ, Stern HM, et al. Analysis of Fcγ receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin Cancer Res. 2012;18(12):3478–86. doi: 10.1158/1078-0432.CCR-11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norton N, Olson RM, Pegram M, et al. Association studies of Fcγ receptor polymorphisms with outcome in HER2+ breast cancer patients treated with trastuzumab in NCCTG (Alliance) Trial N9831. Cancer Immunol Res. 2014;2(10):962–9. doi: 10.1158/2326-6066.CIR-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni L, Bianchini G, Valagussa P, et al. Adaptive immune system and immune checkpoints are associated with response to pertuzumab (P) and trastuzumab (H) in the NeoSphere Study. Cancer Res. 2012;72(24 suppl):S6–7. [Google Scholar]
- 21.Perez EA, Thompson EA, Ballman KV, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J Clin Oncol. 2015;33(7):701–8. doi: 10.1200/JCO.2014.57.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiGiovanna MP, Stern DF, Edgerton SM, Whalen SG, Moore D, II, Thor AD. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol. 2005;23(6):1152–60. doi: 10.1200/JCO.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 23.Nieto Y, Nawaz F, Jones RB, Shpall EJ, Nawaz S. Prognostic significance of overexpression and phosphorylation of epidermal growth factor receptor (EGFR) and the presence of truncated EGFRvIII in locoregionally advanced breast cancer. J Clin Oncol. 2007;25(28):4405–13. doi: 10.1200/JCO.2006.09.8822. [DOI] [PubMed] [Google Scholar]
- 24.Gallardo A, Lerma E, Escuin D, et al. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106(8):1367–73. doi: 10.1038/bjc.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng H, Ballman K, Vassilakopoulou M, et al. EGFR expression is associated with decreased benefit from trastuzumab in the NCCTG N9831 (Alliance) trial. Br J Cancer. 2014;111(6):1065–71. doi: 10.1038/bjc.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61(24):8887–95. [PubMed] [Google Scholar]
- 27.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41):6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 28.Rusnak DW, Affleck K, Cockerill SG, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res. 2001;61(19):7196–203. [PubMed] [Google Scholar]
- 29.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and bio-marker analyses. Breast Cancer Res Treat. 2008;112(3):533–43. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 30.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–30. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Bradbury I, Eidtmann H, et al. NeoALTTO Study Team Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137–46. doi: 10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- 33.Piccart-Gebhart MJ, Holmes AP, Baselga J, et al. First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC) J Clin Oncol. 2014;32:5s. suppl; abstr LBA4. [Google Scholar]
- 34.Garrett JT, Olivares MG, Rinehart C, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108:5021–6. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinkas-Kramarski R, Soussan L, Waterman H, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–67. [PMC free article] [PubMed] [Google Scholar]
- 36.Gala K, Chandarlapaty S. Molecular pathways: HER3 targeted therapy. Clin Cancer Res. 2014;20(6):1410–6. doi: 10.1158/1078-0432.CCR-13-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62:3151–8. [PubMed] [Google Scholar]
- 38.Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–61. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- 39.O’Sullivan CC, Connolly RM. Pertuzumab and its accelerated approval: evolving treatment paradigms and new challenges in the management of HER2- positive breast cancer. Oncology (Williston Park) 2014;28(3):186–94. [PubMed] [Google Scholar]
- 40.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28(7):1138–44. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortés J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(14):1594–600. doi: 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 42.Baselga J, Cortés J, Kim SB, et al. CLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swain SM, Baselga J, Kim SB, et al. CLEOPATRA Study Group Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surmacz E. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin-like growth factor I receptor. Oncogene. 2003;22:6589–97. doi: 10.1038/sj.onc.1206772. [DOI] [PubMed] [Google Scholar]
- 45.Hartog H, Wesseling J, Boezen HM, van der Graaf WT. The insulin-like growth factor 1 receptor in cancer: old focus, new future. Eur J Cancer. 2007;43(13):1895–904. doi: 10.1016/j.ejca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Jerome L, Shiry L, Leyland-Jones B. Deregulation of the IGF axis in cancer: epidemiological evidence and potential therapeutic interventions. Endocr Relat Cancer. 2003;10(4):561–78. doi: 10.1677/erc.0.0100561. [DOI] [PubMed] [Google Scholar]
- 47.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–28. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 48.Liu B, Fan Z, Edgerton SM, Yang X, Lind SE, Thor AD. Potent anti-prolifera-tive effects of metformin on trastuzumab resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle. 2011;10:2959–66. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- 49.Chakraborty AK, Zerillo C, DiGiovanna MP. In vitro and in vivo studies of the combination of IGF1R inhibitor figitumumab (CP-751,871) with HER2 inhibitors trastuzumab and neratinib. Breast Cancer Res Treat. 2015;152(3):533–44. doi: 10.1007/s10549-015-3504-2. [DOI] [PubMed] [Google Scholar]
- 50.Shattuck DL, Miller JK, Carraway KL, III, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68(5):1471–7. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang G, Brantley-Sieders DM, Vaught D, et al. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res. 2010;70(1):299–308. doi: 10.1158/0008-5472.CAN-09-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang K, Esteva FJ, Albarracin C, et al. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell. 2010;18(5):423–35. doi: 10.1016/j.ccr.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28:4094–9. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell JP, Karolak MR, Ma Y, et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi M, Liu D, Duan H, et al. The beta2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125:351–62. doi: 10.1007/s10549-010-0822-2. [DOI] [PubMed] [Google Scholar]
- 57.Liu D, Yang Z, Wang T, et al. β2-AR signaling controls trastuzumab resistance-dependent pathway. Oncogene. 2016;35(1):47–58. doi: 10.1038/onc.2015.58. [DOI] [PubMed] [Google Scholar]
- 58.Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24(2):85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 59.Wang YC, Morrison G, Gillihan R, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers – role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011;13(6):R121. doi: 10.1186/bcr3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright C, Nicholson S, Angus B, et al. Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer. 1992;65(1):118–21. doi: 10.1038/bjc.1992.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Houston SJ, Plunkett TA, Barnes DM, Smith P, Rubens RD, Miles DW. Over-expression of c-erbB2 is an independent marker of resistance to endocrine therapy in advanced breast cancer. Br J Cancer. 1999;79(7–8):1220–6. doi: 10.1038/sj.bjc.6690196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomized multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 63.Lee A, Oesterreich S. Crosstalk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res. 2001;7:4429s–35. [PubMed] [Google Scholar]
- 64.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estro-gen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–53. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 65.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphati-dylinositol-3-OH kinase. Nature. 2000;407:538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prat A, Baselga J. The role of hormonal therapy in the management of hormonal- receptor-positive breast cancer with co-expression of HER2. Nat Clin Pract Oncol. 2008;5(9):531–42. doi: 10.1038/ncponc1179. [DOI] [PubMed] [Google Scholar]
- 67.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29(2):217–33. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27(33):5529–37. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 69.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–46. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 70.Huober J, Fasching P, Paepke S, et al. Letrozole in combination with trastuzumab is superior to letrozole monotherapy as first line treatment in patients with hormone-receptor-positive, HER2-positive metastatic breast cancer (MBC) – results of the eLEcTRA trial. Cancer Res. 2009;69 doi: 10.1016/j.breast.2011.07.006. abstr4094. [DOI] [PubMed] [Google Scholar]
- 71.Arribas J, Baselga J, Pedersen K, Parra-Palau JL. p95HER2 and breast cancer. Cancer Res. 2011;71(5):1515–9. doi: 10.1158/0008-5472.CAN-10-3795. [DOI] [PubMed] [Google Scholar]
- 72.Scaltriti M, Rojo F, Ocaña A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 73.Sperinde J, Jin X, Banerjee J, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab treated breast cancer patients. Clin Cancer Res. 2010;16:4226–35. doi: 10.1158/1078-0432.CCR-10-0410. [DOI] [PubMed] [Google Scholar]
- 74.Duman BB, Sahin B, Acikalin A, Ergin M, Zorludemir S. PTEN, Akt, MAPK, p53 and p95 expression to predict trastuzumab resistance in HER2 positive breast cancer. J BUON. 2013;18(1):44–50. [PubMed] [Google Scholar]
- 75.Loibl S, Bruey G, Von Minckwitz G, et al. Validation of p95 as a predictive marker for trastuzumab-based therapy in primary HER2-positive breast cancer. J Clin Oncol. 2011;29(suppl) Abstr530. [Google Scholar]
- 76.Scaltriti M, Chandarlapaty S, Prudkin L, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16(9):2688–95. doi: 10.1158/1078-0432.CCR-09-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guarneri V, Dieci MV, Frassoldati A, et al. Prospective biomarker analysis of the randomized CHER-LOB study evaluating the dual anti-HER2 treatment with trastuzumab and lapatinib plus chemotherapy as neoadjuvant therapy for HER2-positive breast cancer. Oncologist. 2015;20(9):1001–10. doi: 10.1634/theoncologist.2015-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castiglioni F, Tagliabue E, Campiglio M, Pupa SM, Balsari A, Ménard S. Role of exon-16-deleted HER2 in breast carcinomas. Endocr Relat Cancer. 2006;13:221–32. doi: 10.1677/erc.1.01047. [DOI] [PubMed] [Google Scholar]
- 79.Mitra D, Brumlik MJ, Okamgba SU, et al. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther. 2009;8(8):2152–62. doi: 10.1158/1535-7163.MCT-09-0295. [DOI] [PubMed] [Google Scholar]
- 80.Mayer EL, Baurain JF, Sparano J, et al. A phase 2 trial of dasatinib in patients with advanced HER2-positive and/or hormone receptor-positive breast cancer. Clin Cancer Res. 2011;17(21):6897–904. doi: 10.1158/1078-0432.CCR-11-0070. [DOI] [PubMed] [Google Scholar]
- 81.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32(3):517–30. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 82.Modi S, Stopeck AT, Gordon MS, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25(34):5410–7. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 83.Scaltriti M, Serra V, Normant E, et al. Antitumor activity of the Hsp90 inhibitor IPI-504 in HER2-positive trastuzumab-resistant breast cancer. Mol Cancer Ther. 2011;10(5):817–24. doi: 10.1158/1535-7163.MCT-10-0966. [DOI] [PubMed] [Google Scholar]
- 84.Wainberg ZA, Anghel A, Rogers AM, et al. Inhibition of HSP90 with AUY922 induces synergy in HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol Cancer Ther. 2013;12(4):509–19. doi: 10.1158/1535-7163.MCT-12-0507. [DOI] [PubMed] [Google Scholar]
- 85.Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29(3):325–34. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolff AC, Hammond ME, Hicks DG, et al. American Society of Clinical Oncology. College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 87.Hofmann M, Stoss O, Gaiser T, et al. Central HER2 IHC and FISH analysis in a trastuzumab (Herceptin) phase II monotherapy study: assessment of test sensitivity and impact of chromosome 17 polysomy. J Clin Pathol. 2008;61:89–94. doi: 10.1136/jcp.2006.043562. [DOI] [PubMed] [Google Scholar]
- 88.Kaufman PA, Broadwater G, Lezon-Geyda LG. CALGB 150002: correlation of HER2 and chromosome 17 (ch17) copy number with trastuzumab (T) efficacy in CALGB 9840, paclitaxel (P) with or without T in HER2þ and HER2- metastatic breast cancer (MBC) J Clin Oncol. 2007;25(18 suppl) abstract1009. [Google Scholar]
- 89.Hanna WM, Rüschoff J, Bilous M, et al. HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod Pathol. 2014;27(1):4–18. doi: 10.1038/modpathol.2013.103. [DOI] [PubMed] [Google Scholar]
- 90.Vance GH, Barry TS, Bloom KJ, et al. College of American Pathologists Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch Pathol Lab Med. 2009;133:611–2. doi: 10.5858/133.4.611. [DOI] [PubMed] [Google Scholar]
- 91.Bartlett AI, Starcyznski J, Robson T, et al. Heterogeneous HER2 gene amplification: impact on patient outcome and a clinically relevant definition. Am J Clin Pathol. 2011;136:266–74. doi: 10.1309/AJCP0EN6AQMWETZZ. [DOI] [PubMed] [Google Scholar]
- 92.Seol H, Lee HJ, Choi Y, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. 2012;25:938–48. doi: 10.1038/modpathol.2012.36. [DOI] [PubMed] [Google Scholar]
- 93.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2- positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–84. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 94.Perez EA, Baehner FL, Butler SM. The relationship between quantitative human epidermal growth factor receptor 2 gene expression by the 21-gene reverse transcriptase polymerase chain reaction assay and adjuvant trastuzumab benefit in Alliance N9831. Breast Cancer Res. 2015;17(1):133. doi: 10.1186/s13058-015-0643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1998;95:15587–91. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13(6):224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 98.Gewinner C, Wang ZC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–25. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Liu Y, Du Y, Yin W, Lu J. The predictive role of phosphatase and tensin homolog (PTEN) loss, phosphoinositol-3 (PI3) kinase (PIK3CA) mutation, and PI3K pathway activation in sensitivity to trastuzumab in HER2-positive breast cancer: a meta-analysis. Curr Med Res Opin. 2013;29(6):633–42. doi: 10.1185/03007995.2013.794775. [DOI] [PubMed] [Google Scholar]
- 101.Majewski IJ, Nuciforo P, Mittempergher L, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33(12):1334–9. doi: 10.1200/JCO.2014.55.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baselga J, Cortés J, Im SA, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32(33):3753–61. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 103.Martínez MT, Pérez-Fidalgo JA, Martín-Martorell P, et al. Treatment of HER2 positive advanced breast cancer with T-DM1: a review of the literature. Crit Rev Oncol Hematol. 2016;97:96–106. doi: 10.1016/j.critrevonc.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 104.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baselga J, Verma S, Ro R. Abstract LB-63: relationship between tumor bio-markers (BM) and efficacy in EMILIA, a phase III study of trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) Cancer Res. 2013;73:LB–63. doi: 10.1158/1078-0432.CCR-15-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dave B, Migliaccio I, Gutierrez MC, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29(2):166–73. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hurvitz SA, Andre F, Jiang Z, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16(7):816–29. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 108.André F, O’Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(6):580–91. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 109.Faber AC, Corcoran RB, Ebi H, et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 2011;1(4):352–65. doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park SH, Ito K, Olcott W, Katsyv I, Halstead-Nussloch G, Irie HY. PTK6 inhibition promotes apoptosis of lapatinib-resistant Her2(+) breast cancer cells by inducing Bim. Breast Cancer Res. 2015;17:86. doi: 10.1186/s13058-015-0594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamel S, Bouchard A, Ferrario C, et al. Both t-Darpp and DARPP-32 can cause resistance to trastuzumab in breast cancer cells and are frequently expressed in primary breast cancers. Breast Cancer Res Treat. 2010;120:47–57. doi: 10.1007/s10549-009-0364-7. [DOI] [PubMed] [Google Scholar]
- 112.Christenson JL, Denny EC, Kane SE. t-Darpp overexpression in HER2-positive breast cancer confers a survival advantage in lapatinib. Oncotarget. 2015;6(32):33134–45. doi: 10.18632/oncotarget.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scaltriti M, Eichhorn PJ, Cortes J, et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci USA. 2011;108:3761–6. doi: 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]