Abstract
Background
Metabolic syndrome (MetS) is associated with a higher risk of all-cause mortality. High-sensitivity C-reactive protein (hsCRP) is a prototypic marker of inflammation usually increased in MetS. Women with MetS-related diseases present higher hsCRP levels than men with MetS-related diseases, suggesting sex differences in inflammatory markers. However, it is unclear whether serum hsCRP levels are already increased in men and/or women with MetS risk factors and without overt diseases or under pharmacological treatment.
Objective
To determine the impact of the number of MetS risk factors on serum hsCRP levels in women and men.
Methods
One hundred and eighteen subjects (70 men and 48 women; 36 ± 1 years) were divided into four groups according to the number of MetS risk factors: healthy group (CT; no risk factors), MetS ≤ 2, MetS = 3, and MetS ≥ 4. Blood was drawn after 12 hours of fasting for measurement of biochemical variables and hsCRP levels, which were determined by immunoturbidimetric assay.
Results
The groups with MetS risk factors presented higher serum hsCRP levels when compared with the CT group (p < 0.02). There were no differences in hsCRP levels among groups with MetS risk factors (p > 0.05). The best linear regression model to explain the association between MetS risk factors and hsCRP levels included waist circumference and HDL cholesterol (r = 0.40, p < 0.01). Women with MetS risk factors presented higher hsCRP levels when compared with men (psex < 0.01).
Conclusions
Despite the absence of overt diseases and pharmacological treatment, subjects with MetS risk factors already presented increased hsCRP levels, which were significantly higher in women than men at similar conditions.
Keywords: Metabolic Syndrome, Risk Factors, Sex Characteristics, Protein C
Introduction
Metabolic syndrome (MetS) is a cluster of metabolic risk factors that includes high blood pressure, hyperglycemia, dyslipidemia, and abdominal obesity. When these risk factors are present together, the probability of future cardiovascular problems becomes greater than with any of the factors alone.1,2 Previous studies estimate that 40% of North Americans3 and 25% of Europeans4 or Latin Americans5 may present MetS by the time they reach the age of 60 years. Currently, most efforts are directed towards early detection and treatment of individuals with established MetS to avoid the development of cardiovascular disease.
Patients with MetS usually present increased levels of high-sensitivity C-reactive protein (hsCRP), which is a prototypic marker of inflammation.6 Several studies have shown that there is a clear relationship between metabolic disorders and higher hsCRP levels.7,8 It has also been shown that women with cardiometabolic risks, i.e. those with MetS, diabetes, or hypertension, usually present higher hsCRP levels than men with MetS-related diseases, suggesting sex differences in inflammatory markers.9 However, it is unclear whether serum hsCRP levels are already increased in subjects with MetS risk factors and without overt diseases or under pharmacological treatment. Also, information about hsCRP levels in men and women with MetS risk factors are inconclusive.
Cardiometabolic diseases seem to have a cumulative effect on serum hsCRP levels. It has been demonstrated that hsCRP levels are higher in patients presenting simultaneously MetS and type 2 diabetes than in those with MetS alone.10 However, it is unknown whether the number of MetS risk factors (two or less, three, or four or more factors) can influence the levels of serum hsCRP. Considering these aspects together, this study aimed to determine the effects of the number of MetS risk factors on serum hsCRP levels in women and men. We hypothesized that serum hsCRP levels would increase according to the number of MetS risk factors in women.
Methods
Ethical approval
The study protocol was approved by the ethics committee of Fluminense Federal University and conformed to the standards set by the latest revision of the Declaration of Helsinki. All subjects gave written informed consent before participating in the study.
Sample
Subjects were recruited through advertisements at the University and in local newspapers. One hundred and eighteen subjects (70 men and 48 women) aged 36 ± 1 years were enrolled. We considered the following risk factors of MetS:11 1) waist circumference ≥ 90 cm in men and ≥ 80 in women; 2) serum triglycerides levels ≥ 150 mg/dL; 3) serum HDL cholesterol levels < 40 mg/dL in men and < 50 mg/dL in women; 4) systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg; and 5) fasting serum glucose levels ≥ 100 mg/dL. We divided the subjects into four groups according to the number of MetS risk factors: healthy group (CT; no risk factors); MetS ≤ 2 (two or fewer risk factors); MetS = 3 (three risk factors); and MetS ≥ 4 (four or more risk factors). Other inclusion criteria included the absence of any diagnosed disease, recent infection, use of medication (except contraceptives) or smoking, and the presence of regular menstrual cycles (in women) and sedentary lifestyle (defined as lack of engagement in exercise activities lasting ≥ 30 min, three times per week during the last 3 months).
Measurements
The subjects visited the laboratory three times. On the first visit, a physician conducted an evaluation that included assessment of clinical history and resting electrocardiogram (CardioCare 2000, Bionet, Tustin, CA, USA). On the second visit, the patients underwent a physical evaluation. Anthropometric variables, such as weight and height, were measured using a calibrated medical beam scale (Welmy, Santa Bárbara d´Oeste, SP, Brazil). Body mass index (BMI) was calculated as weight (in kilograms) divided by the squared height (in meters). Waist circumference was measured at the midpoint between the iliac crest and the lower (XII) rib. Blood pressure was measured twice, once in each arm, on two separate days (at the first and second visits) and with the patient in the upright sitting position. On the third visit, blood was drawn from the subjects.
Biochemical blood analyses and hsCRP
Blood was drawn from an anterior cubital vein in the morning after a 12-hour fast. Cholesterol and its subfractions (HDL cholesterol, low-density lipoprotein [LDL] cholesterol, and very-low-density lipoprotein [VLDL] cholesterol) as well as triglycerides and glucose were determined using enzymatic colorimetric methods. Serum levels of hsCRP were measured by immunoturbidimetric assay (Tina-quant® latex, Roche, Basel, Switzerland).
Statistical methods
The data distribution was assessed by the Shapiro-Wilk test. A total sample size of 110 subjects was necessary to detect differences on CRP concentration among the groups (group main effect), considering a one-way ANOVA p value of 0.05 and power of 0.90. Two-way repeated measures ANOVA was also used to compare the variable hsCRP among MetS risk factors groups between males and females. Associations between hsCRP and individual components of MetS were determined by multiple linear regression analysis. Data are presented as mean ± standard error of the mean (SEM). Significance was accepted at a 0.05 level. All analyses were performed with the software Statistica (version 8, StatSoft Inc., Oklahoma, USA).
Results
Table 1 presents the anthropometric, metabolic, and hemodynamic variables. The groups matched for sex and age (p > 0.05). Waist circumference, BMI, VLDL cholesterol, and triglycerides were significantly different in the CT groups compared with the MetS risk factors groups. In addition, the MetS = 3 and MetS ≥ 4 groups also had higher BMI, waist circumference, systolic blood pressure, and serum levels of VLDL cholesterol, triglycerides, and glucose, as well as lower serum HDL cholesterol levels compared with the MetS ≤ 2 group (p < 0.05).
Table 1.
Biochemical and hemodynamic characteristics of healthy subjects and individuals with MetS risk factors
| Variables | Groups | |||
|---|---|---|---|---|
| CT | MetS ≤ 2 | MetS = 3 | MetS ≥ 4 | |
| N (M/W) | 18 (11/7) | 67 (34/33) | 23 (19/4) | 10 (6/4) |
| Age (years) | 33 ± 2 | 36 ± 1 | 37 ± 1 | 39 ± 1 |
| BMI (kg/m2) | 22.90 ± 0.62 | 28.76 ± 0.40* | 32.26 ± 0.88*† | 31.15 ± 0.97*† |
| Waist circumference (cm) | 78.98 ± 1.88 | 95.02 ± 1.12* | 105.86 ± 1.70*† | 103.2 ±1.54*† |
| SBP (mmHg) | 114 ± 1 | 116 ± 1 | 126 ± 3*† | 129 ± 1*† |
| DBP (mmHg) | 75 ± 1 | 76 ± 1 | 81 ± 2 | 85 ± 3*† |
| Total cholesterol (mg/dL) | 174.89 ± 6.77 | 193.30 ± 4.94 | 207.22 ± 8.53* | 215.30 ± 8.66* |
| HDL-c (mg/dL) | 56.67 ± 2.60 | 53.85 ± 1.47 | 41.48 ± 1.54*† | 38 ± 2.82*† |
| LDL-c (mg/dL) | 102.06 ± 6.68 | 119.77 ± 4.50 | 124 ± 7.63 | 138.38 ± 8.43* |
| VLDL-c (mg/dL) | 13.11 ± 1.25 | 19.56 ± 0.85* | 41.87 ± 3.24*† | 46.13 ± 4.42*† |
| Triglycerides (mg/dL) | 54.83 ± 2.35 | 98.52 ± 4.51* | 209.09 ± 16.28*† | 230.88 ± 22.26*† |
| Glucose (mg/dL) | 86.72 ± 1.57 | 87.61 ± 0.76 | 95.76 ± 2.39*† | 97 ± 4.40*† |
Values are displayed as mean ± SEM. CT: healthy subjects; MeTS: metabolic syndrome; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol.
p < 0.05 vs. CT;
p < 0.05 vs. MetS ≤ 2.
The groups with MetS risk factors presented higher serum hsCRP levels compared with the CT group (p ≤ 0.02). However, there were no differences in hsCRP levels among groups with MetS risk factors (Figure 1; p > 0.05). When the analysis was adjusted for BMI, similar results were noted (data not shown).
Figure 1.
Distribution of serum hsCRP levels according to number of MetS risk factors. (*) p < 0.05 vs. CT.
Stepwise multivariate regression analysis of serum hsCRP levels and MetS risk factors demonstrated that waist circumference and HDL cholesterol levels were the major predictors of increased hsCRP levels [y = -1.214+0.13*(waist circumference)+ 0.0006*(HDL cholesterol)] (r = 0.40, p < 0.01).
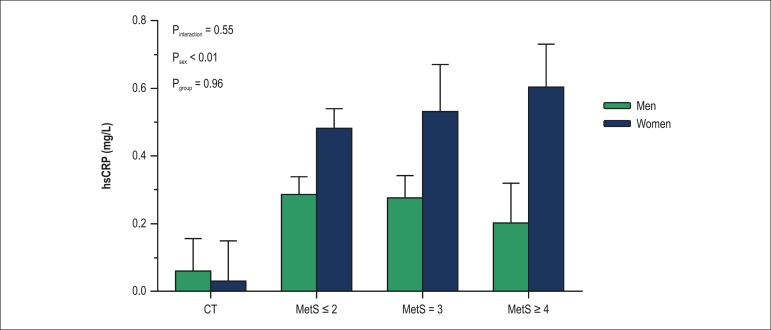
Regarding sex-differences on hsCRP levels, no difference was observed between women and men in the CT group (p = 0.84), whereas in the groups with MetS- related risk factors, women presented higher levels of hsCRP when compared with men (psex < 0.01). However, hsCRP levels were still similar among the groups with MetS risk factors (p > 0.05) (Figure 2).
Figure 2.
- Serum hsCRP levels in men and women according to the number of MetS risk factors. hsCRP: high-sensitivity C-reactive protein; CT: healthy subjects; MetS: metabolic syndrome; MetS ≤ 2, subjects with one or two MetS risk factors; MetS = 3, subjects with three MetS risk factors; MetS ≥ 4, subjects with four or five MetS risk factors.
Discussion
In this study, we tested the hypothesis that serum levels of hsCRP would increase according to the number of MetS risk factors in women. New findings of our study were threefold: 1) hsCRP levels were already higher in subjects with MetS risk factors when compared with controls; 2) the number of MetS risk factors did not influence the levels of hsCRP; 3) women with MetS risk factors presented higher hsCRP levels when compared with men with MetS risk factors.
Previous studies have shown associations between markers of inflammation and components of MetS.12,13 CRP levels in subjects with MetS have also been reported to be four times higher than those in healthy subjects.12 Our study demonstrated that subjects with MetS risk factors, even without preexisting diseases or under pharmacological treatment, already present early changes in hsCRP levels. Taken together, these data suggest that MetS risk factors may be associated with systemic low-grade inflammation.
We found no differences in hsCRP levels among groups with MetS risk factors. In contrast, other studies have demonstrated that CRP levels are positively associated with the number of MetS components.7,14 These studies have also demonstrated a gradual increase in CRP levels with the number of MetS components. It is important to observe that subjects in these other studies presented overt cardiometabolic diseases and/or were taking regular medications. Thus, increased serum CRP levels could be associated with the number of cardiometabolic diseases.
Waist circumference and HDL colesterol levels were the best predictors to explain the increase in hsCRP levels in subjects with MetS risk factors. Nakamura et al. have shown that among the MetS components, waist circumference is the main determinant of increase in CRP concentrations.15 Several studies have reported an inverse relationship between levels of HDL cholesterol and CRP in healthy individuals and subjects with MetS, suggesting that low HDL cholesterol levels may favor the inflammatory process.16,17
In a previous study from our group, we have shown that subjects with MetS, even without overt diseases or under pharmacological treatment, already present an early endothelial dysfunction, demonstrated by a longer time to peak diameter and an increased sE-selectin level.18 Endothelial dysfunction appears to stimulate an inappropriate secretion of proinflammatory and anti-inflammatory adipocytokines in subjects with MetS19 and may lead to a systemic inflammatory condition, which activates genes encoding CRP and other agents in the acute phase.
Regarding sex differences on hsCRP levels in groups with MetS risk factors, women presented higher levels of hsCRP when compared with men. Han et al. also demonstrated that CRP levels predict the development of MetS in women but not in men.20 The sex differences observed in these studies could be explained by endogenous synthesis of estrogen, a hormone that might play a role on the inflammatory process in women. An alternative explanation would be that women might have a greater amount of total body adipose tissue compared with men, which could be the source of proinflammatory cytokines.20
We must mention a limitation of our study. Values of BMI were different among the CT and MetS risk factors groups (MetS ≤ 2, MetS = 3, and MetS ≥ 4). This is important, since it is well known that obesity per se induces an inflammatory response and increases the serum levels of hsCRP.13 However, we obtained similar results when we performed a BMI-adjusted analysis.
Conclusion
Despite the absence of overt diseases and pharmacological treatment, subjects with MetS risk factors presented increased hsCRP levels when compared with healthy subjects. Waist circumference and HDL cholesterol were identified as independent predictors of increased serum hsCRP levels in subjects with MetS risk factors. Moreover, women with MetS risk factors presented higher hsCRP levels than men in the same condition.
These results indicate that an unspecific and subclinical inflammatory process is already present in early stages of the natural history of MetS, through the presence of high hsCRP levels. Measurements of this acute phase inflammatory protein may help determine an individual's cardiovascular risk and implement effective preventive strategies to avoid the development of cardiometabolic diseases, mainly in women.
Acknowledgements
The authors appreciate the time and effort dedicated by all volunteer subjects in this study. Also, we would like to thank the people who work at the Laboratory of Exercise Sciences.
Footnotes
Author contributions
Conception and design of the research: Garcia VP, Sales ARK, Rocha NG, Nóbrega ACL; Acquisition of data: Garcia VP, Rocha HNM, Sales ARK, Rocha NG; Analysis and interpretation of the data, Writing of the manuscript and Critical revision of the manuscript for intellectual content: Garcia VP, Rocha HNM, Sales ARK, Rocha NG, Nóbrega ACL; Statistical analysis: Garcia VP, Rocha HNM, Rocha NG, Nóbrega ACL; Obtaining financing: Nóbrega ACL.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by CAPES, CNPq, FAPERJ and FINEP.
Study Association
This article is part of the thesis of monograph submitted by Vinicius Pacheco Garcia, from Universidade Federal Fluminense.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 2.Sposito AC, Caramelli B, Fonseca FA, Bertolami MC, Afiune A, Neto, Souza AD, et al. IV Brazilian Guideline for dyslipidemia and atherosclerosis prevention: Department of Atherosclerosis of Brazilian Society of Cardiology. Arq Bras Cardiol. 2007;88(1):2–19. doi: 10.1590/s0066-782x2007000700002. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2(3):180–193. doi: 10.1111/j.1753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 4.Martinez MA, Puig JG, Mora M, Aragon R, O'Dogherty P, Anton JL, et al. MAPA (Monitorización Ambulatoria de la Presión Arterial) Working Group. Metabolic syndrome: prevalence, associated factors, and C-reactive protein: the MADRIC (MADrid RIesgo Cardiovascular) Study. Metabolism. 2008;57(9):1232–1240. doi: 10.1016/j.metabol.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Escobedo J, Schargrodsky H, Champagne B, Silva H, Boissonnet CP, Vinueza R, et al. Prevalence of the metabolic syndrome in Latin America and its association with sub-clinical carotid atherosclerosis: the CARMELA cross sectional study. Cardiovasc Diabetol. 2009;8:52–52. doi: 10.1186/1475-2840-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97(2A):3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Frohlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, et al. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23(12):1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 8.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40(11):1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 9.Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Sr.Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110(4):380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 10.Nakano S, Kuboki K, Matsumoto T, Nishimura C, Yoshino G. Small, dense LDL and high-sensitivity C-reactive protein (hs-CRP) in metabolic syndrome with type 2 diabetes mellitus. J Atheroscler Thromb. 2010;17(4):410–415. doi: 10.5551/jat.1891. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Vu JD, Vu JB, Pio JR, Malik S, Franklin SS, Chen RS, et al. Impact of C-reactive protein on the likelihood of peripheral arterial disease in United States adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am J Cardiol. 2005;96(5):655–658. doi: 10.1016/j.amjcard.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Bahia L, Aguiar L, Villela N, Bottino D, Godoy-Matos A, Geloneze B. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics (Sao Paulo) 2006;61(5):433–440. doi: 10.1590/s1807-59322006000500010. [DOI] [PubMed] [Google Scholar]
- 14.Yang T, Chu CH, Hsieh PC, Hsu CH, Chou YC, Yang SH, et al. C-reactive protein concentration as a significant correlate for metabolic syndrome: a Chinese population-based study. Endocrine. 2013;43(2):351–359. doi: 10.1007/s12020-012-9743-7. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, Ito H, Egami Y, Kaji Y, Maruyama T, Koike G, et al. Waist circumference is the main determinant of elevated C-reactive protein in metabolic syndrome. Diabetes Res Clin Pract. 2008;79(2):330–336. doi: 10.1016/j.diabres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Lorda P, Bulló M, Balanzà R, Salas-Salvadó J. C-reactive protein, adiposity and cardiovascular risk factors in a Mediterranean population. Int J Obes (Lond) 2006;30(3):468–474. doi: 10.1038/sj.ijo.0803182. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Parish R, Mansi I, Yu H, Kennen EM, Davis T, et al. Non-high-density lipoprotein cholesterol in patients with metabolic syndrome. J Investig Med. 2008;56(7):931–936. doi: 10.2310/JIM.0b013e318182080a. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes IA, Sales AR, Rocha NG, Silva BM, Vianna LC, da Nobrega AC. Preserved flow-mediated dilation but delayed time-to-peak diameter in individuals with metabolic syndrome. Clin Physiol Funct Imaging. 2014;34(4):270–276. doi: 10.1111/cpf.12092. [DOI] [PubMed] [Google Scholar]
- 19.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18(14):1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 20.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25(11):2016–2021. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]