Abstract
OBJECTIVE:
Children who require chronic mechanical ventilation via tracheostomy are medically complex and require prolonged hospitalization, placing a heavy burden on caregivers and hospital systems. We developed an interdisciplinary Ventilator Care Program to relieve this burden, through improved communication and standardized care. We hypothesized that a standardized team approach to the discharge of tracheostomy- and ventilator-dependent children would decrease length of stay (LOS), reduce patient costs, and improve safety.
METHODS:
We used process mapping to standardize the discharge process for children requiring chronic ventilation. Interventions included developing education materials, a Chronic Ventilation Road Map for caregivers, utilization of the electronic medical record to track discharge readiness, team-based care coordination, and timely case management to arrange home nursing. We aimed to decrease overall and pediatric respiratory care unit LOS as the primary outcomes. We also analyzed secondary outcomes (mortality, emergency department visits, unplanned readmissions), and per-patient hospital costs during 2-year “preintervention” and “postintervention” periods (n = 18 and 30, respectively).
RESULTS:
Patient demographics were not different between groups. As compared with the preintervention cohort, the overall LOS decreased 42% (P = .002). Pediatric respiratory care unit LOS decreased 56% (P = .001). As a result, unplanned readmissions, emergency department visits, and mortality were not increased. Direct costs per hospitalization were decreased by an average of 43% (P = .01).
CONCLUSIONS:
Although LOS remained high, a standardized discharge process for chronically ventilated children by an interdisciplinary Ventilator Care Program team resulted in decreased LOS and costs without a negative impact on patient safety.
The decision to initiate chronic ventilation via tracheostomy tube often results in prolonged hospitalization to achieve medical stability, train caregivers, provide adequate home nursing, and coordinate complex outpatient care. Extended inpatient care is costly, specifically as compared with providing long-term mechanical ventilation at home.1–5 Thus, although such patients present infrequently, children who require tracheostomy and chronic ventilator therapy pose substantial challenges and burdens on caregivers and health care systems.6–8
Children may require chronic ventilation in the neonatal setting for a number of clinical indications including severe bronchopulmonary dysplasia (BPD), congenital diaphragmatic hernia, airway malacia, neuromuscular disease, and other forms of respiratory insufficiency.9–11 Traumatic brain injury, paralysis, central neurologic conditions, progression of chronic respiratory insufficiency, as well as postinfectious sequelae may result in the need for chronic ventilation in older children.12,13 Regardless of the primary cause of chronic respiratory failure, children with tracheostomy who require chronic ventilation are medically complex with significant morbidity and mortality.14–16
Hospital practice patterns vary as to whether chronically ventilated children are cared for exclusively in neonatal and pediatric intensive care units or are transferred to step-down units and/or inpatient wards once medical stability has been achieved.17 Regardless, the overall length of stay (LOS) tends to be many months or years for the most complex patients. In some centers, those with stable chronic ventilation requirements may be transferred to long-term acute care facilities.18
Our interdisciplinary Ventilator Care Program (VCP) was formed in 2006 as a collaboration between pulmonology, neonatology, otolaryngology, nursing, respiratory therapy, and others. The program originated in the NICU and initially aimed to improve communication, standardize approaches to the common morbidities associated with the need for chronic ventilation, and improve outcomes for chronically ventilated neonates. Due to the need for coordinated interdisciplinary care, the program expanded to include care of older children. Since its inception, we have held weekly interdisciplinary inpatient rounds to discuss the complex care of these children. With input from the primary medical service, nurses, therapists, and caregivers, the VCP team attempts to accurately describe each child’s current status and progress for optimal medical decision-making. The pulmonary and neonatology directors of the VCP lead weekly rounds, provide longitudinal perspective, and collaborate with the primary inpatient attending and care teams to manage and coordinate the multisystem care of each patient. Emphasis is placed on major decisions, such as the timing of surgical procedures, ventilation strategies, transition to the portable ventilator, and discharge planning. Weekly plans are reviewed after each child is discussed.
Our VCP team approach has resulted in a dramatic increase in survival for infants with severe BPD.19 However, the duration of the inpatient stays for these children remained prolonged, and significant variation in practice patterns were observed between patients, with respect to discharge teaching methods, the use of informal handouts, and the acquisition of home nursing. In an effort to standardize care, we used process mapping, a method previously shown to reduce such variance in care in the emergency department (ED) and other settings.20,21 We hypothesized that implementation of a standardized discharge process would result in a timely and safe home discharge after initiation of chronic ventilation.
Methods
We developed a formal quality improvement project with the goal of measuring LOS, outcomes, and per-patient hospital costs before and after implementation of a standardized discharge process for our identified patient population. The institutional quality review panel approved the project, determined that it did not involve human subjects research, and authorized publication of this quality report.
Study Design, Setting, and Population
We identified patients who were discharged from the hospital from our tertiary care children’s hospital after initiation of chronic ventilation during a 2-year “preintervention” period (March 2011 to February 2013) and during a “postintervention” period (March 2013 to February 2015). Children who were weaned from invasive mechanical ventilation before discharge, those mechanically ventilated at home before admission, those discharged on continuous intravenous (IV) infusions, and those who were back-transported to other hospitals before home discharge were excluded. Death between initiation of chronic ventilation and hospital discharge was also recorded. Patients who had a tracheostomy (without mechanical ventilation) before hospital admission were included if chronic ventilation was initiated during the inpatient stay.
Children in both groups were diagnosed with chronic respiratory failure or insufficiency due to neonatal lung disease, neuromuscular disease, central hypoventilation, or other disorders and treated in either our neonatal or PICU for the acute illness. Once a decision was made to pursue chronic ventilation, a tracheostomy was placed by an otolaryngologist and the first tracheostomy tube change was completed 5 to 7 days after surgery and before transfer to the pediatric respiratory care unit (PRCU). ICU care continued until each child was medically stable for home discharge on a home ventilator. Our institution utilizes Trilogy (Philips Respironics, Murrysville, PA) and Pulmonetics LTV (CareFusion, San Diego, CA) ventilators for chronic ventilation. Stability was determined by consensus during weekly VCP rounds and consisted of breathing comfortably with an oxygen requirement (fraction of inspired oxygen) of <0.4, maintained off continuous IV medications, and without episodes of hypoxemia for ∼2 weeks. Maximal ventilator settings for transfer were not specified. At this time, patients were transferred to the PRCU, a section of the inpatient pulmonary floor where education and discharge planning are completed. Our program insists that patients transfer to our PRCU for focused training and demonstration of caregiver competency rather than discharging home directly from the ICU.
Patient Identification
Subjects were identified by surgical billing code for tracheostomy placement (or admission with a tracheostomy) combined with a hospital charge for mechanical ventilation on a hospital day when the child was not admitted to an ICU. This list was compared with the VCP’s inpatient census for the study time period and no discrepancies were identified.
Interventions
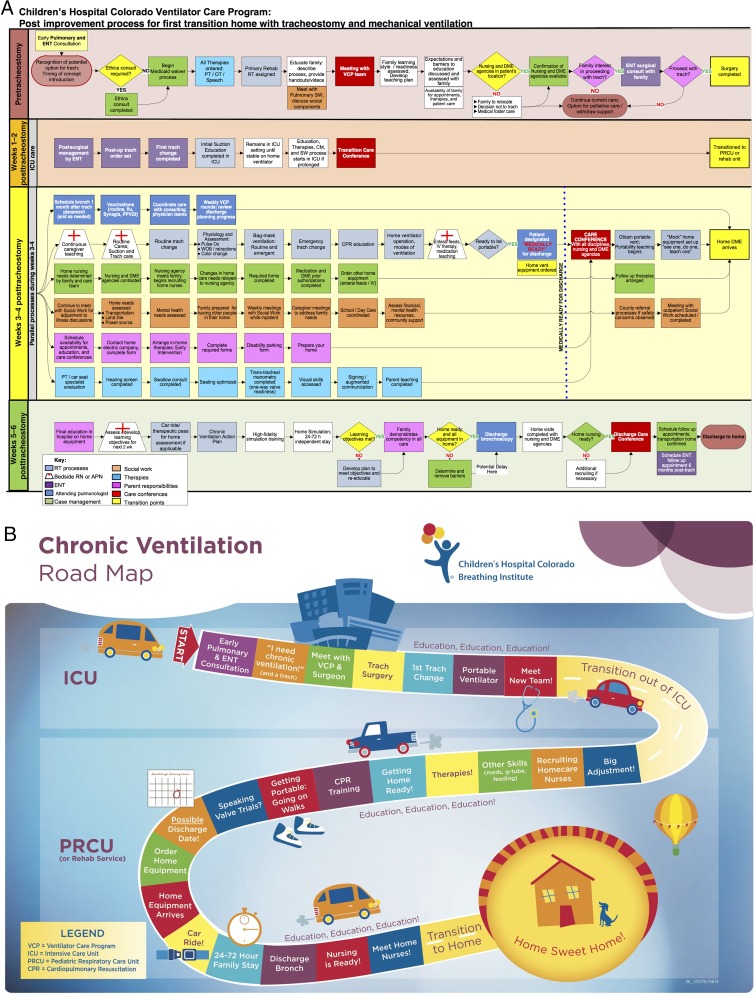
We formed a VCP administrative team consisting of the medical director, inpatient and outpatient advanced practice nurses, and the director of respiratory care. We began holding planning meetings twice each month in July 2012 to plan the quality improvement project. In January 2013, our team began working with a process improvement specialist to build future state process maps capturing discharge processes of the population of interest (Fig 1A). We met with physician representatives from the involved medical disciplines (pulmonology, neonatology, critical care, otolaryngology, pediatric residents), as well as respiratory and developmental therapists, nurses, case managers, social workers, and families. A formal process map was then created and approved by the team.
FIGURE 1.
A, Process map. VCP team members participated in process mapping sessions to determine the necessary steps for a safe discharge. In the figure, color-coding indicates the provider responsible for completing each task. ENT, Ear Nose Throat specialist (otolaryngologist); PT, physical therapy; OT, occupational therapy; RT, respiratory therapy; SW, social work; DME, durable medical equipment; CM, case management; Ox, oximetry; WOB, work of breathing. B, Chronic ventilation road map. The road map outlines the discharge process in a “family friendly” manner. The map emphasizes the collaborative team approach to care and the focus on caregiver education.
In collaboration with family partners, we developed formal discharge materials to align with institutional policies and procedures. We also reviewed printed educational materials from other institutions to ensure completeness of our formal handouts and then developed unique instructional videos (materials available upon request from the corresponding author). All handouts and videos were produced in both English and Spanish. We designed a “Chronic Ventilation Road Map” to communicate the discharge process in family-friendly language to families and providers (Fig 1B). A laminated copy of the road map was placed in each child’s hospital room and referenced by family and clinicians throughout the discharge education process to show progress over time.
Standardized team-based care coordination was optimized by using a dedicated advanced practice nurse and rehabilitation respiratory therapist to assist with education and track progress of all families. The electronic medical record was used in a number of ways to facilitate care coordination activities. A Best Practice Alert was created to encourage VCP consultation whenever a chronically ventilated child is hospitalized. A Discharge Readiness Report and documentation flowsheet track each child’s progress toward discharge. This report is projected on a screen during weekly VCP rounds for the team to review and update. A separate Caregiver Skills Checklist documents both the instruction provided to caregivers and the caregiver’s demonstration of competency in each skill. Standardized Care Coordination notes were used during weekly meetings and care conferences.
Tofil et al22 used high-fidelity simulation training to provide caregiver education within a home ventilator program. As part of our formal training process, we collaborated with our hospital’s high-fidelity simulation laboratory to pilot similar training for caregivers of VCP patients. Beginning in April of 2014, caregivers from each family completed 2 emergency simulation modules that focused on management of a plugged tracheostomy tube and ventilator malfunction. Skills reinforced by the training included suctioning, changing the tracheostomy tube, performing bag-tracheostomy ventilation, activating the emergency medical response system, and performing full cardiopulmonary resuscitation.
Arrangement of private duty nursing (PDN) and durable medical equipment was prioritized. As soon as the need for chronic ventilation was determined, the ICU case manager and respiratory therapist identified the appropriate home care agencies and made initial contact. Case management also ensured that each child’s insurance coverage was appropriate for the provision of in-home PDN.
Although family care conferences took place before our formal quality improvement project, we began to require 2 essential conferences with each family, a Transition Care Conference (when a child is ready to transfer from the ICU to the PRCU) and a Discharge Care Conference (in the days before discharge). Additional care conferences were scheduled as needed. Because we prioritized the arrangement of PDN to occur earlier in the hospitalization, PDN staff members were able to attend these critical meetings. Every effort was made to have a representative from the PDN agency present at the Transition and Discharge Care Conferences.
Data Collection
A dashboard was developed to collect patient data for tracking of quality metrics on an ongoing basis. Protected health information was stored securely on a password protected hospital network. The primary study outcomes were overall hospital LOS and PRCU LOS (the time from ICU transfer to discharge home). Secondary outcomes included the time from tracheostomy placement to hospital discharge and balancing metrics of patient safety: 1-year mortality, ED visits (within 6 months), and unplanned hospital readmissions (within 30 days and 12 months). Direct hospital costs were also compared. If a child already had a tracheostomy tube at admission, the time from tracheostomy placement to discharge was considered to be equal to the overall LOS. The hospital readmission rate was calculated by dividing the total readmissions in the given time period by the number of patients in a cohort, permitting readmission rates of greater than 100%. Planned hospitalizations for ventilator weaning, decannulation, or other anticipated reasons were excluded. In the postintervention cohort, 12-month unplanned readmissions and mortality were only available for 16 of 30 patients, as a full year had not passed since discharge at the time of data collection. The hospital finance department provided cost data by using the EPSi software package (Allscripts, Chicago, IL). Direct hospital costs did not include physician charges. In addition to the reported quality metrics, we reviewed each patient’s electronic medical record to obtain the demographic and clinical data reported.
Statistical Analysis
Primary and secondary outcome measures were compared with 2-sample t testing and 1-year mortality was compared with a χ2 test for association. Nonparametric demographic data were compared by Mann-Whitney testing and presented as medians with ranges. Outcomes were reviewed over time utilizing statistical process control I-charts. SD and control limits were calculated with the average moving range method. All standard rules for special cause variation were applied to control charts. Centerlines were shifted at the point of statistical special cause. All statistical testing and I-charts were completed by using Minitab Statistical Software version 17 (State College, PA). Significance level was set at α = 0.05.
Results
A total of 48 subjects were studied; 18 in the preintervention cohort and 30 in the postintervention cohort. Six additional children (3 in each cohort) died in the hospital after initiation of chronic ventilation, but before initial discharge. One child was discharged on IV treprostinil and was excluded. One child in the preintervention cohort was weaned from mechanical ventilation before discharge due to lack of homecare services in a rural area and was thus excluded. Two children (1 in each cohort) were back-transported to regional hospitals in other states and were thus also excluded. No children were transferred to subacute care, as a facility for chronically ventilated children does not exist in our state. Patient characteristics were similar between study groups (Table 1). There were more male children in both cohorts.
TABLE 1.
Patient Characteristics
| Preintervention, n = 18 | Postintervention, n = 30 | P | |
|---|---|---|---|
| Characteristics | |||
| Gender (boy, girl) | 14, 4 | 19, 11 | .35 |
| Gestational age, median (range), wk | 34.5 (23–40) | 33 (23–40) | .44 |
| Birth weight, median (range), g | 2236 (425–4049) | 1419 (340–3400) | .35 |
| Age at admission, median (range), y | 0.3 (0–10.8) | 0.5 (0–25.2) | .32 |
| Age at discharge, median (range), y | 1.1 (0.6–11.2) | 1.0 (0.4–25.3) | .48 |
| Preexisting tracheostomy, % (n) | 11 (2) | 20 (6) | .69 |
| Procedures, % (n) | |||
| Ductus arteriosus ligated | 28 (5) | 23 (7) | .75 |
| Gastrostomy tube | 94 (17) | 97 (29) | .99 |
| Gastric fundoplication | 78 (14) | 62 (18)a | .35 |
| ECMO during admission | 6 (1) (7 d) | 0 (0) | .19 |
| Diagnoses, % (n) | |||
| BPD | 44 (8) | 53 (16) | .77 |
| Congenital diaphragmatic hernia | 11 (2) | 3 (1) | .55 |
| Neuromuscular disease | 11 (2) | 10 (3) | .90 |
| Spinal cord injury/trauma | 11 (2) | 3 (1) | .28 |
| Congenital heart disease | 28 (5) | 17 (5) | .47 |
| Genetic/chromosomal anomaly | 17 (3) | 27 (8) | .50 |
| Oncology/bone marrow transplant | 11 (2) | 0 (0) | .14 |
| Airway malaciaa | 17 (3) | 37 (11) | .20 |
| Pulmonary hypertension | 33 (6) | 23 (7) | .51 |
| Upper airway obstruction | 11 (2) | 27 (8) | .28 |
| Pulmonary interstitial glycogenosis | 0 (0) | 7 (2) | .52 |
| Intrauterine growth restriction | 11 (2) | 7 (2) | .62 |
| Seizure disorder/infantile spasms | 11 (2) | 17 (5) | .60 |
| Postoperative extubation failure | 6 (1) | 3 (1) | .71 |
| Training | |||
| High-fidelity simulation completed | 0 (0) | 57 (17) | <.001 |
Comparison of the preintervention (March 2011 to February 2013) and postintervention (March 2013 to February 2015) cohorts. ECMO, extracorporeal membrane oxygenation.
Fundoplication status unknown for 1 older subject in the postintervention cohort.
As shown in Table 1, severe BPD after premature birth was the most common indication for chronic ventilation. Tracheomalacia and bronchomalacia were more frequently diagnosed in the postintervention group. Pulmonary hypertension complicated the course of many children.
The 2 primary study outcomes, overall LOS and PRCU LOS, were significantly decreased in the postintervention cohort (Table 2). Mean overall LOS decreased from 249 to 143.4 days (42% reduction, P = .002), and PRCU LOS was decreased from 111.8 to 49.7 days (56% reduction, P = .001). The statistical process control I-chart for overall LOS (Fig 2A) reveals that some patients continued to require lengthy hospitalizations in excess of 300 days even after the intervention. However, the I-chart in Figure 2B demonstrates a sustained reduction in PRCU LOS over the 2-year period after implementation of the standardized process. The time from tracheostomy placement to discharge was also decreased from 196.8 to 116.1 days (41% reduction, P = .003). LOS within the ICU was decreased by 32% after the intervention, but this difference was not statistically significant due to the large variance within each study group.
TABLE 2.
Patient Outcomes Before and After Implementation of Standardized Discharge Process
| Preintervention, n = 18 | Postintervention, n = 30 | P | |
|---|---|---|---|
| Primary outcomes | |||
| Overall hospital LOS, mean (SD), d | 249 (117) | 143.4 (97) | .002 |
| PRCU LOS, mean (SD), d | 111.8 (73) | 49.7 (33) | .001 |
| Secondary outcomes | |||
| Tracheostomy placement to discharge, mean (SD), d | 197 (99) | 116 (73) | .003 |
| ICU LOS, mean (SD), d | 137 (140) | 94 (97) | .18 |
| 1-y mortality,a % (n) | 17 (3) | 0 (0) | .09 |
| ED visits within 6 mo, % (n) | 61 (11) | 48 (12) | .48 |
| Readmissions within 30 d, % (n) | 17 (3) | 17 (5) | .99 |
| Readmissions within 12 mo,a % (n) | 161 (29) | 88 (14) | .13 |
| Direct cost per patient -×$1000, mean (SD) | 590 (371) | 336 (284) | .01 |
Primary and secondary outcomes in the preintervention and postintervention cohorts. Hospital readmission rates were calculated by dividing the total readmissions in a given time period by the number of patients in the cohort, permitting readmission rates of greater than 100%.
One-year mortality and 12-mo readmission data were only available for 16 patients in the postintervention cohort, because a full-year had not passed when the data were collected.
FIGURE 2.
Statistical process control I-charts demonstrate LOS and PRCU LOS for all patients. Each dot represents an individual hospital admission. The primary intervention involved implementation of the new standardized process with road map and education handouts. Red dots indicate failed tests for special cause. A, Mean overall LOS was significantly reduced after the intervention (Table 2), but LOS remained elevated for some patients after the intervention. B, The intervention resulted in a sustained reduction in PRCU LOS as demonstrated by the centerline shift. UCL, upper control limit.
The secondary outcomes were not negatively impacted by the intervention (Table 2). One-year mortality after discharge was 17% in the preintervention cohort (1 patient with cardiopulmonary arrest at home; 2 with septic shock) and 0% in the 16 patients with available data at the writing of this article (P = .09). The 12-month unplanned readmission rate in these patients was reduced by 47%, but this change was not statistically significant (P = .13). ED visits within 6 months and the 30-day unplanned readmission rate remained unchanged. Mean direct hospital costs were significantly decreased by 43% per hospitalization (P = .01). A portion of this cost reduction may have been due to a variety of hospital-wide resource stewardship initiatives unrelated to this particular study.
Discussion
We studied the impact of a novel standardized discharged process for ventilator dependent children within an interdisciplinary VCP at our institution. Through an organized quality improvement intervention, we achieved a striking reduction in the duration of hospitalization required for implementation of chronic ventilation via tracheostomy. Furthermore, by emphasizing caregiver education and consistency of care, morbidity and mortality were not increased as a result of the shorter hospitalization.
The medical management of children with chronic respiratory failure can be complex.23 Although we demonstrate here the benefits of a standardized discharge process, the importance of interdisciplinary collaborative decision-making to develop a customized care plan for each child cannot be overemphasized. Many of these children suffer from severe parenchymal lung disease, airways disease, congenital heart disease, and pulmonary hypertension. The VCP team requests that the neonatology or critical care primary team consult pulmonology and otolaryngology before tracheostomy placement to ensure that caregivers understand the benefits, risks, and time course of chronic ventilation including the lengthy hospital stay. Gastrostomy tube feedings are nearly always necessary, and empirical gastric fundoplication is requested for children at greatest risk of aspiration in the setting of gastroesophageal reflux. Our hospital’s interdisciplinary pulmonary hypertension team (consisting of pulmonary, cardiology, and intensivist providers) is consulted for children with pulmonary vascular disease. The role of developmental therapists (physical, occupational, and speech–language pathology) who see the children regularly is critical for determining medical stability, readiness for transition to the home ventilator, and discharge timing.
Our results reveal that overall LOS was significantly lower in the postintervention cohort, but the corresponding I-chart failed to reveal a sustained reduction. This is because the most severely affected children, particularly those born extremely premature, have multisystem disease and will continue to require prolonged hospitalization to reach medical stability. The medical complexity of a child is expected to result in greater costs. However, the sustained decrease in PRCU LOS after our quality improvement intervention reveals that some of the high cost is due to clinical inefficiencies and logistical delays in arranging for PDN and durable medical equipment. Thus, timely care coordination is critical for addressing these issues and controlling costs.
Fortunately, the majority of chronically ventilated children will improve over time.11,12,24,25 We view tracheostomy placement, not as a care failure, but rather as a proactive way to support children through important developmental time periods to optimize long-term outcomes.26 Tracheostomy placement is only part of an organized strategy to provide chronic ventilation allowing the focus to shift from acute care issues toward enhancement of long-term outcomes. Significant delays in providing the ventilatory support necessary to manage chronic respiratory insufficiency, air hunger, and dyspnea can adversely affect long-term developmental outcomes, and tracheostomy placement permits sustained mechanical ventilation, relieves distress, and provides the respiratory stability necessary to enhance neurocognitive, behavioral, and developmental outcomes. Rather than reserving tracheostomy placement for the most futile of cases, this proactive approach has led to many successful outcomes. Despite this, disease severity was not different between study groups and thus not the reason for our positive results.
Given the relatively small number of children who require chronic ventilation, conducting randomized controlled trials in this population is challenging. This quality improvement project did not test the utility of a specific clinical intervention, but rather focused on the improvements observed with implementation of a formal quality improvement initiative. In line with the Plan-Do-Study-Act cycle approach to quality assurance, this project is ongoing and will continue to monitor outcomes in this population.27 Some of the improvements in care, such as high-fidelity simulation training, were implemented after the formal intervention point. Improvements currently in progress include the development of additional educational videos, a partnership with our child life specialists to create a program for siblings of ventilator-dependent children including age-appropriate handouts and videos, and a bilingual video providing the fathers’ perspective.
Although not directly related to the discharge process, we also have an interdisciplinary VCP outpatient clinic. The outpatient team consists of a pediatric pulmonologist, nurse practitioner, respiratory therapist, outpatient nurse, and speech–language pathologist. A dedicated dietitian and social worker are available as needed. Quality improvement is an ongoing priority of the VCP clinic and we speculate that this contributed to why quality/safety metrics were not diminished in the setting of a more timely hospital discharge.
Process mapping with the VCP team members was highly useful for identifying the steps involved in discharging a child with chronic ventilation. However, the process as outlined in the map is does not encompass all aspects of care and is only an example of how such care can be arranged. Any institution that cares for similar patients must account for regional and center-based differences.
Conclusions
We used a formal quality improvement initiative to demonstrate that implementation of a standardized discharge process for children requiring chronic home ventilation via tracheostomy tube reduced LOS and hospital costs. Importantly, this intervention did not compromise patient safety as measured by mortality, ED visits, and hospital readmissions.
Acknowledgments
The authors thank John Kinsella, MD, Dan Hyman, MD, Cloy Van Eman, RRT, Monte Leidholm, RRT, Marilyn Willis, RN, Roberta Cox, RRT, Alicia Grenolds, PNP, Jessica Dawson, RN, Sasha Jacobs-Lowry, RN, and Heather Richards for their many contributions to the VCP and its patients.
Glossary
- BPD
bronchopulmonary dysplasia
- ED
emergency department
- IV
intravenous
- LOS
length of stay
- PDN
private duty nursing
- PRCU
pediatric respiratory care unit
- VCP
ventilator care program
Footnotes
Dr Baker conceptualized and designed the study, cofounded the Ventilator Care Program, drafted the manuscript, and supervised the data collection and data analyses; Ms Martin contributed to study design, performed the initial data analyses, and critically revised the manuscript; Ms Thrasher contributed to study design, cofounded the Ventilator Care Program, and critically revised the manuscript; Ms Moore and Ms Baker contributed to study design and critically revised the manuscript; Drs Abman and Gien contributed to study design, cofounded the Ventilator Care Program, and critically revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Institutes of Health grants NIH K12-HL090147-01 (to Dr Baker), NIH K23-HL121090-01 (to Dr Baker), and NIH 5K08-HL102261 (to Dr Gien); Children’s Hospital Colorado Patient Safety grant (to Dr Baker); and University of Colorado School of Medicine Bridge Funding (to Dr Baker). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bach JR, Intintola P, Alba AS, Holland IE. The ventilator-assisted individual. Cost analysis of institutionalization vs rehabilitation and in-home management. Chest. 1992;101(1):26–30 [DOI] [PubMed] [Google Scholar]
- 2.King AC. Long-term home mechanical ventilation in the United States. Respir Care. 2012;57(6):921–930; discussion 930–922 [DOI] [PubMed] [Google Scholar]
- 3.Benneyworth BD, Gebremariam A, Clark SJ, Shanley TP, Davis MM. Inpatient health care utilization for children dependent on long-term mechanical ventilation. Pediatrics. 2011;127(6). Available at: www.pediatrics.org/cgi/content/full/127/6/e1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin R, Sayal A, Syed F, et al. How long does it take to initiate a child on long-term invasive ventilation? Results from a Canadian pediatric home ventilation program. Can Respir J. 2015;22(2):103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6). Available at: www.pediatrics.org/cgi/content/full/130/6/e1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gowans M, Keenan HT, Bratton SL. The population prevalence of children receiving invasive home ventilation in Utah. Pediatr Pulmonol. 2007;42(3):231–236 [DOI] [PubMed] [Google Scholar]
- 7.Cox CE, Carson SS, Govert JA, Chelluri L, Sanders GD. An economic evaluation of prolonged mechanical ventilation. Crit Care Med. 2007;35(8):1918–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Pelt DC, Milbrandt EB, Qin L, et al. Informal caregiver burden among survivors of prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175(2):167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–368 [DOI] [PubMed] [Google Scholar]
- 10.Allen J, Zwerdling R, Ehrenkranz R, et al. ; American Thoracic Society . Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168(3):356–396 [DOI] [PubMed] [Google Scholar]
- 11.Cristea AI, Carroll AE, Davis SD, Swigonski NL, Ackerman VL. Outcomes of children with severe bronchopulmonary dysplasia who were ventilator dependent at home. Pediatrics. 2013;132(3). Available at: www.pediatrics.org/cgi/content/full/132/3/e727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin RS, Fitton CM. Tracheostomy and home ventilation in children. Semin Neonatol. 2003;8(2):127–135 [DOI] [PubMed] [Google Scholar]
- 13.Overman AE, Liu M, Kurachek SC, et al. Tracheostomy for infants requiring prolonged mechanical ventilation: 10 years’ experience. Pediatrics. 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1491 [DOI] [PubMed] [Google Scholar]
- 14.Edwards EA, O’Toole M, Wallis C. Sending children home on tracheostomy dependent ventilation: pitfalls and outcomes. Arch Dis Child. 2004;89(3):251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards JD, Kun SS, Keens TG. Outcomes and causes of death in children on home mechanical ventilation via tracheostomy: an institutional and literature review. J Pediatr. 2010;157(6):955–959.e2 [DOI] [PubMed] [Google Scholar]
- 16.Frates RC Jr, Splaingard ML, Smith EO, Harrison GM. Outcome of home mechanical ventilation in children. J Pediatr. 1985;106(5):850–856 [DOI] [PubMed] [Google Scholar]
- 17.Edwards JD, Rivanis C, Kun SS, Caughey AB, Keens TG. Costs of hospitalized ventilator-dependent children: differences between a ventilator ward and intensive care unit. Pediatr Pulmonol. 2011;46(4):356–361 [DOI] [PubMed] [Google Scholar]
- 18.Seneff MG, Wagner D, Thompson D, Honeycutt C, Silver MR. The impact of long-term acute-care facilities on the outcome and cost of care for patients undergoing prolonged mechanical ventilation. Crit Care Med. 2000;28(2):342–350 [DOI] [PubMed] [Google Scholar]
- 19.Gien J, Kinsella JP, Grenolds A, Abman SH, Baker CD Improved Survival Using a Multidisciplinary Care Team for Infants with Tracheostomy-Dependent Severe Bronchopulmonary Dysplasia. E-PAS2014:1534.579
- 20.Dagher M, Lloyd RJ. Managing negative outcome by reducing variances in the emergency department. QRB Qual Rev Bull. 1991;17(1):15–21 [DOI] [PubMed] [Google Scholar]
- 21.Hilton B. The benefits of business process mapping. Admin Radiol. 1993;12(11):31–34 [PubMed] [Google Scholar]
- 22.Tofil NM, Rutledge C, Zinkan JL, et al. Ventilator caregiver education through the use of high-fidelity pediatric simulators: a pilot study. Clin Pediatr (Phila). 2013;52(11):1038–1043 [DOI] [PubMed] [Google Scholar]
- 23.Abman SH, Nelin LD. Management of Severe BPD. In: Bancalari E, ed. The Newborn Lung: Neonatology Questions and Controversies, 2nd ed. Philadelphia, PA: Elsevier; 2012:21.21–21.29 [Google Scholar]
- 24.Schreiner MS, Downes JJ, Kettrick RG, Ise C, Voit R. Chronic respiratory failure in infants with prolonged ventilator dependency. JAMA. 1987;258(23):3398–3404 [PubMed] [Google Scholar]
- 25.Amin R, Sayal P, Syed F, Chaves A, Moraes TJ, MacLusky I. Pediatric long-term home mechanical ventilation: twenty years of follow-up from one Canadian center. Pediatr Pulmonol. 2014;49(8):816–824 [DOI] [PubMed] [Google Scholar]
- 26.Gien J, Abman SH, Baker CD. Interdisciplinary care for ventilator-dependent infants with chronic lung disease. J Pediatr. 2014;165(6):1274–1275 [DOI] [PubMed] [Google Scholar]
- 27.Guinane CS, Sikes JI, Wilson RK. Using the PDSA cycle to standardize a quality assurance program in a quality improvement-driven environment. Jt Comm J Qual Improv. 1994;20(12):696–705 [DOI] [PubMed] [Google Scholar]