Abstract
BACKGROUND:
The Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial found that recurrent urinary tract infections (rUTI) with resistant organisms were more common in the trimethoprim-sulfamethoxazole prophylaxis (TSP) arm. We describe factors associated with trimethoprim-sulfamethoxazole (TMP-SMX) resistance of rUTIs in RIVUR.
METHODS:
Children aged 2 to 71 months with first or second UTI (index UTI) and grade I to IV vesicoureteral reflux (VUR) were randomized to TSP or placebo and followed for 2 years. Factors associated with TMP-SMX–resistant rUTI were evaluated.
RESULTS:
Among 571 included children, 48% were <12 months old, 43% had grade II VUR, and 38% had grade III VUR. Recurrent UTI occurred in 34 of 278 children receiving TSP versus 67 of 293 children receiving placebo. Among those with rUTI, 76% (26/34) of subjects receiving TSP had TMP-SMX–resistant organisms versus 28% (19/67) of subjects receiving placebo (P < .001). The proportion of TMP-SMX–resistant rUTI decreased over time: in the TSP arm, 96% were resistant during the initial 6 months versus 38% resistant during the final 6 months; corresponding proportions for the placebo arm were 32% and 11%. Among children receiving TSP, 7 (13%) of 55 with TMP-SMX–resistant index UTI had rUTI, whereas 27 (12%) of 223 with TMP-SMX–susceptible index UTI had rUTI (adjusted hazard ratio 1.38, 95% confidence interval 0.54–3.56). Corresponding proportions in placebo arm were 17 (26%) of 65 and 50 (22%) of 228 (adjusted hazard ratio 1.33, 95% confidence interval 0.74–2.38).
CONCLUSIONS:
Although TMP-SMX resistance is more common among children treated with TSP versus placebo, resistance decreased over time. Among children treated with TSP, there was no significant difference in UTI recurrence between those with TMP-SMX–resistant index UTI versus TMP-SMX–susceptible UTI.
What’s Known on This Subject:
Although antimicrobial prophylaxis is effective in preventing recurrent urinary tract infection (UTI) in children with vesicoureteral reflux, increased antibiotic resistance is a concern. However, little is known about patterns of resistance over time with prophylaxis, and whether index UTI resistance affects prophylaxis efficacy.
What This Study Adds:
Although trimethoprim-sulfamethoxazole (TMP-SMX) resistance is more common among children with reflux treated with TMP-SMX prophylaxis, resistance decreased over time, in both treatment and placebo groups. Index UTI TMP-SMX resistance was not associated with recurrent UTI, among those treated with TMP-SMX prophylaxis.
Children diagnosed with vesicoureteral reflux (VUR) after a urinary tract infection (UTI) are commonly treated with antimicrobial prophylaxis, to reduce the risk of recurrent UTI (rUTI). Although there has been controversy in recent years regarding utilization of antimicrobial prophylaxis to prevent rUTI, the recent Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial demonstrated convincingly that the incidence of recurrent febrile or symptomatic UTI (F/SUTI) is lower among children with VUR treated with trimethoprim-sulfamethoxazole prophylaxis (TSP) compared with those receiving placebo.1
RIVUR trial outcomes were also significant for the finding that among participants who developed a first recurrence of F/SUTI with Escherichia coli, the proportion of isolates that were resistant to trimethoprim-sulfamethoxazole (TMP-SMX) was 63% with TSP and 19% with placebo (P < .001). However, space limitations in the primary RIVUR results report precluded extensive discussion of characteristics and resistance patterns of recurrent F/SUTIs in the trial.
Data regarding TMP-SMX resistance of the index (preenrollment) F/SUTI were also collected. In clinical practice, some assume that the antibiotic-resistance pattern of the initial UTI should be used to determine the appropriate agent for prophylaxis, rather than the side-effect profile or ease of administration of the agent. Limited evidence is available to support this assumption,2 and the problem is difficult to study in an uncontrolled retrospective setting, as the clinical choice of prophylaxis agent is so often based on the resistance pattern of the index UTI, rendering the sample biased. The RIVUR study allowed for a natural experiment, because all children randomized to the active agent received TMP-SMX regardless of the resistance pattern of the index UTI, giving us the opportunity to analyze rUTI rates in this unbiased sample.
The aims of this analysis were twofold: (1) to characterize patterns of antimicrobial resistance among recurrent F/SUTIs in the RIVUR trial, and (2) to determine whether resistance of the index UTI to TMP-SMX reduces the effectiveness of TSP in preventing recurrent F/SUTI.
Methods
Data Source and Patient Population
The rationale and design of the RIVUR trial have been published previously.3,4 Briefly, children aged 2 to 71 months with a history of confirmed first or second febrile or symptomatic UTI, and grade I to IV VUR, were enrolled at 19 centers in the United States. Enrolled children were randomized to either TMP-SMX or placebo and followed for 2 years, with primary outcome of recurrence of F/SUTI, and secondary outcomes of renal scarring, failure of TSP, and antimicrobial resistance. Children with non-VUR urologic anomalies, or index F/SUTI occurring >112 days before randomization, were excluded. Of the 607 children randomized in the RIVUR trial, this analysis includes 571 children who had complete data for index UTI resistance; 278 children in the TSP arm and 293 in the placebo arm. Subjects were analyzed as randomized (not strictly intention-to-treat), as we excluded those without index UTI sensitivity data.
UTI Definitions
Both index and recurrent F/SUTIs were defined as clinical events meeting all of the following criteria: pyuria on urinalysis, culture-proven infection with a single organism (≥50 000 colony-forming units per milliliter for catheterized or suprapubic aspirate specimens, ≥100 000 colony-forming units per milliliter for clean voided specimens), and fever (≥38°C) or urinary tract symptoms (suprapubic, abdominal, or flank pain or tenderness; or urinary urgency, frequency, or hesitancy; or dysuria; or foul-smelling urine; or in infants ≤4 months old: failure to thrive, dehydration, or hypothermia) within 24 hours before or after urine collection.
Resistance of both index and recurrent F/SUTIs was based on the antibiotic susceptibility profile (the “antibiogram”) from the urine culture obtained in the course of clinical care. These tests were performed by local clinical laboratories; urine cultures were not processed in a central facility. Among children enrolled in the RIVUR trial after their second F/SUTI, resistance classification was based on the second F/SUTI, which represented the index F/SUTI for the trial. F/SUTIs caused by Enterococcus were assumed to be resistant to TMP-SMX, based on biological mechanisms inherent to this organism. TMP susceptibility alone was reported for 8.8% of recurrent F/SUTIs; for analytic purposes these were grouped together with those reporting TMP-SMX susceptibility.
Timing of resistance for recurrent F/SUTI was assessed by comparing 6-month enrollment periods, focusing in particular on resistance during the first 6 months versus resistance during the last 6 months (study period 18–24 months).
Statistical Analysis
We compared participants’ characteristics by using Fisher’s exact test to compare categorical variables, by using a 3-way comparison of those without recurrent F/SUTI versus those with at least 1 resistant recurrent F/SUTI versus those with only susceptible recurrent F/SUTI, as well as a 2-way comparison between those with at least 1 resistant recurrent F/SUTI versus those with only susceptible recurrent F/SUTI (among those with a recurrent F/SUTI).
To compare incidence of recurrent F/SUTI between children with TMP-SMX–resistant versus TMP-SMX–sensitive index F/SUTI, we calculated unadjusted and adjusted hazard ratios (HRs) for the TMP-SMX group only, by using Cox proportional hazards regression.. Adjusted HRs account for febrile (versus nonfebrile) index UTI, resistant (versus susceptible) index UTI, age, race, ethnicity, VUR grade, bladder and bowel dysfunction (BBD), and administrative site. BBD was a time-dependent measure (yes, no, not toilet trained, unknown), allowed to change up to 4 times over the course of the follow-up period, and presented as person-years of follow-up time. Because most subjects did not have the primary outcome (recurrent F/SUTI), we could not calculate median event-free survival, and so we calculated and reported time to 10% incidence (the enrollment time at which 10% of at-risk subjects had experienced the outcome). The Mantel-Haenszel correlation statistic was used to compare treatment-group–adjusted rates of resistance over 4 time periods during the study.
All tests were 2-sided. P values were not adjusted for multiple testing. Calculations were performed by using SAS version 9.3 (SAS Institute, Inc, Cary, NC).
Results
Characteristics of the Sample
Demographic and clinical characteristics of RIVUR participants have been previously reported.1 For the current study, we excluded 36 children who lacked adequate index UTI resistance data; compared with the included sample (n = 571), the excluded children (n = 36) had a higher proportion randomized to active treatment (67% vs 49%), fewer index UTIs caused by E coli (81% vs 89%), a higher proportion with recurrent F/SUTI infections (28% vs 18%), and were more likely to be white (94% vs 80%). Among included children (Table 1), almost half (48%) were <1 year of age at enrollment and 92% were girls. Because the cohort was relatively young at enrollment, most (78%) were not toilet trained and therefore could not be classified with respect to BBD at enrollment; of the 122 who were toilet trained, 68 (56%) met the definition of BBD. Most children had grade II (43%) or grade III (38%) VUR. The index UTI was resistant to TMP-SMX in 21% of cases (20% among the TMP-SMX group, 22% among the placebo group).
TABLE 1.
Characteristics of RIVUR Subjects by rUTIs
| Overall, n = 571, n (%) | No rUTI, n = 470, n (%) | At Least 1 Resistant rUTI, n = 45, n (%) | Subjects With Only Susceptible rUTIs, n = 56, n (%) | Overall Pa | R vs S Pb | |
|---|---|---|---|---|---|---|
| Total no. of patients | <.001 | <.001 | ||||
| TMP-SMX | 278 (49) | 244 (52) | 26 (58) | 8 (14) | ||
| Placebo | 293 (51) | 226 (48) | 19 (42) | 48 (86) | ||
| Age at enrollment, mo | .01 | .90 | ||||
| <12 | 275 (48) | 233 (50) | 19 (42) | 23 (41) | ||
| 12–35 | 179 (31) | 154 (33) | 12 (27) | 13 (23) | ||
| 36–72 | 117 (20) | 83 (18) | 14 (31) | 20 (36) | ||
| Gender | .35 | .32 | ||||
| Circumcised boys | 17 (3) | 17 (4) | 0 (0) | 0 (0) | ||
| Girls | 526 (92) | 429 (91) | 42 (93) | 55 (98) | ||
| Uncircumcised boys | 28 (5) | 24 (5) | 3 (7) | 1 (2) | ||
| Race | .05 | .03 | ||||
| Nonwhite | 113 (20) | 96 (21) | 12 (27) | 5 (9) | ||
| White | 448 (80) | 364 (79) | 33 (73) | 51 (91) | ||
| Hispanic ethnicity | .63 | .75 | ||||
| Hispanic | 75 (13) | 65 (14) | 5 (11) | 5 (9) | ||
| Other | 493 (87) | 402 (86) | 40 (89) | 51 (91) | ||
| Highest VUR grade | .32 | >.99 | ||||
| I | 62 (11) | 52 (11) | 4 (9) | 6 (11) | ||
| II | 244 (43) | 211 (45) | 15 (33) | 18 (33) | ||
| III | 218 (38) | 172 (37) | 21 (47) | 25 (45) | ||
| IV | 44 (8) | 33 (7) | 5 (11) | 6 (11) | ||
| VUR laterality | .93 | >.99 | ||||
| Bilateral | 271 (48) | 225 (48) | 20 (45) | 26 (47) | ||
| Unilateral | 292 (52) | 239 (52) | 24 (55) | 29 (53) | ||
| Index UTI organism | .66 | .92 | ||||
| E coli | 509 (89) | 417 (89) | 41 (91) | 51 (91) | ||
| Enterococcus species | 13 (2) | 12 (3) | 1 (2) | 0 (0) | ||
| Klebsiella species | 18 (3) | 15 (3) | 1 (2) | 2 (4) | ||
| Proteus species | 14 (2) | 14 (3) | 0 (0) | 0 (0) | ||
| Other | 17 (3) | 12 (3) | 2 (4) | 3 (5) | ||
| Index UTI symptoms | .50 | .41 | ||||
| Febrile | 490 (86) | 405 (86) | 36 (80) | 49 (88) | ||
| Nonfebrile | 81 (14) | 65 (14) | 9 (20) | 7 (13) | ||
| Index UTI | .21 | .16 | ||||
| Resistant | 120 (21) | 96 (20) | 14 (31) | 10 (18) | ||
| Susceptible | 451 (79) | 374 (80) | 31 (69) | 46 (82) | ||
| BBD | .003 | .83 | ||||
| No BBD | 54 (10) | 42 (9) | 6 (14) | 6 (11) | ||
| BBD | 68 (12) | 45 (10) | 9 (20) | 14 (25) | ||
| Not toilet trained | 441 (78) | 376 (81) | 29 (66) | 36 (64) | ||
| History of constipation | .72 | .82 | ||||
| No | 401 (71) | 326 (70) | 34 (76) | 41 (73) | ||
| Yes | 166 (29) | 140 (30) | 11 (24) | 15 (27) | ||
| Treated for constipation | .72 | .75 | ||||
| No | 491 (87) | 401 (86) | 41 (91) | 49 (88) | ||
| Yes | 76 (13) | 65 (14) | 4 (9) | 7 (13) | ||
| No. of rUTIsc | ||||||
| 0 | 470 (82) | 470 (100) | 0 (0) | 0 (0) | ||
| 1 | 64 (11) | 0 (0) | 26 (58) | 38 (68) | ||
| 2 | 27 (5) | 0 (0) | 12 (27) | 15 (27) | ||
| 3 | 5 (1) | 0 (0) | 4 (9) | 1 (2) | ||
| 4 | 5 (1) | 0 (0) | 3 (7) | 2 (4) |
P values for an overall comparison of no rUTI, at least 1 resistant UTI, and only susceptible UTIs were computed using Fisher's exact test.
P value for a comparison of at least 1 resistant UTI versus only susceptible UTIs using Fisher's exact test.
P values were not computed for number of rUTIs.
Characteristics of rUTI
Factors associated with incidence of recurrent F/SUTI are shown in Table 1. A total of 470 children had no recurrent F/SUTI during the study period, whereas 101 (18%) had at least 1 recurrent F/SUTI. A number of children had multiple recurrences, including 27 children with 2 recurrences, 5 children with 3 recurrences, and 5 children with 4 recurrences. Additional characteristics of recurrent F/SUTI are shown in Table 2. Although 67% of recurrences were febrile, the remainder presented with symptoms other than fever. E coli was the causative uropathogen isolated in most (82%) recurrences, with a range of bacteria making up the remainder, including Klebsiella, Enterococcus, and Proteus species. Enterococcus, assumed to be TMP-SMX resistant and not tested based on the metabolism of the organism, comprised 12% (n = 7) of the resistant recurrent F/SUTIs. Most recurrent F/SUTI episodes were treated as outpatients, but 35% of events involved either hospitalization or an emergency department visit. Almost half of the recurrences occurred during the first 6 months after enrollment; the remaining recurrences were split relatively evenly between the other three 6-month blocks.
TABLE 2.
Characteristics of rUTIs
| All rUTI, n = 147, n (%) | Resistant rUTI, n = 58, n (%) | Susceptible rUTI, n = 89, n (%) | Pa | |
|---|---|---|---|---|
| Time from RIVUR enrollment, d | .004 | |||
| 0–182 | 70 (48) | 37 (64) | 33 (37) | |
| 183–365 | 27 (18) | 11 (19) | 16 (18) | |
| 366–547 | 24 (16) | 5 (9) | 19 (21) | |
| >547 | 26 (18) | 5 (9) | 21 (24) | |
| Clinical presentation rUTI | .15 | |||
| Febrile | 38 (26) | 18 (31) | 20 (22) | |
| Symptomatic | 49 (33) | 14 (24) | 35 (39) | |
| Febrile AND symptomatic | 60 (41) | 26 (45) | 34 (38) | |
| Recurrent UTI organismb | ||||
| E coli | 121 (82) | 42 (72) | 79 (89) | .01 |
| Klebsiella | 10 (7) | 5 (9) | 5 (6) | .52 |
| Enterococcus | 7 (5) | 7 (12) | 0 (0) | .001 |
| Proteus | 3 (2) | 0 (0) | 3 (3) | .28 |
| Enterobacter | 2 (1) | 1 (2) | 1 (1) | >.99 |
| Citrobacter | 1 (1) | 1 (2) | 0 (0) | .39 |
| CNS | 1 (1) | 1 (2) | 0 (0) | .39 |
| Aerobic GN Enterobacteriaceae | 1 (1) | 1 (2) | 0 (0) | .39 |
| Morganella | 1 (1) | 0 (0) | 1 (1) | >.99 |
| Hospitalization or ED visit for rUTI | 51 (35) | 20 (34) | 31 (35) | >.99 |
CNS, central nervous system; ED, emergency department; GN, gram-negative.
P values were computed using Fisher's exact test.
For UTI organisms, P values were computed for dummy variables E coli vs not E coli, Klebsiella vs not Klebsiella, and so forth.
Antibiotic Susceptibility Among Recurrent F/SUTIs
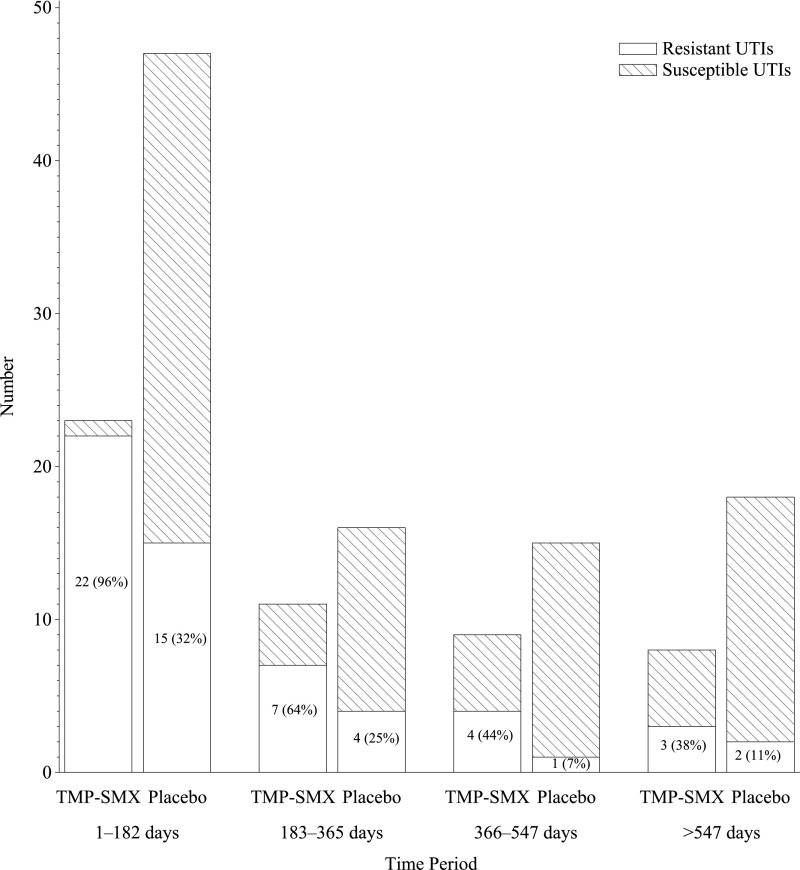
Among the 101 children who experienced recurrent F/SUTI, 45 (45%) had at least 1 recurrent F/SUTI caused by a TMP-SMX–resistant uropathogen, whereas 56 (55%) had recurrences caused only by TMP-SMX–susceptible uropathogens. The associations of subject characteristics with TMP-SMX resistance among recurrent F/SUTI are shown in Table 1 and Table 2. The overall proportion of RIVUR participants who had recurrent F/SUTI caused by a TMP-SMX–resistant uropathogen was slightly larger in the TSP arm (26 [9%] of 278 vs 19 [6%] of 293, odds ratio 1.46, 95% confidence interval [CI] 0.80–2.6). Conversely, the proportion with only susceptible recurrent F/SUTI was higher in the placebo group (16% vs 3%, P < .0001). Among children who experienced recurrent F/SUTI, those in the TSP arm were more likely to have a recurrence caused by a TMP-SMX–resistant uropathogen (26/34, 76%) compared with children in the placebo arm (19 [28%] of 67; P < .001). However, the proportion of recurrent F/SUTI in both the TSP and placebo arms caused by TMP-SMX–resistant uropathogens decreased over the course of the study (Fig 1). For children in the TSP arm, 96% of recurrent F/SUTIs during the initial 6 months were caused by a TMP-SMX–resistant uropathogen; during the final 6 months, only 38% of recurrent F/SUTIs were caused by TMP-SMX–resistant organisms. Corresponding proportions during the same time periods for children in the placebo arm were 32% during the initial 6 months and 11% during the final 6 months. The Mantel-Haenszel correlation statistic (P < .0001) also pointed to a substantial decrease in resistant infections over time.
FIGURE 1.
Proportion of rUTI episodes resistant to TMP-SMX as a function of the timing of the episode (divided into 6-month blocks after enrollment), stratified by TMP-SMX treatment arm versus placebo arm.
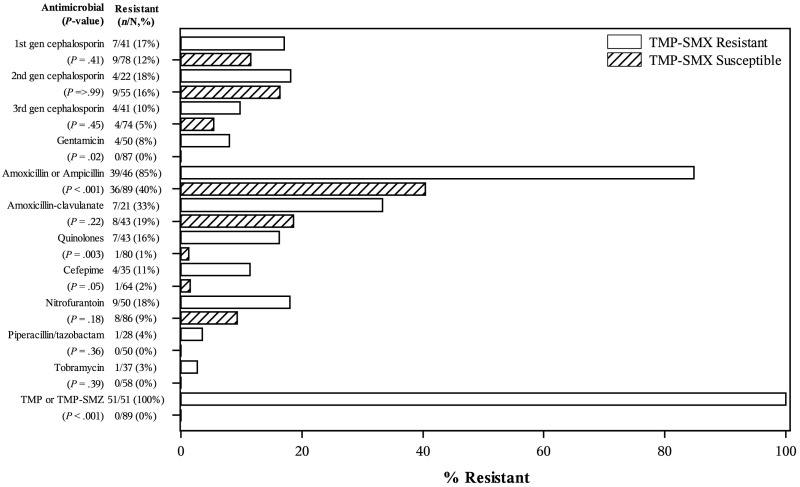
Figure 2 also shows the susceptibility patterns for other antimicrobial classes among recurrent F/SUTI; TMP-SMX resistance was significantly associated with resistance to ampicillin/amoxicillin (P < .001), gentamicin (P = .02), quinolones (P = .003), and cefepime (P = .05).
FIGURE 2.
Plot showing association of resistance to other antimicrobial agents with TMP-SMX resistance, among recurrent F/SUTI.
Association of TMP-SMX Resistance in the Index F/SUTI With Recurrence of F/SUTI Among Children in the TSP Treatment Arm
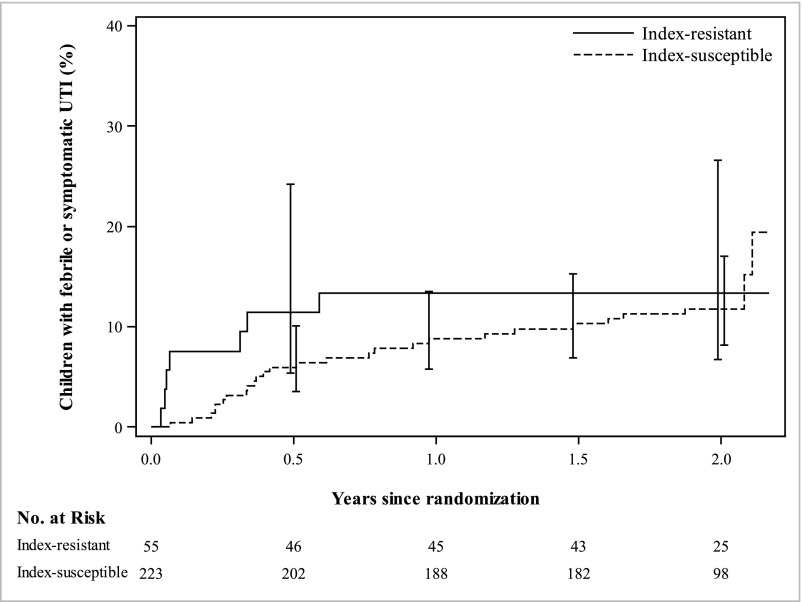
TMP-SMX resistance status of the index F/SUTI was not associated with recurrence of F/SUTI among those treated with TSP (Table 3). Among children in the TSP group who had an index F/SUTI resistant to TMP-SMX, 7 (13%) of 55 had a recurrent F/SUTI; among children in the TSP group who had an index F/SUTI susceptible to TMP-SMX, 27 (12%) of 223 had a recurrent F/SUTI. The survival analysis for recurrence of F/SUTI, comparing index-resistant to index-susceptible children in the TSP treatment arm only, adjusting for febrile index UTI, age, race/ethnicity, VUR grade, BBD, and geographic site, showed an HR of 1.38 (95% CI 0.54–3.56). Most recurrent F/SUTIs in the index-resistant group occurred early in the study period, whereas recurrences in the index-susceptible group were spread throughout the length of the study period (Fig 3); however, the overall proportion with recurrent F/SUTI was similar between index-resistant and index-susceptible groups. Limiting this analysis to only the first 6 months of the study period still did not show a significant difference (HR 2.45, 95% CI 0.77–7.85).
TABLE 3.
Incidence of rUTI, Stratified by Resistant Versus Susceptible Index UTI, Among RIVUR Subjects Randomized to TMP-SMX Treatment, With Cox Proportional Hazards Survival Analysis
| Outcome/Exposure | Unadjusted | Adjusteda | |||
|---|---|---|---|---|---|
| No. With rUTI/No. at Risk (%) | Time to 10% incidence daysb | HR (95% CI) | HR (95% CI) | Pc | |
| Recurrent UTI | |||||
| All treatment arm subjects | 34/278 (12) | 428 | N/A | N/A | N/A |
| Index UTI TMP-SMX resistant | 7/ 55 (13) | 123 | 1.13 (0.49–2.60) | 1.38 (0.54–3.56) | .50 |
| Index UTI TMP-SMX susceptible | 27/223 (12) | 541 | Reference | Reference | N/A |
Adjusted for febrile index UTI, age, race, ethnicity, VUR, BBD, and site.
The time interval (days) between enrollment and a 10% incidence of events.
Calculated using the Wald χ2 test statistic.
FIGURE 3.
Survival plot showing proportion of children experiencing rUTI during follow-up period, comparing those with susceptible versus resistant index UTI, among children in the TSP treatment arm of the RIVUR study.
Discussion
This analysis of the RIVUR cohort data found that, although rUTIs occurring in the TSP treatment group were more likely to be due to TMP-SMX–resistant organisms, the overall incidence of TMP-SMX–resistant rUTI was only slightly higher in the treatment group compared with placebo (9% vs 6%). The proportion of TMP-SMX–resistant recurrent F/SUTIs decreased over the course of the study, in both the placebo and TSP treatment groups. We also found that, among those in the TSP treatment arm, there was no difference in the incidence of recurrent F/SUTI between those with TMP-SMX–resistant versus –susceptible index F/SUTI.
This study takes advantage of the robust prospective data collection of the RIVUR trial. Entry criteria were strict and carefully documented, the cohort was well-characterized, children were followed closely during the study (clinic visits every 6 months and phone contacts every 2 months), and retention was excellent. However, certain limitations should be recognized. As noted in the methods section, the resistance classifications were based on antibiograms provided by the clinical laboratories in which care was received. There was no RIVUR-specific standardization of culture or resistance evaluation techniques. We can assume that clinical laboratories followed techniques and operating procedures in accordance with state regulations and those promoted by professional organizations such as the Clinical and Laboratory Standards Institute, but we have no way of independently verifying the techniques used. The RIVUR trial itself had limitations, including limited generalizability to children with different demographic or clinical characteristics, and our findings apply only to children with documented grade I to IV VUR. Although compliance with study medication was carefully documented by using surveys administered every 2 months, absolute certainty with respect to intake of study drug cannot be achieved because biosampling was not performed for compliance assessment purposes.
Previous randomized trials of prophylaxis to prevent rUTI in children have generally shown that, as in the RIVUR trial, resistance rates were higher in the treatment group. However, almost none of these studies have been as thorough in documentation of outcomes as the RIVUR trial, or of this duration; most reported very limited resistance data. Roussey-Kesler et al5 found that the infecting organism was resistant to TMP-SMX in 73% of treatment group recurrences, and 49% of control group recurrences. Craig et al2 conducted a trial of prophylaxis among children with a history of UTI (but not necessarily VUR) and also found that resistance to TMP-SMX was more common in UTIs of children randomized to the treatment group (67% vs 25% in the placebo group). Montini et al,6 Garin et al,7 and Pennisi et al8 each reported that recurrences in the treatment arms were more likely to be caused by resistant pathogens, although these authors did not report detailed resistance data. In the 3-armed Swedish Reflux Study, Brandström et al9 observed that although resistant rUTIs were more prevalent among girls on prophylaxis compared with those on surveillance (7/8 vs 9/24 rUTIs, P = .038), there was no difference in resistance between those in the prophylaxis group and those in the endoscopic treatment group, who were not exposed to chronic antibiotics (7/8 vs 5/10 rUTIs, P = .15).
Although the RIVUR trial used TMP-SMX as the sole prophylaxis agent, resistance patterns may be different when other agents are used. Cheng et al10 found that among children with VUR who were treated with prophylaxis by using a variety of agents, rUTI with organisms producing extended-spectrum β-lactamase was more common among children receiving cephalosporin prophylaxis compared with those on TMP-SMX, and that resistance among the TMP-SMX group overall increased minimally. Another study of breakthrough UTI in 56 children found resistant uropathogenic organisms in 78% of those on cefixime, 37% of those on cephalexin, and 37% of those on TMP-SMX.11
Differences in pathogen type between TMP-SMX–resistant and –susceptible infections were largely driven by Enterococcus, which, based on the metabolism of the organism, was not tested with respect to TMP-SMX resistance. Therefore we classified Enterococcus as resistant by definition. Excluding Enterococcus infections, proportions of E coli were relatively similar (82% E coli [42/51] among TMP-SMX–resistant rUTI versus 89% E coli [79/89] among TMP-SMX–susceptible rUTI, P = .31).
The detailed RIVUR trial data allowed us to perform unique analyses of time patterns of TMP-SMX resistance. We observed that the proportion of rUTIs that were TMP-SMX resistant decreased over the course of the study, in both the TSP treatment arm and the placebo arm, with much larger decreases in the TSP group. One explanation may be decreased medication compliance over time, from 90% compliance during the initial 2 months, to 59% compliance during the last 2 months (compliance was defined as the percentage of doses reportedly administered to each subject during the preceding 2-month time period, averaged over the entire group). We might expect the proportion of TMP-SMX–resistant rUTIs to decrease along with the decrease in exposure to the agent. However, this explanation is weakened by the fact that the same trend of decreasing TMP-SMX resistance was observed in the placebo group (albeit less dramatically than in the treatment group). The mechanism of diminishing resistance in both groups is uncertain, and may be different in each group.
Another key question addressed by this analysis is whether rUTIs are more common if the index UTI was resistant to the agent used for prophylaxis. Craig et al2 reported that if the index UTI was TMP-SMX sensitive, TSP was associated with decreased rUTI; but, if the index UTI was TMP-SMX resistant, then TSP was not effective. However, these authors did not report the impact of index UTI resistance on recurrence for the treatment group specifically. The Campbell-Walsh Urology textbook12 states (without citing any published data) that the agent chosen for prophylaxis should be different from the one used to treat the index UTI, because the fecal flora are likely to be resistant to the therapeutic agent and thus the risk of rUTI is high if the same agent is used for prophylaxis. Clinicians routinely must decide whether to use the index UTI antibiogram to guide their choice of prophylaxis agent. Our results failed to demonstrate any difference in overall incidence of rUTI among the TSP arm based on index UTI resistance to TMP-SMX; although the timing pattern of rUTI differed somewhat between the groups, the overall incidence of UTI was similar, suggesting that TSP is a reasonable option for prophylaxis in children with TMP-SMX–resistant index UTI. In practice, the choice of prophylaxis agent is based on a number of factors, including the patient’s age, allergies, the prescriber’s experience and comfort with certain agents over others, and the history of previous treatment with antibiotics.
Conclusions
Although rUTIs that occurred among children with VUR receiving TSP were more likely to be caused by TMP-SMX–resistant organisms than rUTIs among children in the placebo group, there was no clinically significant difference between groups in the proportion of children who experienced TMP-SMX–resistant rUTI. The frequency of TMP-SMX–resistant infections decreased substantially as duration of therapy increased, in both the treatment and placebo arms. Resistance of the index UTI to TMP-SMX does not reduce the efficacy of prophylaxis with this agent to prevent rUTI among children with VUR.
Acknowledgments
The authors thank the RIVUR participants and their families, and participating physicians, investigators, and staff for making this research possible
Glossary
- BBD
bladder and bowel dysfunction
- CI
confidence interval
- F/SUTI
febrile or symptomatic urinary tract infection
- HR
hazard ratio
- RIVUR
Randomized Intervention for Children with Vesicoureteral Reflux Trial
- rUTI
recurrent urinary tract infection
- TMP-SMX
trimethoprim-sulfamethoxazole
- TSP
trimethoprim-sulfamethoxazole prophylaxis
- UTI
urinary tract infection
- VUR
vesicoureteral reflux
Footnotes
Dr Nelson contributed to the design of the study, directed the analysis, drafted the initial manuscript, and approved the final manuscript as submitted; Drs Hoberman, Shaikh, Keren, Mathews, Greenfield, Mattoo, and Moxey-Mims contributed to the design of the study, reviewed the data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted; Mr Gotman devised the statistical analytic plan, performed the data analysis, critically reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr Ivanova oversaw development of the statistical analytic plan and data analysis, critically reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr Carpenter contributed to the design of the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted; and Dr Chesney contributed to the design of the study, critically reviewed and revised the manuscript, and approved early drafts of the manuscript. Dr. Chesney died before the manuscript was finalized.
This trial has been registered at clinicaltrials.gov (identifier NCT00405704).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants U01 DK074059, U01 DK074053, U01 DK074082, U01 DK074064, U01 DK074062, and U01 DK074063 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services. This trial was also supported by the University of Pittsburgh Clinical and Translational Science Award (UL1RR024153 and UL1TR000005) and the Children’s Hospital of Philadelphia Clinical and Translational Science Award (UL1TR000003), both from the National Center for Research Resources, now at the National Center for Advancing Translational Sciences, National Institutes of Health. Dr Nelson is supported by grant K23-DK088943 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hoberman A, Greenfield SP, Mattoo TK, et al. ; RIVUR Trial Investigators . Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370(25):2367–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig JC, Simpson JM, Williams GJ, et al. ; Prevention of Recurrent Urinary Tract Infection in Children with Vesicoureteric Reflux and Normal Renal Tracts (PRIVENT) Investigators . Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748–1759 [DOI] [PubMed] [Google Scholar]
- 3.Keren R, Carpenter MA, Hoberman A, et al. Rationale and design issues of the Randomized Intervention for Children With Vesicoureteral Reflux (RIVUR) study. Pediatrics. 2008;122(suppl 5):S240–S250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpenter MA, Hoberman A, Mattoo TK, et al. ; RIVUR Trial Investigators . The RIVUR trial: profile and baseline clinical associations of children with vesicoureteral reflux. Pediatrics. 2013;132(1). Available at: www.pediatrics.org/cgi/content/full/132/1/e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roussey-Kesler G, Gadjos V, Idres N, et al. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux: results from a prospective randomized study. J Urol. 2008;179(2):674–679, discussion 679 [DOI] [PubMed] [Google Scholar]
- 6.Montini G, Rigon L, Zucchetta P, et al. ; IRIS Group . Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics. 2008;122(5):1064–1071 [DOI] [PubMed] [Google Scholar]
- 7.Garin EH, Olavarria F, Garcia Nieto V, Valenciano B, Campos A, Young L. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006;117(3):626–632 [DOI] [PubMed] [Google Scholar]
- 8.Pennesi M, Travan L, Peratoner L, et al. ; North East Italy Prophylaxis in VUR study group . Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics. 2008;121(6). Available at: www.pediatrics.org/cgi/content/full/121/6/e1489 [DOI] [PubMed] [Google Scholar]
- 9.Brandström P, Esbjörner E, Herthelius M, Swerkersson S, Jodal U, Hansson S. The Swedish reflux trial in children: III. Urinary tract infection pattern. J Urol. 2010;184(1):286–291 [DOI] [PubMed] [Google Scholar]
- 10.Cheng CH, Tsai MH, Huang YC, et al. Antibiotic resistance patterns of community-acquired urinary tract infections in children with vesicoureteral reflux receiving prophylactic antibiotic therapy. Pediatrics. 2008;122(6):1212–1217 [DOI] [PubMed] [Google Scholar]
- 11.Nateghian AR, Robinson JL, Mohandessi S, Hooman N. Resistance pattern of breakthrough urinary tract infections in children on antibiotic prophylaxis. J Infect Public Health. 2009;2(3):147–152 [DOI] [PubMed] [Google Scholar]
- 12.Shortliffe LMD. Infection and inflammation of the pediatric genitourinary tract. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CP, eds. Campbell-Walsh Urology. Philadelphia, PA: Saunders; 2011 [Google Scholar]