Abstract
BACKGROUND AND OBJECTIVE:
Insufficient iron levels for optimal fetal and infant development is a concern during pregnancy and infancy. The goal of this study was to assess the effects of iron supplementation in pregnancy and/or infancy on motor development at 9 months.
METHODS:
The study was a randomized controlled trial (RCT) of infancy iron supplementation linked to an RCT of pregnancy iron supplementation, conducted in Hebei, China. A total of 1482 infants were randomly assigned to receive placebo (n = 730) or supplemental iron (n = 752) from 6 weeks to 9 months. Gross motor development (assessed by using the Peabody Developmental Motor Scale, Second Edition, instrument) was the primary outcome. Neurologic integrity and motor quality were secondary outcomes.
RESULTS:
Motor outcome was available for 1196 infants, divided into 4 supplementation period groups: (1) placebo in pregnancy/placebo in infancy (n = 288); (2) placebo in pregnancy/iron in infancy (n = 305); (3) iron in pregnancy/placebo in infancy (n = 298); and (4) iron in pregnancy/iron in infancy (n = 305). Using the Peabody Developmental Motor Scale, instrument, iron supplementation in infancy but not pregnancy improved gross motor scores: overall, P < .001; reflexes, P = .03; stationary, P < .001; and locomotion, P < .001. Iron supplementation in infancy improved motor scores by 0.3 SD compared with no supplementation or supplementation during pregnancy alone. Effects of iron supplementation in infancy alone were similar to effects with iron in both pregnancy and infancy.
CONCLUSIONS:
The RCT design supports the causal inference that iron supplementation in infancy, with or without iron supplementation in pregnancy, improved gross motor test scores at 9 months.
What’s Known on This Subject:
Iron deficiency in infancy is associated with poorer motor development. Some randomized controlled trials (RCTs) of iron supplementation in infancy show positive effects on motor behavior, but others do not. Few RCTs of iron supplementation in pregnancy reported motor outcomes.
What This Study Adds:
The study linked an infancy RCT to a pregnancy RCT of iron supplementation to support causal inferences about developmental impacts of timing and duration. Iron supplementation in infancy, regardless of supplementation in pregnancy, improved gross motor development at 9 months.
The acquisition of varied and successful motor skills in early childhood, especially gross motor skills such as locomotion,1 affects the development of cognitive2 and socio-emotional capabilities.3 Movement is considered to be a vehicle for improving knowledge of self and environment4–6 such that being active assists in learning how to learn.7 Many factors, including nutrition, contribute to motor development.8 Lack of sufficient iron, which is common during pregnancy and infancy, may have adverse effects through iron’s role in muscle and brain function.9–12 Establishing a causal connection between lack of iron and lower developmental test scores in humans largely depends on randomized controlled trials (RCTs) of iron supplementation. In a 2010 expert review that organized RCTs of iron supplementation in infancy according to duration and child age,13 6 of 8 pertinent RCTs reported benefits on motor development. The investigators considered the evidence sufficient to conclude that long-term (>2 months) iron supplementation during infancy improves motor development. There is little research on motor outcomes in infancy with iron supplementation during pregnancy. A recent summary included 4 RCTs14 and found only 1 that assessed motor development in infancy.15 Maternal iron/folate supplementation (14 weeks’ gestation to delivery) did not improve infant motor scores in the first or second year.15,16 Together with the RCTs of iron supplementation in infancy, these findings suggest motor development benefits from iron supplementation during infancy but not pregnancy.
The present study focused on developmental impacts of timing and duration of iron supplementation by linking an RCT of iron supplementation in infancy to an RCT of iron supplementation in pregnancy. Iron status and growth outcomes were reported previously. Pregnancy iron supplementation reduced iron deficiency (ID) and iron-deficiency anemia in mothers but had little impact on fetal neonatal iron status17; infancy iron supplementation reduced ID at 9 months, with no added benefit of pregnancy iron supplementation.18 There were no adverse effects of iron supplementation on infant health or growth overall or among infants who were iron-sufficient at birth. We report here the effects on gross motor development, neurologic integrity, and quality of motor behavior at 9 months. This assessment was more comprehensive than previous studies, reflecting that motor development requires behavioral and motor control as well as skill development. We hypothesized that the greatest impact would be when iron supplementation coincided with the period of rapid change in motor development (ie, during infancy). We also predicted greater benefits with iron supplementation during pregnancy and infancy than supplementation in only 1 period.
Methods
Study Setting and Design
The study (an RCT of infancy iron supplementation connected to an RCT of pregnancy iron supplementation) was designed to support causal inferences regarding the developmental effects of reducing ID in the fetus, young infant, or during both periods. The design resulted in 4 groups based on period of supplementation in pregnancy and/or infancy: (1) placebo in pregnancy/placebo in infancy (placebo/placebo); (2) placebo in pregnancy/iron in infancy (placebo/iron); (3) iron in pregnancy/placebo in infancy (iron/placebo); and (4) iron in pregnancy/iron in infancy (iron/iron). The study, conducted in rural Hebei Province, China, was approved by the ethics committees of the University of Michigan and Peking University First Hospital. The RCTs are briefly described here; full details were reported previously.17,18
Participants
Participants were infants born to women in the pregnancy RCT. The pregnancy RCT enrolled 2371 women with uncomplicated singleton pregnancies between June 2009 and December 2011 who were randomized to receive iron/folate or placebo/folate. Most attrition was due to mothers giving birth in a nonparticipating hospital. In the infancy RCT, 1482 infants were enrolled between December 2009 and June 2012 and were randomly assigned to receive placebo (n = 730) or supplemental iron (n = 752) from 6 weeks to 9 months. Infants with cord ferritin concentrations suggesting brain ID (<35 µg/L) were excluded. At 9 months, 1276 infants provided outcome data (September 2010–March 2013).18
Enrollment and Informed Consent
Mothers were informed of the infant development study at prenatal visits. After delivery, project staff provided further information and obtained signed informed consent.
Randomization and Masking
Infants were randomly assigned 1:1 to the iron or placebo group by a University of Michigan biostatistician (N.K.) using PROC SURVEYSELECT in SAS (SAS Institute, Inc, Cary, NC). The code was broken only after study completion. Supplements were in identical dark-colored bottles, and participants and personnel were unaware of group assignment.
Interventions
All participating pregnant women received daily folate (0.40 mg) and either iron (300 mg ferrous sulfate) or placebo from enrollment to birth.17 Infants received a single daily dose of ∼1 mg/kg of elemental iron as an iron protein succinylate oral solution (Ferplex,Italfarmico, S.A., Madrid, Spain) or carrier (placebo) from 6 weeks to 9 months.18
Study Outcomes
The primary motor outcome was gross motor development, assessed by using the Peabody Developmental Motor Scale, Second Edition (PDMS-2), instrument.19 Secondary outcomes were neurologic integrity, evaluated by using the Infant Neurologic International Battery (INFANIB),20 and motor quality, assessed by using the Behavior Rating Scale (BRS) of the Bayley Scales of Infant Development, Second Edition.21
The PDMS-2 gross motor dimension at 9 months provides an overall motor quotient derived from 3 subscales (reflexes, stationary, and locomotion). Reflexes reflect automatic reactions to environmental events (eg, righting reflex, parachute reflex). Stationary assesses postural control within the center of gravity and equilibrium (eg, sitting while manipulating a toy, transitioning to sit from prone). Locomotion covers moving from 1 place to another (eg, crawling, sitting to crawling or standing).19 A Chinese version of the PDMS-2 instrument is routinely used at Peking University First Hospital to track motor development and intervention effects in the rehabilitation clinic. The clinic follows the standard definition of ceiling but also elicits each child’s optimal performance by administering a preset maximum number of items in each subscale based on age. Passed items above ceiling for each subscale are included.22 In our study, only a few infants (31 of 1195) passed items above ceiling, solely in the locomotion subscale. Using scores with passes above ceiling did not affect PDMS-2 outcome in the RCT. Therefore, scoring was preserved as customary at Peking University First Hospital. Because almost all infants were similar in age at the 9-month assessment, the PDMS-2 outcomes are presented as raw scores, controlling for age in days.
The INFANIB assesses infant neurologic integrity. The total score of overall neurologic integrity is a composite derived from 20 items within 5 factors (spasticity/muscle tone, head and trunk control, vestibular function, legs/lower limb function, and French angles [shoulder and hip angles]). Items are scored 1 to 5 (abnormal to normal). Results are expressed as raw subscale and total scores, controlling for age.20
The BRS motor quality factor is based on examiner ratings of infant motor performance. The factor is generated from 8 items related to muscle tone and movement control and quality. Items are rated 1 to 5, with higher values indicating more consistently appropriate behavior.21 Results are expressed as the BRS motor quality factor total raw score, controlling for age.
Developmental testing occurred in dedicated rooms at the Maternity and Child Health Care Center. Infants were accompanied by a parent/guardian and given time for adjusting to the setting, frequent breaks, naps, and/or feeding. US and Chinese investigators trained Chinese supervisory personnel, who then jointly trained coders/testers and provided ongoing supervision. Reliability was assessed before and during testing; reliability levels were ≥90%.
Sample Size
Gross motor outcomes were available for 1196 infants. This sample size was sufficient to detect small effect size differences of ≥0.16 SD between the 2 groups in the pregnancy RCT and ≥0.23 SD for any pairwise comparison among the 4 pregnancy/infancy groups.
Statistical Methods
The primary analytic approach was based on intention to treat. A χ2 test and analysis of variance model were used to test for overall group differences in demographic and biologic data. An analysis of covariance model was used to test for group differences for primary and secondary outcomes, controlling for age at testing. SAS PROC GLMSELECT with stepwise inclusion was used to determine if additional background variables should be included in the final models. Planned pairwise comparisons were conducted if the overall statistical test results were significant. For effects of supplementation timing, key contrasts were: (1) iron/placebo versus placebo/iron, followed by (2) iron/placebo versus placebo/placebo and (3) placebo/iron versus placebo/placebo to confirm a supplementation effect. For duration, key contrasts were: (1) iron/iron versus iron/placebo and (2) iron/iron versus placebo/iron, followed by (3) iron/iron versus placebo/placebo. Two different types of effect size measures were used: partial η squared (η2) to express the magnitude of the overall association between group and dependent variable in the analysis of variance model (effect sizes were low [0.01], medium [0.06], and large [0.14])23 and Cohen’s d to indicate the difference between 2 group means in pooled SD units (small, <0.2; medium, 0.5; and large, 0.8).24 Based on primary findings, logistic regression was used to estimate the relative risk (95% confidence interval) of scoring in the lowest quartile for gross motor development based on iron supplementation in infancy. In addition, multiple/logistic regression was used to model relations between bottles of iron received and outcomes. Significance was set at P < .05.
Results
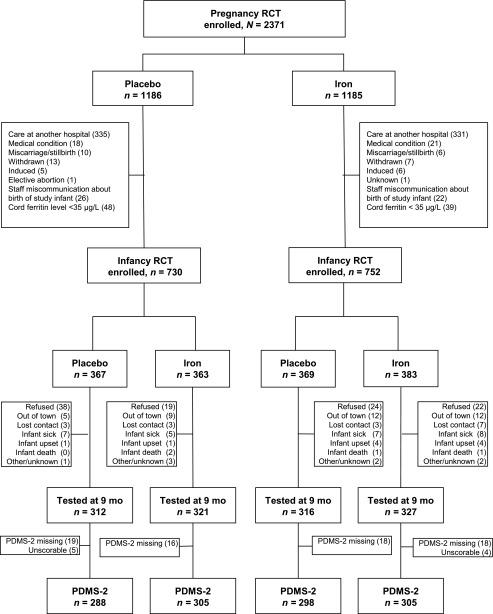
Attrition in the pregnancy RCT was largely due to women who gave birth at a nonparticipating hospital.17 The main reason for the 14% overall attrition in the infancy RCT was refusal or withdrawal (Fig 1). There was no differential attrition according to RCT group. Of the 1276 infants assessed for iron status or growth at 9 months, 1196 had data on gross motor development: placebo/placebo, n = 288; placebo/iron, n = 305; iron/placebo, n = 298; and iron/iron, n = 305. Of the 80 infants who were assessed at 9 months but did not provide gross motor development data, the PDMS-2 tool was not administered for 67 and was not scorable for 13.
FIGURE 1.
Infancy RCT: flowchart of participants.
Participant Characteristics
The groups were similar in background characteristics at birth (Table 1). Most infants were first-born. Both genders were included and approximately equally represented. Almost all were born at term (>37 weeks’ gestation) and weighed 3.36 kg on average. At 9 months, there was a suggestive overall difference in age at gross motor developmental testing. The placebo/iron and iron/iron groups averaged 1.7 days younger than the iron/placebo and placebo/placebo groups (P = .02). Mean infant weight-for-age z score was 0.89. More than 80% of the infants were breastfeeding at the time of the 9-month assessment. Mothers averaged ∼25 years of age, and most completed middle school. Most families were stressed financially; 84% had incomes below the local county threshold for public housing assistance.25 Family support of child development was similar across groups.
TABLE 1.
Infant and Family Characteristics
| Characteristic | PP (n = 288) | PI (n = 305) | IP (n = 298) | II (n = 305) | Pa |
|---|---|---|---|---|---|
| Infant characteristics at birth | |||||
| Male sex | 155/288 (54) | 141/305 (46) | 149/298 (50) | 158/305 (52) | .29 |
| Birth weight, g | 3373.3 ± 373.3 | 3368.2 ± 375.3 | 3329.5 ± 370.1 | 3379.8 ± 350.2 | .39 |
| Gestational age, wk | 39.7 ± 1.1 | 39.7 ± 1.1 | 39.8 ± 1.1 | 39.7 ± 1.1 | .69 |
| First/only child | 217/283 (77) | 235/298 (79) | 240/293 (82) | 231/300 (77) | .18 |
| ID: serum ferritin <75 µg/L or zinc protoporphyrin/heme >118 µmol/mol | 118/288 (41) | 127/305 (42) | 131/298 (44) | 127/305 (42) | .89 |
| Infant characteristics at 9 mo | |||||
| Age at testing, mo | 9.31 ± 0.42 | 9.29 ± 0.41 | 9.34 ± 0.49 | 9.25 ± 0.40 | .06 |
| 9-mo weight-for-age z score | 0.88 ± 1.02 | 0.79 ± 1.09 | 0.92 ± 0.98 | 0.97 ± 0.98 | .15 |
| Milk feeding patternb | .50 | ||||
| Only breast milk | 108/217 (50) | 115/238 (48) | 114/230 (50) | 134/237 (57) | |
| Breast milk and formula | 73/217 (34) | 78/238 (33) | 71/230 (31) | 70/237 (29) | |
| Only formula | 36/217 (17) | 45/238 (19) | 45/230 (20) | 33/237 (14) | |
| IDc | 195/286 (68)d,e | 179/298 (60)d,f | 204/296 (69)e | 175/300 (58)f | .01 |
| Anemia, hemoglobin <110 g/L | 129/286 (45)d | 101/298 (34)e | 118/296 (40)d,e | 119/300 (40)d,e | .05 |
| ID anemiag | 108/265 (41)d | 81/278 (29)e | 99/277 (36)d,e | 101/282 (36)d,e | .04 |
| Maternal and family characteristics | |||||
| Maternal age, y | 24.6 ± 4.0) | 24.8 ± 3.5) | 24.6 ± 3.8) | 25.1 ± 4.0 | .44 |
| Maternal education, high school or higher | 89/287 (31) | 115/299 (38) | 98/297 (33) | 96/302 (32) | .21 |
| Net family income, ≤50 000 yuan/y | 243/284 (86) | 245/300(82) | 236/288 (82) | 255/295 (86) | .27 |
| Maternal mood total score at 9 mo (maximum = 30, possible depression ≥10)h | 6.09 ± 4.54 | 6.06 ± 4.48) | 5.60 ± 4.37 | 6.38 ± 4.35 | .21 |
| Home Observation for Measurement of the Environment total score at 9 mo (maximum = 45) | 31.5 ± 4.0 | 31.8 ± 4.0 | 31.3 ± 4.0 | 31.5 ± 3.9 | .51 |
| Maternal ID: body iron <0 mg/kgi | 174/284 (61.3)d | 204/304 (67.1)d | 119/294 (40.5)e | 128/302 (42.4)e | <.001 |
Values are n/total (%) for categorical values and mean ± SD for continuous variables. The n values vary due to missing data. II, iron in pregnancy/iron in infancy; IP, iron in pregnancy/placebo in infancy; PI, placebo in pregnancy/iron in infancy; PP, placebo in pregnancy/placebo in infancy.
Analysis of variance for continuous variables, x2 test for categorical variables.
Feeding solid foods was generally initiated between 4 and 6 months of age. Solids were typically not iron fortified at the time.
ID was defined as ≥2 abnormal iron measurements (mean corpuscular volume <74 fl, zinc protoporphyrin/heme >69 µmol/mol heme, serum ferritin <12 µg/L).
Groups with same superscript do not differ; different letters indicate statistically significant difference (P < .05).
Groups with same superscript do not differ; different letters indicate statistically significant difference (P < .05).
Groups with same superscript do not differ; different letters indicate statistically significant difference (P < .05).
Anemia by cutoff defined as hemoglobin <110 g/L, and ID was defined as ≥2 abnormal iron measurements (mean corpuscular volume <74 fl, zinc protoporphyrin/heme >69 µmol/mol heme, serum ferritin <12 µg/L).
Maternal mood evaluated by using the Edinburgh Postnatal Depression Scale.26
Body iron was calculated by using serum ferritin and soluble transferring receptor (sTfR), according to the formula of Cook et al27: body iron (mg/kg) = – [log10(sTfR*1000/ferritin) – 2.8229]/0.1207.
The groups differed in iron status, as expected by the RCT designs (Table 1). In keeping with the findings of improved maternal iron status with iron supplementation in the pregnancy RCT,17 there was more maternal ID in the placebo/iron and placebo/placebo groups than in the iron/placebo and iron/iron groups (P < .001). However, there were no group differences in fetal-neonatal iron status at birth. Neonatal iron status was generally poor, as indicated by cord ferritin levels <75 µg/L or zinc protoporphyrin/heme ratio >118 µmol/mol in >40%. In keeping with hematology findings in the infancy RCT,18 iron status was worse in the groups that did not receive iron supplementation in infancy. Nonetheless, ID remained common: 59% in groups receiving iron in infancy (placebo/iron and iron/iron) versus 69% in infancy placebo groups (iron/placebo and placebo/placebo) (P < .001).
Study Outcomes
Groups that received iron in infancy (placebo/iron and iron/iron) reported significantly better PDMS-2 scores than those that did not (iron only during pregnancy [iron/placebo] or in neither time period [placebo/placebo]). The pattern was similar for overall gross motor score (P < .001; p-η2 = 0.02) and for the subscales: reflexes (P = .03; p-η2 = 0.01), stationary (P < .001; p-η2 = 0.03), and locomotion (P < .001; p-η2 = 0.02) (Table 2).
TABLE 2.
Primary Outcome: Gross Motor Development Assessed According to the PDMS-2 at 9 Months
| Subscale | Mean (95% CI) | Pa | Effect Size d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Timing | Duration | ||||||||||
| PP (n =288) | PI (n =305) | IP (n =298) | II (n =305) | IP Versus PI | IP versus PP | PI Versus PP | II Versus IP | II Versus PI | II Versus PP | ||
| Reflexes | 14.4 (14.3–14.6) | 14.6 (14.5–14.7) | 14.4 (14.3–14.6) | 14.7 (14.5–14.8) | .03 | 0.16 | 0.00 | 0.16 | 0.18b | 0.03 | 0.19b |
| Stationary | 33.5 (33.4–33.7) | 34.0 (33.8–34.1) | 33.4 (33.3–33.6) | 34.0 (33.8–34.1) | <.001 | 0.35b | 0.08 | 0.28b | 0.37b | 0.01 | 0.29b |
| Locomotion | 39.5 (38.7–40.2) | 41.4 (40.7–42.2) | 39.5 (38.8–40.3) | 41.3 (40.5–42.0) | <.001 | 0.30b | 0.01 | 0.30b | 0.27b | 0.03 | 0.28b |
| Overall gross motor | 87.5 (86.5–88.4) | 90.0 (89.0–90.9) | 87.4 (86.4–88.3) | 89.9 (89.0–90.8) | <.001 | 0.31b | 0.01 | 0.30b | 0.30b | 0.01 | 0.29b |
CI, confidence interval; II, iron in pregnancy/iron in infancy; IP, iron in pregnancy/placebo in infancy; PI, placebo in pregnancy/iron in infancy; PP, placebo in pregnancy/placebo in infancy. Subscale n values vary slightly due to missing data.
Analysis of covariance model covarying age at testing. Pairwise comparisons expressed as effect size d (difference between means divided by pooled SD).
Significant difference between groups, P < .05.
The timing analysis highlighted the benefits of iron supplementation on gross motor development in infancy. The placebo/iron group had higher PDMS-2 scores than the iron/placebo group (iron only during pregnancy), and placebo/iron group scores were also higher than the placebo/placebo group scores. The duration analysis indicated no added benefit of iron supplementation in either pregnancy or infancy; the placebo/iron and iron/iron groups did not differ from each other, and both were higher than the placebo/placebo group.
To further characterize the beneficial effect of infancy iron supplementation on gross motor performance, we analyzed the proportion of infants in each group with subscale scores in the lowest quartile according to PDMS-2 norms.19 For reflexes and stationary, <2% of infants had such low scores. However, 22.1% of locomotion scores were below the 25th percentile cutoff. The proportions were significantly lower in groups that received supplemental iron in infancy, compared with groups that did not (P < .001): placebo/iron, 50 (16%) of 305; iron/iron, 57 (19%) of 305; placebo/placebo, 70 (24%) of 288; and iron/placebo, 87 (29%) of 298. The risk of being in the lowest quartile was reduced by 36% in placebo/iron and iron/iron groups, compared with the iron/placebo and placebo/placebo groups (relative risk, 0.64 [95% confidence interval, 0.52–0.80]).
There were no group differences in overall neurologic integrity (INFANIB total score, P = .43). However, the groups differed in the head and trunk factor, which is most related to gross motor development (P < .001). Scores were better in groups receiving iron supplementation in infancy compared with groups that did not. Motor quality (examiner BRS ratings) did not show group differences (P = .93). There were no statistically significant relations between the number of bottles of iron received and motor outcomes.
Discussion
The uniqueness of our study design (an infancy RCT built upon a pregnancy RCT) addresses specific questions regarding timing and duration of iron supplementation and supports causal inferences. We found that iron supplementation from 6 weeks to 9 months had a positive effect on overall gross motor development at 9 months. The effect was similar whether supplementation was provided only during infancy or also to mothers during pregnancy. The benefits were mostly related to stationary and locomotor skills. Furthermore, iron supplementation during infancy reduced the proportion of children in the lowest quartile for the locomotor subscale, regardless of whether their mothers received iron supplementation during pregnancy.
These results do not seem attributable to other factors. The groups were similar with respect to background characteristics at birth; group differences in maternal ID were as expected based on results of the pregnancy RCT. The groups were also similar in family and infant characteristics at 9 months. It is unlikely that the small difference in testing age accounted for the findings. Age was a covariate in all analyses and did not remain significant in any model. Furthermore, the groups exhibiting more advanced motor development (placebo/iron and iron/iron) were the youngest, albeit only by a few days.
The results confirm our hypothesis that the greatest impact would be when iron supplementation coincided with the period of rapid change in motor development; that is, infancy. Our findings of better motor outcome with iron supplementation in infancy are in agreement with a 2010 expert review of previous RCTs.13 However, the results did not confirm our prediction of greater benefits with iron supplementation during both pregnancy and infancy. The lack of benefit of iron supplementation during pregnancy on motor development is consistent with the sole relevant RCT in a recent summary.14
There are several possible explanations for motor benefits of iron supplementation in infancy but not pregnancy. Brain areas mature at different times and need iron at different rates.11,12 Various motor domains (eg, reflexes, sensory integration, postural control, motor activity, motor coordination and planning) are subserved by different brain areas and networks. Based on current understanding, the complex brain areas and pathways involved in gross motor development mature most rapidly in the first year of life, thus requiring more iron and increasing vulnerability to lack of iron.28 Iron is specifically required for oligodendrocyte function and myelin formation.29–32 Consequently, neural pathways that are involved in motor skill acquisition, such as the corticospinal and corticostriatal tracts, may be more vulnerable to effects of ID in infancy than during gestation because these pathways are not completely myelinated at birth.33,34 Iron supplementation in infancy might also improve gross motor development indirectly by reducing concurrent behaviors associated with ID, such as withdrawal35 and lower spontaneous motor activity.36 Better motor scores in the placebo/iron and iron/iron groups did not seem to result from the potential effects on maternal behavior of iron supplementation in pregnancy. Although mothers in the iron/placebo group had better iron status than those in the placebo/iron group and might have been more proactive about their children’s development, as suggested by Perez et al,37 their infants did not exhibit better motor development at 9 months.
Gross motor development was assessed by using 3 different measures to include aspects of neuromotor development (INFANIB) and motor behavior (BRS motor quality factor) as well as global development (PDMS-2 gross motor). Although there were no group differences in the total INFANIB score, closer examination of the factor most related to gross motor development (head and trunk control) demonstrated a pattern similar to PDMS-2 results. We found no differences in BRS motor quality factor, in contrast to our previous results in a small observational study.38 The BRS is not a direct assessment of motor skills and may thus be less sensitive to the development of specific motor skills and more influenced by tester expertise and experience.
Several gross motor skills developing at ∼9 months seemed sensitive to iron supplementation in infancy. Our PDMS-2 locomotor findings denote better crawling in infants who receive iron supplementation during infancy. Onset of standing with lateral progression (ie, cruising) also occurs at ∼9 months, as does the ability to pull from sitting to standing.39–41 Our stationary subscale findings imply better performance in transitioning from sitting to standing with iron supplementation in infancy. A benefit of iron supplementation in infancy on earlier onset of specific motor milestones has been reported in some previous RCTs.42–44 Similarly, an association between better iron status in infancy and earlier onset of walking was reported in 2 observational studies.45,46 These locomotor-related benefits of iron supplementation may enhance infant development in other domains (eg, cognitive, social-emotional).2,3,40,47
Our results may not be directly comparable to previous RCTs of iron supplementation due to differences in several respects: we used a different motor assessment (PDMS-2 vs Bayley or motor milestones in other studies), our population was growing well and mainly breast-fed, and the prevalence of ID was higher than in some other studies. The hematologic response to iron supplementation was less than typically observed in other infant RCTs.48 The likely explanation for the limited reduction in prevalence of ID was a combination of poor iron status at birth, high iron needs for growth, and insufficient supplemental iron intake.18 Furthermore, the magnitude of the effects we observed might not generalize to other populations. For instance, effects might be stronger in samples with a greater reduction in ID with iron supplementation. Because infant complementary foods were not generally iron fortified at the time of our study, and delayed cord clamping was not routine, the results do not contribute to the discussion regarding alternatives to iron supplementation. In any case, our results regarding timing and duration require replication, and further research is needed on the mechanisms whereby iron supplementation can improve infant motor development.
Conclusions
The RCT design of this study supports the causal inference that iron supplementation in infancy, with or without iron supplementation in pregnancy, improved gross motor test scores at 9 months. The study confirms developmental benefits of routine iron supplementation in infancy, perhaps especially in settings in which iron deficiency is common.
Acknowledgments
We thank Drs Gengli Zhao and Min Zhou for overall direction of the pregnancy RCT; Drs Zhixiang Zhang, Twila Tardif, and Xing Li for help coordinating the pregnancy and infancy RCTs and overall supervision of the infancy RCT; and Dr Guobin Xu for supervision of laboratory measures of iron status. We also appreciate the dedicated efforts of the project physicians and nurses at Sanhe Maternal and Child Health Center.
Glossary
- BRS
Behavior Rating Scale
- ID
iron deficiency
- INFANIB
Infant Neurologic International Battery
- PDMS-2
Peabody Developmental Motor Scale, Second Edition
- RCT
randomized controlled trial
Footnotes
Dr Angulo-Barroso conceptualized and designed the study, acquired data, interpreted data, and drafted and revised the manuscript; Dr Li conceptualized and designed the study, acquired data, interpreted data, and critically reviewed the manuscript; Dr Santos interpreted the data and drafted and revised the manuscript; Ms Bian coordinated and supervised data collection and critically revised the manuscript; Ms Sturza and Mr Richards analyzed data and revised the statistical analysis sections critically for important intellectual content; Dr Jiang acquired data and critically reviewed the laboratory assessment of iron status sections; Dr Kaciroti conceptualized and designed the study, analyzed data, and revised the statistical analysis sections critically for important intellectual content; and Dr Lozoff conceptualized and designed the study, interpreted data, revised the manuscript, organized funding of the study, and provided overall supervision of the research group. All authors approved the final manuscript as submitted. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The content is solely the responsibility of the authors and does not necessarily represent the official views of funding sources. The authors had full control of primary data and did not have an agreement with the funders that limited their ability to complete the research as planned.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00613717).
FINANCIAL DISCLOSURE: Dr Lozoff was an unpaid speaker at 2 seminars supported by Lee’s Pharmaceutical Holdings Limited. The topic was iron deficiency and child development (Shanghai, April 11, 2010, and Beijing, May 15, 2011). The company covered hotel accommodations and, for the 2011 seminar, internal airfare between Hangzhou and Beijing. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: A grant from the National Institutes of Health (R01 HD052069), which included funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Office of Dietary Supplements, provided support for the infancy study and laboratory measures of iron status for mothers and infants (Dr Lozoff, Principal Investigator). Vifor Pharma, Ltd provided financial support for the pregnancy study. The São Paulo Research Foundation–FAPESP/Brazil (2014/00018-0) and Methodist University of Piracicaba–UNIMEP/Brazil provided financial support for Dr Santos. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Anderson DI, Campos JJ, Witherington DC, et al The role of locomotion in psychological development. Front Psychol 2013;4:440 [DOI] [PMC free article] [PubMed]
- 2.Clearfield MW. The role of crawling and walking experience in infant spatial memory. J Exp Child Psychol. 2004;89(3):214–241 [DOI] [PubMed] [Google Scholar]
- 3.Clearfield MW. Learning to walk changes infants’ social interactions. Infant Behav Dev. 2011;34(1):15–25 [DOI] [PubMed] [Google Scholar]
- 4.Gibson JJ. The Ecological Approach to Visual Perception. Boston, MA: Houghton Mifflin; 1979 [Google Scholar]
- 5.Piaget J. The Construction of Reality in the Child. New York, NY: Basic Books; 1954 [Google Scholar]
- 6.Thelen E. Motor development as foundation and future of developmental psychology. Int J Behav Dev. 2000;24(4):385–397 [Google Scholar]
- 7.Adolph KE. Learning to move. Curr Dir Psychol Sci. 2008;17(3):213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angulo-Barroso RM, Schapiro L, Liang W, et al. Motor development in 9-month-old infants in relation to cultural differences and iron status. Dev Psychobiol. 2011;53(2):196–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434(3):365–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(2S-2):568S–579S, discussion 580S [DOI] [PubMed] [Google Scholar]
- 11.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165 [DOI] [PubMed] [Google Scholar]
- 12.Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev Psychopathol. 2015;27(2):411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grantham-McGregor S, Baker-Henningham H. Iron deficiency in childhood: causes and consequences for childhood development. Annales Nestle. 2010;68:105–119 [Google Scholar]
- 14.Saint SE, Frick JE Prenatal supplementation and its effects on early childhood cognitive outcome. In: Wallace TC. Dietary Supplements in Health Promotion. Boca Raton, FL: Taylor and Francis Group; 2015:75-104
- 15.Li Q, Yan H, Zeng L, et al. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow-up evaluation of a double-blind, randomized, controlled trial. Pediatrics. 2009;123(4). Available at: www.pediatrics.org/cgi/content/full/123/4/e685 [DOI] [PubMed] [Google Scholar]
- 16.Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/3/e755 [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Xu G, Zhou M, et al. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J Nutr. 2015;145(8):1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lozoff B, Jiang Y, Li X, et al. Low-dose iron supplementation in infancy modestly increases infant iron status at 9 months without decreasing growth or increasing illness in a randomized clinical trial in rural China [published online ahead of print January 20, 2016]. J Nutr. doi:10.3945/jn.115.223917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folio MK, Fewell R. Peabody Developmental Motor Scales: Examiner’s Manual. 2nd ed. Austin, TX: PRO-ED, Inc; 2000 [Google Scholar]
- 20.Ellison PH, Horn JL, Browning CA. Construction of an Infant Neurological International Battery (INFANIB) for the assessment of neurological integrity in infancy. Phys Ther. 1985;65(9):1326–1331 [DOI] [PubMed] [Google Scholar]
- 21.Bayley N. Bayley Scales of Infant Development. San Antonio, TX: The Psychological Corporation; 1993 [Google Scholar]
- 22.Zhao G, Bian Y, Li M. Impact of passing items above the ceiling on the assessment results of Peabody developmental motor scales [in Chinese]. Beijing Da Xue Xue Bao. 2013;45(6):928–932 [PubMed] [Google Scholar]
- 23.Murphy KR, Myors B. Statistical Power Analysis: A Simple and General Model for Traditional and Modern Hypothesis Tests. Mahwah, NJ: Lawrence Erlbaum; 2004 [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1977 [Google Scholar]
- 25.Sanhe People’s Government Sanhe public housing benefits guidelines. 2013. Available at: www.he.xinhuanet.com/zfwq/sanhe/zhengwu/zhengwu/2013-10/21/c_117803056.htm. Accessed on June 15, 2014
- 26.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786 [DOI] [PubMed] [Google Scholar]
- 27.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364 [DOI] [PubMed] [Google Scholar]
- 28.Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(suppl 3):511–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. 2003;53(2):217–223 [DOI] [PubMed] [Google Scholar]
- 30.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58 [DOI] [PubMed] [Google Scholar]
- 31.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17(2):83–93 [DOI] [PubMed] [Google Scholar]
- 32.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr. 1998;68(3):683–690 [DOI] [PubMed] [Google Scholar]
- 33.Cai J, Zhang YP, Shields LB, et al. Correlation between electrophysiological properties, morphological maturation, and olig gene changes during postnatal motor tract development. Dev Neurobiol. 2013;73(9):713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothwell J. Control of Human Voluntary Movement. London, UK: Chapman & Hall; 1994 [Google Scholar]
- 35.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Dev. 1998;69(1):24–36 [PubMed] [Google Scholar]
- 36.Angulo-Kinzler RM, Peirano P, Lin E, Garrido M, Lozoff B. Spontaneous motor activity in human infants with iron-deficiency anemia. Early Hum Dev. 2002;66(2):67–79 [DOI] [PubMed] [Google Scholar]
- 37.Perez EM, Hendricks MK, Beard JL, et al. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135(4):850–855 [DOI] [PubMed] [Google Scholar]
- 38.Shafir T, Angulo-Barroso R, Jing Y, Angelilli ML, Jacobson SW, Lozoff B. Iron deficiency and infant motor development. Early Hum Dev. 2008;84(7):479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO Multicentre Growth Reference Study Group . WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;450(suppl 450):86–95 [DOI] [PubMed] [Google Scholar]
- 40.Bertenthal BI, Campos JJ, Barrett KC. Self-produced locomotion: an organizer of emotional cognitive, and social development in infancy. In: Emde R, Harmon R, eds. Continuity and Discontinuities in Development. New York, NY: Plenum; 1984 [Google Scholar]
- 41.Brill B. Motor development and cultural attitudes. Themes in Motor Development. 1986;35:297–313 [Google Scholar]
- 42.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112(4):846–854 [PubMed] [Google Scholar]
- 43.Olney DK, Pollitt E, Kariger PK, et al. Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5- to 11-mo old. J Nutr. 2006;136(9):2427–2434 [DOI] [PubMed] [Google Scholar]
- 44.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, et al. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ. 2001;323(7326):1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kariger PK, Stoltzfus RJ, Olney D, et al. Iron deficiency and physical growth predict attainment of walking but not crawling in poorly nourished Zanzibari infants. J Nutr. 2005;135(4):814–819 [DOI] [PubMed] [Google Scholar]
- 46.Siegel EH, Stoltzfus RJ, Kariger PK, et al. Growth indices, anemia, and diet independently predict motor milestone acquisition of infants in south central Nepal. J Nutr. 2005;135(12):2840–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biringen Z, Emde RN, Campos JJ, Appelbaum MI. Affective reorganization in the infant, the mother, and the dyad: the role of upright locomotion and its timing. Child Dev. 1995;66(2):499–514 [PubMed] [Google Scholar]
- 48.Pasricha SR, Hayes E, Kalumba K, Biggs BA. Effect of daily iron supplementation on health in children aged 4-23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Glob Health. 2013;1(2):e77–e86 [DOI] [PubMed] [Google Scholar]