Abstract
BACKGROUND:
Viral upper and lower respiratory tract infections (URI, LRI) are common in infants. We determined the prevalence of viral URI and its complications, including acute otitis media (AOM) and LRI, and assessed the effect of bacterial-viral interactions, and genetic and environmental risks on AOM development.
METHODS:
Healthy infants were enrolled from near birth and followed to the first episode of AOM up to 12 months of age. Nasopharyngeal specimens were collected at monthly intervals (months 1–6, 9) and during viral URI episodes for bacterial culture and viral polymerase chain reaction studies. Subjects were followed closely for AOM development.
RESULTS:
A total of 367 infants were followed for 286 child-years; 887 URI (305 infants) and 180 AOM episodes (143 infants) were documented. Prevalence of URI, LRI, and AOM in the first year was 3.2, 0.25, and 0.67 per child-year, respectively. Cumulative AOM incidence by ages 3, 6, and 12 months was 6%, 23%, and 46%. Infants with and without AOM had 4.7 and 2.3 URI episodes per child-year, respectively (P < .002). Pathogenic bacterial colonization rates by month were significantly higher in infants with AOM (P < .005). Breastfeeding reduced both URI and AOM risks (P < .05). Significant bacterial-viral interactions occurred with Moraxella catarrhalis and a variety of respiratory viruses and altered URI and AOM risks.
CONCLUSIONS:
Almost half of infants experienced AOM by age 1. Important AOM risk factors included frequent viral URI, pathogenic bacterial colonization, and lack of breastfeeding. Bacterial-viral interactions may play a significant role in AOM pathogenesis and deserve further investigation.
What’s Known on This Subject:
Viral upper respiratory tract infection is often complicated by bacterial infections. Medical progress has been made in the past few decades in pediatric vaccination and viral diagnostics. Birth cohort studies on respiratory infections mostly came from outside the United States.
What This Study Adds:
Updated prevalence of upper respiratory tract infection, acute otitis media, and lower respiratory tract infection in American infants in the pneumococcal conjugate vaccine era. New information on the dynamics of pathogenic bacterial colonization, viral and bacterial interactions, and acute otitis media risk factors.
Viral and bacterial infections of the respiratory tract are important causes of morbidity in the first year of life. Infants are colonized with pathogenic bacteria from the first few months and are continuously exposed to respiratory viruses.1–5 Evidence to date suggests that viruses and bacteria interact, leading to upper and lower respiratory tract infections (URIs and LRIs, respectively).6–9 Although LRI is often associated with hospitalization and high morbidity in young infants, it is less prevalent than URI, which is exceedingly common and often leads to bacterial complications, such as acute bacterial sinusitis and acute otitis media (AOM).10,11
AOM is one of the most common childhood infections, the leading cause of visits to doctors by children, and the most common reason children consume antibiotics or undergo surgery. Development of AOM early in life increases the risk for recurrent or chronic AOM later.12,13 Preventive efforts in the past decades may have led to reduction in AOM incidence. These include the advent of pneumococcal conjugate vaccines (PCV)14,15 and routine use of influenza vaccines in infants and children,16 declining smoking rates,17 and increasing breastfeeding rates.18 Data on incidence of otitis media (OM) and socioeconomic impact of OM are derived from earlier studies.12,19,20 It is important to have contemporary data on the incidence and impact of OM.
The purpose of this study was to determine the incidence of viral URI and its complications in the first year of life in a healthy, prospective birth cohort and to assess the effect of bacterial-viral interactions, and genetic and environmental risks on the development of AOM.
Methods
Study Design
This was a prospective, longitudinal study performed between October 2008 and March 2014.21 The study was approved by the University of Texas Medical Branch Institutional Review Board. Healthy infants were recruited at age <1 month from the newborn nursery or primary care pediatric clinics at University of Texas Medical Branch. Excluded were infants with preterm birth, major medical problems, or anatomic defects. Before enrollment, subjects were prescreened for polymorphisms of tumor necrosis factor α (TNFα−308) and interleukin-6 (IL-6−174) genes, which we previously found associated with AOM susceptibility.22,23 The plan was to recruit equal numbers of infants with TNF−308 polymorphism and wild type, but the matching attempt was discontinued because of the effects of Hurricane Ike (September 2008) on displacement and reduction of the Galveston population.
Subjects were followed to the first AOM episode or at least 6 months; subjects without AOM were followed to age 12 months. Data collected at enrollment and at each contact included the following: family history of AOM, number of siblings, day care attendance, cigarette smoke exposure, and breast versus formula feeding. Study personnel visited the homes monthly to collect nasopharyngeal swabs at months 1 to 6 and 9. Parents notified the study team for each URI: nasal congestion, rhinorrhea, cough, and/or sore throat, with or without constitutional symptoms such as fever, decreased appetite, and restless sleep. At URI onset, and 3 to 5 days later, the subject was seen by the study physician; time and travel were compensated. The investigator performed otoscopic examination and tympanometry, and nasopharyngeal specimens were collected for microbiological studies. Study personnel called parents weekly for 4 weeks after URI onset to assess for possible AOM symptoms. Parents were encouraged to bring the subject for examination whenever they suspected the infant might have an ear infection. Investigators trained in otoscopy (TC, JP, or DM) made the AOM diagnosis based on acute symptoms (eg, fever, irritability, otalgia), signs of tympanic membrane inflammation (intense redness, bulging, opaque tympanic membrane), and presence of middle ear fluid as documented by pneumatic otoscopy and/or tympanometry. Video-otoscopy was attempted in all cases. AOM complicating URI was defined as AOM that occurred within 28 days of URI onset,24,25 unless a new URI occurred during this period, in which case AOM was a complication of the most recent URI. LRI was defined as a lower respiratory tract illness preceded by URI.
Study personnel called the parents twice monthly to identify missed URI, AOM, or LRI episodes. At subject discharge, the electronic medical record was reviewed for any missed URI, AOM, or LRI.
Nasopharyngeal swab specimens were tested for bacteria by culture, and for viruses by polymerase chain reaction, as previously described.21 Polymerase chain reaction assays detected 13 respiratory viruses, including adenovirus; bocavirus; coronaviruses 229E, NL63, and OC43; enterovirus; human metapneumovirus (MPV); influenza A and B; parainfluenza viruses 1 and 3; respiratory syncytial virus (RSV); and rhinovirus (RV).
Statistics
The rate of bacterial colonization between AOM and no-AOM groups was compared by using linear trend in the logistic model. The effect of colonization on presence of AOM was estimated by using a mixed model with month and AOM status as factors along with an interaction effect; subject was a random intercept effect. The number of URIs was compared between subjects with and without AOM by using a standard Poisson model. Because the encounters for determining URI as well as viral and bacterial presence were not uniform or lasted the same amount of time, a multivariate recurring events Cox proportional hazards model was used to compare the incidences of URI and AOM by viral or bacterial copresence; the subject was considered a random effect. A similar multivariate hazards model was used to assess the effect of static or changing environmental factors as well as genotype. Statistical significance was declared with P < .05. All calculations and models were run in R (cran.r-project.org), using add-on libraries such as survival and mgcv.
Results
A total of 367 subjects were enrolled and followed; of these, 311 (85%) completed the study and 56 (15%) were lost to follow-up. Twenty-one percent of subjects were followed for 6 months, 14% until the first AOM episode between age 6 and 12 months, and 65% for 12 months. Characteristics of the subjects, follow-up duration, and the number of URI episodes and complications are shown in Table 1. Environmental factor data are shown in Supplemental Table 7.
TABLE 1.
Subject Characteristics, and Number of URI, AOM, and LRI Episodes
| Total | Completed Subjects | Dropped Subjects | |
|---|---|---|---|
| No. of subjects | 367 | 311 | 56 |
| Boys | 199 (54)a | 168 (54) | 31 (55) |
| Race | |||
| White | 278 (76) | 235 (76) | 43 (77) |
| Black | 84 (23) | 72 (23) | 12 (21) |
| Asian | 5 (1) | 4 (1) | 1 (2) |
| Ethnicity | |||
| Hispanic/Latino | 183 (50) | 159 (51) | 24 (43) |
| Not Hispanic/Latino | 184 (50) | 152 (49) | 32 (57) |
| TNF−308 polymorphism (363 tested) | 104 (29) | 89 (29) | 15 (31) |
| IL-6–174 polymorphism (364 tested) | 163 (45) | 139 (45) | 24 (48) |
| Patient months | 3436 | 3204 | 232 |
| First 6 mo | 2078 | 1866 | 212 |
| 6–12 mo | 1358 | 1338 | 20 |
| URI episodes | 887 (305)b | 859 (287) | 28 (19) |
| First 6 mo | 605 | 577 | 28 |
| 6–12 mo | 283 | 283 | 0 |
| AOM episodes | 180 (143) | 180 (143) | 0 (0) |
| First 6 mo | 87 | 87 | 0 |
| 6–12 mo | 93 | 93 | 0 |
| LRI episodes | 66 (51) | 66 (51) | 0 (0) |
| First 6 mo | 38 | 38 | 0 |
| 6–12 mo | 28 | 28 | 0 |
No statistically significant difference in demographic and gene polymorphism data in completed subjects and dropped subjects.
Percentage of total.
Number of patients.
URI and Complications
Of 887 URI episodes (305 infants), the investigators saw 413 (48%); the remaining were documented by phone or medical record review. Table 2 summarizes details regarding age and cumulative incidence of the first URI, AOM, and LRI. Subjects experienced between 1 and 9 URI episodes (mean = 3); URI prevalence was 3.7 and 3.2 per child-year in the first 6 and 12 months, respectively.
TABLE 2.
The First Episode of URI, AOM, and LRI in 311 Subjects and Cumulative Incidence by Month
| Age at the First Episode | URI Episodes | AOM Episodes | LRI Episodes | |||
|---|---|---|---|---|---|---|
| n | Cumulative Incidence | n | Cumulative Incidence | n | Cumulative Incidence | |
| % (CI) | % (CI) | % (CI) | ||||
| 1st mo of life | 44 | 14 (10.7–18.5) | 0 | 0.0 | 4 | 1 (0.48–3.4) |
| 2nd mo | 60 | 33 (28.5–39.0) | 5 | 2 (0.68–3.8) | 3 | 2 (1.1–4.7) |
| 3rd mo | 50 | 50 (44.1–55.2) | 15 | 6 (4.2–9.8) | 6 | 4 (2.4–7.1) |
| 4th mo | 37 | 61 (56.0–66.8) | 16 | 12 (8.5–11.6) | 6 | 6 (3.9–9.4) |
| 5th mo | 28 | 70 (65.3–74.5) | 20 | 18 (14.2–22.7) | 8 | 9 (6.0–12.4) |
| 6th mo | 21 | 77 (72.4–81.7) | 14 | 23 (18.3–27.6) | 6 | 11 (7.7–14.6) |
| >6–12 mo of age | 47 | 92 (89.0–94.9) | 73 | 46 (40.7–51.7) | 18 | 18 (13.9–23.0)a |
| No. of subjects | 287a | 143 | 51 | |||
CI, confidence interval. Cumulative incidence = % of subjects with at least 1 episode of URI, AOM, or LRI by specific age.
Only 203 subjects were followed to 12 mo.
Sinusitis or LRI occurring within 28 days of URI onset were considered URI complications. Sinusitis was diagnosed only clinically (mostly by nonstudy physicians), based on persistence and/or severity of symptoms. Clinical sinusitis was recorded after 41 URI episodes in 37 subjects (4.6% of URI episodes). Because of the uncertainty in the diagnosis of sinusitis in this age group, no further details on sinusitis are reported.
There were 66 LRI episodes (51 subjects) that occurred after URI; the rate of LRI complicating URI was 7.6%. The age at the first LRI diagnosis is shown in Table 2; the LRI diagnoses are shown in Supplemental Table 8. The prevalence of LRI in the first 6 and 12 months of life was 0.25 and 0.24 per child-year, respectively.
AOM Incidence and Prevalence
Overall, 143 infants (46%) experienced 180 AOM episodes, all but 2 within 28 days of a preceding URI. Two AOM episodes were diagnosed without history of preceding URI. The rate of AOM after URI was 21% (178 of 859). AOM was diagnosed, on average, 5 days after URI onset (median = 3 days). Investigators diagnosed 104 AOMs (58%); others diagnosed 76, 22 of these with LRI were diagnosed by the hospital physicians. Of AOM episodes diagnosed by others, 93% documented solid evidence of AOM, including bulging, purulent fluid/otorrhea, and/or opacification; 7% lacked documentation. Having no evidence to invalidate the diagnosis, we did not exclude these cases from further analyses. The cumulative incidence of AOM (percentage of subjects with at least 1 AOM episode) by specific age is shown in Table 2. The prevalence of AOM at ages 6 and 12 months was 0.56 and 0.67 per patient-year, respectively.
Factors Affecting AOM Occurrences in the First Year of Life
Nasopharyngeal Bacterial Colonization
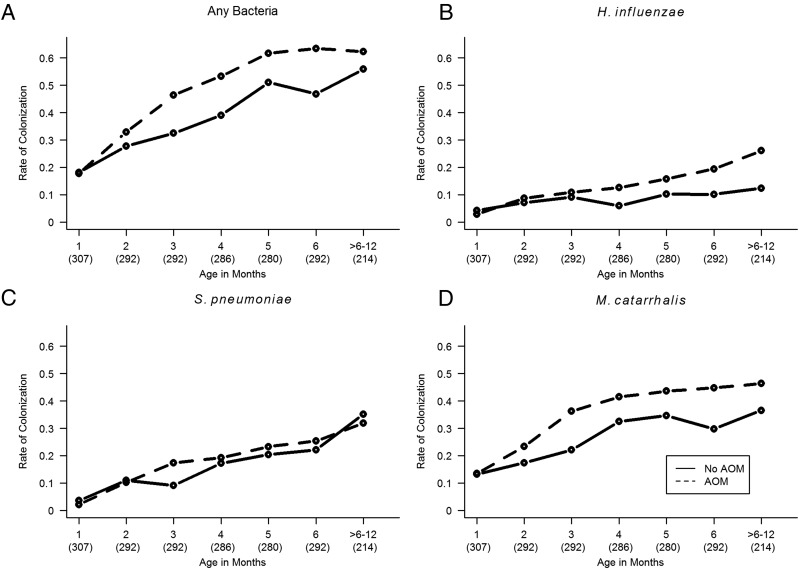
Bacterial culture results from nasopharyngeal swabs collected monthly and during URI/AOM episodes in subjects with (n = 143) and without AOM (n = 168) in the first year of life are shown in Fig 1. Colonization rates with 3 pathogenic bacteria increased with age. Infants with AOM were colonized more often with pathogenic bacteria overall (P < .005), with Haemophilus influenzae (P < .001), and with Moraxella catarrhalis (P = .015). Statistically significant difference was not detected for Streptococcus pneumoniae (P = .54).
FIGURE 1.
Rate of nasopharyngeal bacterial colonization by age in subjects with (n = 143, dotted line) and without (n = 168, solid line) AOM in the first year of life. A, Colonization with any pathogenic bacteria; B, colonization with H influenzae; C, S pneumoniae; D, M catarrhalis. Numbers in parentheses are numbers of nasopharyngeal swabs; age was adjusted to the nearest month.
Viral URI
A total of 311 subjects had 859 URI episodes. There were 466 URI episodes in 143 subjects with AOM and 393 episodes in 168 subjects without AOM. Subjects with AOM had significantly more frequent URIs than those without (4.7 vs 2.3 episodes per child-year; P < .002). Types of viruses and viral load in URI with or without AOM from cases seen by the study group were previously reported.21 Overall, 2153 specimens from 362 subjects were tested21; viruses were detected in 76% of URI samples: 60% with a single virus and 40% with multiple viruses. Viruses in single-virus samples were as follows: RV (55%), RSV (11%), parainfluenza (8%), coronavirus (8%), MPV (7%), adenovirus (4%), enterovirus (3%), bocavirus (2%), and influenza (2%).
Viral and Bacterial Interactions During URI and AOM
Of 413 URI episodes seen by the study group, bacterial and viral data, obtained within 7 days of URI onset, were available in 395 (96%) episodes. Tables 3 and 4 report hazard ratios (HRs), comparing viral and/or bacterial pathogens, against reference. Table 3, top, shows HRs during URI events by presence of bacteria or viruses. The presence of any virus, M catarrhalis, or S pneumoniae was associated with an increased URI risk. Data from this model also suggested significant interactions between M catarrhalis (but not other bacteria) and RSV, RV, and MPV; therefore, we further analyzed these specific interactions within each subset (Table 3, bottom). Presence of RSV, or M catarrhalis, but not both, increased URI risk compared with neither. There was no significant difference detected between cases with RSV, compared with M catarrhalis on URI risk in this subset. For RV and MPV, presence of M catarrhalis, virus, or both significantly increased URI risk, compared with neither. Also, the presence of RV or MPV increased URI risk, compared with presence of M catarrhalis without virus.
TABLE 3.
HRs for URI by Presence of Virus and/or Bacteria in the Nasopharynx (n = 395)
| Presence of Virus and/or Bacteria | n | % | HR (95% CI) | HR (95% CI) | HR (95% CI) |
|---|---|---|---|---|---|
| No virus or bacteria | 50 | 13 | ref | — | — |
| Any virus | 295 | 75 | 8.70 (6.20–12.2) | — | — |
| M catarrhalisa | 211 | 53 | 6.10 (4.04–9.23) | — | — |
| S pneumoniaea | 98 | 25 | 2.18 (1.44–3.31) | — | — |
| H influenzaea | 70 | 18 | 1.15 (0.89–1.48) | — | — |
| No RSVb or M catarrhalis | 188 | 48 | ref | — | — |
| No RSV, M catarrhalis | 177 | 45 | 1.33 (1.08–1.63) | ref | — |
| RSV, no M catarrhalis | 15 | 4 | 1.73 (1.15–2.60) | 1.30 (0.85–2.00) | ref |
| RSV and M catarrhalis | 15 | 4 | 1.24 (0.84–1.83) | 0.93 (0.62–1.40) | 0.72 (0.50–1.18) |
| No RVb or M catarrhalis | 116 | 29 | ref | — | — |
| No RV, M catarrhalis | 98 | 25 | 1.63 (1.25–2.13) | ref | — |
| RV, no M catarrhalis | 87 | 22 | 2.58 (1.98–3.38) | 1.48 (1.14–2.19) | ref |
| RV and M catarrhalis | 94 | 24 | 2.51 (1.98–3.18) | 1.54 (1.16–2.04) | 0.97 (0.76–1.25) |
| No MPVb or M catarrhalis | 194 | 49 | ref | — | — |
| No MPV, M catarrhalis | 177 | 45 | 1.33 (1.09–1.62) | ref | — |
| MPV, no M catarrhalis, | 9 | 2 | 3.69 (2.25–6.06) | 2.87 (1.66–4.65) | ref |
| MPV and M catarrhalis | 15 | 4 | 2.03 (1.40–2.94) | 1.53 (1.07–2.20) | 0.55 (0.30–1.00) |
CI, confidence interval
With or without other bacteria.
With or without other viruses.
TABLE 4.
HRs for AOM by Presence of Virus and/or Bacteria in the Nasopharynx (n = 83)
| Presence of Virus and/or Bacteria | n | % | HR (95% CI) | HR (95% CI) | HR (95% CI) |
|---|---|---|---|---|---|
| No virus or bacteria | 14 | 17 | ref | — | — |
| Any virus | 69 | 83 | 1.50 (0.99–2.22) | — | — |
| M catarrhalisa | 50 | 60 | 1.00 (0.62–1.60) | — | — |
| S pneumoniaea | 29 | 35 | 165.0 (45.0–601) | — | — |
| H influenzaea | 23 | 28 | 1.48 (0.99–2.22) | — | — |
| No RVb or M catarrhalis | 17 | 21 | ref | — | — |
| No RV, M catarrhalis | 21 | 25 | 18.10 (5.50–59.6) | ref | — |
| RV, no M catarrhalis | 16 | 19 | 30.00 (10.0–90.0) | 1.66 (0.73–3.79) | ref |
| RV and M catarrhalis | 29 | 35 | 25.40 (9.30–69.5) | 1.40 (0.71–2.77) | 0.85 (0.50–1.42) |
| No RSVb or M catarrhalis | 31 | 37 | ref | — | — |
| No RSV, M catarrhalis | 42 | 51 | 18.10 (5.50–59.60) | ref | — |
| RSV, no M catarrhalis | 2 | 2 | 56.20 (22.1–142.6) | 3.10 (1.18–4.9) | ref |
| RSV and M catarrhalis | 8 | 10 | 12.70 (4.35–37.13) | 0.70 (0.29–1.73) | 0.23 (0.11–0.48) |
CI, confidence interval
With or without other bacteria.
With or without other viruses.
Table 4 reports AOM risk by presence of virus and/or bacteria. Of 104 AOM episodes diagnosed or confirmed by the study group, there were 83 (80%) episodes with available bacterial and viral data within 7 days of AOM diagnosis and before antibiotic treatment. The presence of any virus or S pneumoniae significantly increased the AOM risk, compared with no pathogens (Table 4, top). Data also suggested interactions of M catarrhalis, but not other bacteria, with RSV and RV. Therefore, we further analyzed these specific subsets (Table 4, bottom). The presence of M catarrhalis, RV, or both increased AOM risk, compared with neither; but the presence of RV did not alter AOM risk, compared with presence of M catarrhalis. The presence of M catarrhalis, RSV, or both increased AOM risk, compared with neither. Also, RSV, compared with M catarrhalis, increased AOM risk, but M catarrhalis with RSV decreased AOM risk, compared with RSV without M catarrhalis.
Environmental and Genetic Factors
We collected environmental risk data at enrollment and during monthly visits, URI and AOM visits, and phone interviews, an average of 12 data encounters per subject. Environmental and genetic factors associated with URI and AOM risks, modeled through a multivariate logistic mixed model, are shown in Tables 5 and 6. We analyzed data in the first 6 months of life because all infants were followed until 6 months of age (n = 239 infants); available data after 6 months were not uniform, as the subjects completed the study after the first AOM episode was diagnosed. Day care attendance and multiple siblings were associated with increased URI risk. Decreased URI risk was associated with birth after February 2010, exclusive breastfeeding >6 months, increased breastfeeding duration, and increased length of time to exclusive formula feeding.
TABLE 5.
Environmental and Genetic Factors Associated With URI in the First 6 Months (n = 239 Infants)
| Factor | n | HR (95% CI) | P |
|---|---|---|---|
| Cigarette exposurea | 54 | 1.15 (0.89–1.49) | .28 |
| Day care attendanceb | 67 | 1.74 (1.44–2.11) | <.0001 |
| One or more sibling(s) in the homec | 86 | 1.07 (1.01–1.14) | .032 |
| Breastfeeding exclusively for at least 6 mod | 22 | 0.63 (0.40–0.99) | .049 |
| Length of breastfeeding, moe | 95 | 0.96 (0.93–0.99) | .0075 |
| Length of time to exclusive formula feeding, mo | 268 | 0.96 (0.93–0.98) | .0009 |
| Length of time to other feeding, mof | 271 | 0.97 (0.93–1.00) | .070 |
| Born after February 2010g | 220 | 0.84 (0.75–0.93) | .0012 |
| TNFα−308 polymorphismh | 81 | 1.19 (0.69–1.01) | .063 |
| IL-6–174 polymorphismh | 127 | 1.06 (0.89–1.27) | .50 |
CI, confidence interval
Any smoking in the household.
Yes/no, any length of time.
Linear function, taking into account the number of siblings.
The length 6 mo was chosen because it optimized the model fit to the data (ie, maximized the likelihood).
Length of breastfeeding, not necessarily exclusively.
Other food than breast milk or formula.
Heptavalent PCV (7) was given before February 2010; all subjects received PCV13 thereafter. Of the subjects completing the study, 73% had received 4 doses of PCV vaccines, 18% received 3 doses, 5% received 2 doses, and 3% received only 1 dose.
Homozygous and heterozygous.
TABLE 6.
Environmental and Genetic Factors Associated With AOM in the First 6 Months (n = 70 Infants)
| Factor | n | HR (95% CI) | P |
|---|---|---|---|
| Cigarette exposurea | 54 | 0.74 (0.48–1.14) | .17 |
| Day care attendanceb | 67 | 1.19 (0.79–1.80) | .41 |
| One or more sibling(s) in the homec | 86 | 1.07 (0.97–1.18) | .20 |
| Breastfeeding exclusively at least 3 mod | 245 | 0.40 (0.18–0.90) | .028 |
| Length of breastfeeding, moe | 95 | 0.85 (0.78–0.93) | .0003 |
| Length of time to exclusive formula feeding, mo | 268 | 0.76 (0.69–0.83) | <.0001 |
| Length of time to other feeding, mof | 271 | 0.89 (0.70–1.00) | .051 |
| Born after February 2010g | 220 | 0.89 (0.71–1.11) | .31 |
| TNFα−308 polymorphismh | 81 | 0.71 (0.49–1.03) | .069 |
| IL-6–174 polymorphismh | 127 | 0.79 (0.55–1.15) | .23 |
CI, confidence interval
Any smoking in the household.
Yes/no, any length of time.
Linear function, taking into account the number of siblings.
The length 3 mo was chosen because it optimized the model fit to the data (ie, maximized the likelihood).
Length of breastfeeding, not necessarily exclusively.
Other food than breast milk or formula.
Heptavalent PCV (7) was given before February 2010; all subjects received PCV13 thereafter. Of the subjects completing the study, 73% had received 4 doses of PCV vaccines, 18% received 3 doses, 5% received 2 doses, and 3% received only 1 dose.
Homozygous and heterozygous.
Seventy infants experienced 87 AOM episodes before age 6 months. Compared with infants without AOM, decreased AOM risk was associated with the following: exclusive breastfeeding >3 months, increased duration of breastfeeding, and increased length of time to exclusive formula feeding. We did not detect any environmental or genetic factor that increased AOM risk in this model.
Discussion
In this large American birth cohort study performed during the PCV and influenza vaccine era, infants experienced 3.2 URIs per child-year. Birth cohort studies on viral respiratory infections have been conducted mostly outside the United States and have focused on LRI and childhood asthma2,3,26–28; we focused on AOM, the most common URI complication. We clearly showed that frequent viral infections, bacterial colonization, and lack of breastfeeding are major AOM risk factors. Our data also suggested that interactions between M catarrhalis and respiratory viruses may alter the risk for both URI and AOM.
Our study provided close follow-up of subjects for URI and AOM and our passive surveillance of data from electronic medical records covered all pediatric practices in Galveston plus review of AOM diagnoses by others. AOM incidence in our study was 6%, 23%, and 46% by ages 3, 6, and 12 months, respectively, an appreciable decrease from incidence in previous studies with similar design. Data from studies in the late 1980s and 1990s reported 18% AOM incidence at age 3 months,29 30% to 39% at 6 months,12,29,30 and 60% to 62% at 1 year.12,29 It is likely that medical interventions in the past few decades, such as the use of pneumococcal and influenza virus vaccines, higher breastfeeding rates,18 and decreased smoking,17 helped reduce AOM incidence. More recent reports from population birth cohort studies using questionnaire or parental interview have reported even lower OM incidences (eg, 5%–16% at 6 months,31,32 and 23% at 1 year of age32).
Viral respiratory infections are common in infants. More than 75% of our subjects had developed a URI by age 6 months while they may still have had maternal antibodies, and >90% had URI by 12 months. LRI was diagnosed in 11% and 18% by age 6 and 12 months, respectively. Because of our focus on AOM, many infants completed the study after the first AOM episode, or at age 6 months. Therefore, the incidence of URI and LRI in the second 6 months of life may not be as accurate as during the first 6 months due to a smaller number of subjects remaining in the study. Nevertheless, our data point to the significant current morbidity of viral respiratory infections and the clear benefit of breastfeeding in reducing both URI and AOM, as has been previously shown. Interestingly, we found that infants born after 2010, who received PCV13 in place of PCV7, experienced a decreased URI risk but not AOM risk. The number of AOMs was small and these results may not be related specifically to PCV immunization. The small number of AOM events may also explain lack of association between day care attendance or number of siblings and increased AOM risk.
Our previous studies of children age 6 months to 9 years had shown associations between TNF−308 and IL-6−174 polymorphisms and OM susceptibility.22,23 The original aim of the current study was to determine the mechanisms by which genetic risk, as represented by TNF−308 renders the host OM susceptible. Unfortunately, our attempt to enroll equal numbers of infants with TNF−308 polymorphism and matched controls was discontinued because of the disruption of the Galveston population after Hurricane Ike (2008). The final proportions of subjects with TNF−308 and IL-6−174 polymorphisms were within populations previously described (3%–44% for TNF−308 and 32%–47% for IL-6−174).22,23,33,34 Interestingly, these polymorphisms did not affect AOM susceptibility in this study. The difference in results may be from the young age of our subjects. Because maternal antibodies protect infants from infections early in life, it is possible that the effects of these polymorphisms on AOM susceptibility are evident only at a later age when AOM incidence is higher (eg, at peak age incidence of 6–36 months). A recent study of 96 infants <9 months of age also did not find TNF−308 and IL-6−174 to be associated with AOM susceptibility.35
Because of the wide variety of respiratory viruses and pathogenic bacteria, analyses of viral-bacterial interactions are complex, especially in studies with longitudinal data. Previous studies have analyzed associations between viral-bacterial interactions and AOM risk by comparing data from cases with and without AOM.6,36 Others have studied viral-bacterial interactions in asymptomatic children.37,38 We analyzed the risk, as represented by HR, during URI and AOM events given the presence of specific bacteria and viruses. Interestingly, we found that not only viruses increased URI risk, M catarrhalis and S pneumoniae also increased URI risk. On the other hand, we found better protection for S pneumoniae (infants born after 2010) associated with decreased URI risk. One recent study of children with URI symptoms reported that rhinitis, nasal congestion, and cough had positive associations with M catarrhalis colonization39; another study reported a positive association between rhinitis and H influenzae colonization.40 These findings taken together suggest that colonizing bacteria may also enhance URI symptoms during viral infection.
During URI, presence of M catarrhalis increased URI risk, regardless of the presence or absence of viruses. Interestingly, the M catarrhalis–RSV interaction showed a significant decrease in AOM risk compared with that with RSV alone, but the numbers were small. These complex viral-bacterial interactions may provide clues to the pathogenesis of URI and AOM, but studies using larger samples are needed. Better knowledge of these mechanisms could lead to interventions such as development of new bacterial and viral vaccines, which would help reduce the burden of these common childhood diseases.
Conclusions
URI was common during infancy and contributed to complications such as AOM, sinusitis, and LRI. Compared with previous decades, the incidence of AOM has decreased. Prolonged breastfeeding was associated with significant reductions of both URI and AOM. S pneumoniae and M catarrhalis were important coinfecting bacteria during URI in infants. Viral-bacterial interactions during URI and AOM may increase the risk of disease manifestations. These complex viral-bacterial interactions require further investigations.
Acknowledgments
We thank the study subjects, their parents, and their primary care physicians who allowed us to study their patients. We also thank Alejandro Diego, Stella Kalu, Linda Ede, Tal Marom, Esther Valdivia, Lilia Rodriquez, and Ying Xiong for their assistance with the study subjects and specimen processing.
Glossary
- AOM
acute otitis media
- HR
hazard ratio
- IL-6
interleukin-6
- LRI
lower respiratory tract infection/illness
- MPV
metapneumovirus
- OM
otitis media
- PCV
pneumococcal conjugate vaccine
- RSV
respiratory syncytial virus
- RV
rhinovirus
- TNF
tumor necrosis factor
- URI
upper respiratory tract infection
Footnotes
Dr Chonmaitree conceptualized and designed the study, oversaw the study conduct, drafted the manuscript, and takes responsibility for the integrity of the data; Dr Trujillo coordinated the data collection, tabulated clinical data, reviewed the manuscript and approved the manuscript as submitted; Dr Jennings carried out the statistical analyses, reviewed the manuscript, and approved the manuscript as submitted; Drs Alvarez-Fernandez and Nokso-Koivisto coordinated the data collection, reviewed the manuscript, and approved the manuscript as submitted; Dr Patel co-conceptualized and co-designed the study, participated in clinical data collection, reviewed and revised the manuscript, and approved the manuscript as submitted; Dr Loeffelholz co-conceptualized and co-designed the study, participated in microbiological data collection and analyses, reviewed the manuscript, and approved the manuscript as submitted; Dr Matalon co-conceptualized and co-designed the study, reviewed the manuscript, and approved the manuscript as submitted; Dr Pyles conceptualized and supervised molecular virology studies, reviewed the manuscript, and approved the manuscript as submitted; Mr Miller co-conceptualized and performed molecular virology studies, reviewed the manuscript, and approved the manuscript as submitted; and Dr McCormick co-conceptualized and co-designed the study, participated in clinical data collection, drafted parts of the manuscript, and approved the manuscript as submitted.
Dr Trujillo’s current affiliation is San Juan City Hospital, San Juan, Puerto Rico; the current affiliation of Dr Alvarez-Fernandez is the University of Puerto Rico, San Juan, Puerto Rico; and the current affiliation of Dr Nokso-Koivisto is the University of Helsinki, Helsinki, Finland.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by the National Institutes of Health research grants R01DC005841 and UL1TR001439. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175(6):1440–1445 [DOI] [PubMed] [Google Scholar]
- 2.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25(8):680–686 [DOI] [PubMed] [Google Scholar]
- 3.Regamey N, Kaiser L, Roiha HL, et al. ; Swiss Paediatric Respiratory Research Group . Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J. 2008;27(2):100–105 [DOI] [PubMed] [Google Scholar]
- 4.Fairchok MP, Martin ET, Chambers S, et al. Epidemiology of viral respiratory tract infections in a prospective cohort of infants and toddlers attending daycare. J Clin Virol. 2010;49(1):16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller EK, Gebretsadik T, Carroll KN, et al. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J. 2013;32(9):950–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011;49(11):3750–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming-Dutra KE, Taylor T, Link-Gelles R, et al. Effect of the 2009 influenza A(H1N1) pandemic on invasive pneumococcal pneumonia. J Infect Dis. 2013;207(7):1135–1143 [DOI] [PubMed] [Google Scholar]
- 8.Esposito S, Zampiero A, Terranova L, et al. Pneumococcal bacterial load colonization as a marker of mixed infection in children with alveolar community-acquired pneumonia and respiratory syncytial virus or rhinovirus infection. Pediatr Infect Dis J. 2013;32(11):1199–1204 [DOI] [PubMed] [Google Scholar]
- 9.Weinberger DM, Grant LR, Steiner CA, et al. Seasonal drivers of pneumococcal disease incidence: impact of bacterial carriage and viral activity. Clin Infect Dis. 2014;58(2):188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marom T, Alvarez-Fernandez PE, Jennings K, Patel JA, McCormick DP, Chonmaitree T. Acute bacterial sinusitis complicating viral upper respiratory tract infection in young children. Pediatr Infect Dis J. 2014;33(8):803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46(6):815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160(1):83–94 [DOI] [PubMed] [Google Scholar]
- 13.Kvaerner KJ, Nafstad P, Hagen JA, Mair IW, Jaakkola JJ. Recurrent acute otitis media: the significance of age at onset. Acta Otolaryngol. 1997;117(4):578–584 [DOI] [PubMed] [Google Scholar]
- 14.Eskola J, Kilpi T, Palmu A, et al. ; Finnish Otitis Media Study Group . Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–409 [DOI] [PubMed] [Google Scholar]
- 15.Marom T, Tan A, Wilkinson GS, Pierson KS, Freeman JL, Chonmaitree T. Trends in otitis media-related health care use in the United States, 2001–2011. JAMA Pediatr. 2014;168(1):68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Academy of Pediatrics Committee on Infectious Diseases . Prevention of influenza: recommendations for influenza immunization of children, 2008–2009. Pediatrics. 2008;122(5):1135–1141 [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014 [Google Scholar]
- 18.National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Breastfeeding Report Card 2013. Available at: www.cdc.gov/breastfeeding/pdf/2013BreastfeedingReportCard.pdf
- 19.Stool SE, Field MJ. The impact of otitis media. Pediatr Infect Dis J. 1989;8(suppl 1):S11–S14 [PubMed] [Google Scholar]
- 20.Gates GA. Cost-effectiveness considerations in otitis media treatment. Otolaryngol Head Neck Surg. 1996;114(4):525–530 [DOI] [PubMed] [Google Scholar]
- 21.Chonmaitree T, Alvarez-Fernandez P, Jennings K, et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clin Infect Dis. 2015;60(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel JA, Nair S, Revai K, et al. Association of proinflammatory cytokine gene polymorphisms with susceptibility to otitis media. Pediatrics. 2006;118(6):2273–2279 [DOI] [PubMed] [Google Scholar]
- 23.Revai K, Patel JA, Grady JJ, Nair S, Matalon R, Chonmaitree T. Association between cytokine gene polymorphisms and risk for upper respiratory tract infection and acute otitis media. Clin Infect Dis. 2009;49(2):257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanyal MA, Henderson FW, Stempel EC, Collier AM, Denny FW. Effect of upper respiratory tract infection on eustachian tube ventilatory function in the preschool child. J Pediatr. 1980;97(1):11–15 [DOI] [PubMed] [Google Scholar]
- 25.Koivunen P, Kontiokari T, Niemelä M, Pokka T, Uhari M. Time to development of acute otitis media during an upper respiratory tract infection in children. Pediatr Infect Dis J. 1999;18(3):303–305 [DOI] [PubMed] [Google Scholar]
- 26.van der Zalm MM, Uiterwaal CS, Wilbrink B, et al. Respiratory pathogens in respiratory tract illnesses during the first year of life: a birth cohort study. Pediatr Infect Dis J. 2009;28(6):472–476 [DOI] [PubMed] [Google Scholar]
- 27.Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mommers M, Thijs C, Stelma F, et al. Timing of infection and development of wheeze, eczema, and atopic sensitization during the first 2 yr of life: the KOALA Birth Cohort Study. Pediatr Allergy Immunol. 2010;21(6):983–989 [DOI] [PubMed] [Google Scholar]
- 29.Duffy LC, Faden H, Wasielewski R, Wolf J, Krystofik D. Exclusive breastfeeding protects against bacterial colonization and day care exposure to otitis media. Pediatrics. 1997;100(4). Available at: www.pediatrics.org/cgi/content/full/100/4/E7 [DOI] [PubMed] [Google Scholar]
- 30.Daly KA, Brown JE, Lindgren BR, Meland MH, Le CT, Giebink GS. Epidemiology of otitis media onset by six months of age. Pediatrics. 1999;103(6 pt 1):1158–1166 [DOI] [PubMed] [Google Scholar]
- 31.Nafstad P, Magnus P, Jaakkola JJ. Early respiratory infections and childhood asthma. Pediatrics. 2000;106(3). Available at: www.pediatrics.org/cgi/content/full/106/3/E38 [DOI] [PubMed] [Google Scholar]
- 32.Todberg T, Koch A, Andersson M, Olsen SF, Lous J, Homøe P. Incidence of otitis media in a contemporary Danish National Birth Cohort. PLoS One. 2014;9(12):e111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen RD. Polymorphism of the human TNF-α promoter—random variation or functional diversity? Mol Immunol. 1999;36(15–16):1017–1027 [DOI] [PubMed] [Google Scholar]
- 34.Huang D, Zheng C, Giscombe R, Matell G, Pirskanen R, Lefvert AK. Polymorphisms at - 174 and in the 3′ flanking region of interleukin-6 (IL-6) gene in patients with myasthenia gravis. J Neuroimmunol. 1999;101(2):197–200 [DOI] [PubMed] [Google Scholar]
- 35.Ilia S, Goulielmos GN, Samonis G, Galanakis E. Polymorphisms in IL-6, IL-10, TNF-α, IFN-γ and TGF-β1 genes and susceptibility to acute otitis media in early infancy. Pediatr Infect Dis J. 2014;33(5):518–521 [DOI] [PubMed] [Google Scholar]
- 36.Ruohola A, Pettigrew MM, Lindholm L, et al. Bacterial and viral interactions within the nasopharynx contribute to the risk of acute otitis media. J Infect. 2013;66(3):247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore HC, Jacoby P, Taylor A, et al. ; Kalgoorlie Otitis Media Research Project Team . The interaction between respiratory viruses and pathogenic bacteria in the upper respiratory tract of asymptomatic Aboriginal and non-Aboriginal children. Pediatr Infect Dis J. 2010;29(6):540–545 [DOI] [PubMed] [Google Scholar]
- 38.van den Bergh MR, Biesbroek G, Rossen JWA, et al. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One. 2012;7(10):e47711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uitti JM, Tähtinen PA, Laine MK, Huovinen P, Ruuskanen O, Ruohola A. Role of nasopharyngeal bacteria and respiratory viruses in acute symptoms of young children. Pediatr Infect Dis J. 2015;34(10):1056–1062 [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues F, Foster D, Nicoli E, et al. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in children attending daycare. Pediatr Infect Dis J. 2013;32(3):227–232 [DOI] [PubMed] [Google Scholar]