Abstract
BACKGROUND AND OBJECTIVE:
In adults, leukocyte telomere length (LTL) is variable, familial, and longer in women and in offspring conceived by older fathers. Although short LTL is associated with atherosclerotic cardiovascular disease, long LTL is associated with major cancers. The prevailing notion is that LTL is a “telomeric clock,” whose movement (expressed in LTL attrition) reflects the pace of aging. Accordingly, individuals with short LTL are considered to be biologically older than their peers. Recent studies suggest that LTL is largely determined before adulthood. We examined whether factors that largely characterize LTL in adults also influence LTL in newborns.
METHODS:
LTL was measured in blood samples from 490 newborns and their parents.
RESULTS:
LTL (mean ± SD) was longer (9.50 ± 0.70 kb) in newborns than in their mothers (7.92 ± 0.67 kb) and fathers (7.70 ± 0.71 kb) (both P < .0001); there was no difference in the variance of LTL among the 3 groups. Newborn LTL correlated more strongly with age-adjusted LTL in mothers (r = 0.47; P < .01) than in fathers (r = 0.36; P < .01) (P for interaction = .02). Newborn LTL was longer by 0.144 kb in girls than in boys (P = .02), and LTL was longer by 0.175 kb in mothers than in fathers (P < .0001). For each 1-year increase in father’s age, newborn LTL increased by 0.016 kb (95% confidence interval: 0.04 to 0.28) (P = .0086).
CONCLUSIONS:
The large LTL variation across newborns challenges the telomeric clock model. Having inherently short or long LTL may be largely determined at birth, anteceding by decades disease manifestation in adults.
What’s Known on This Subject:
In adults, leukocyte telomere length (LTL) is highly variable, heritable, and modified by gender and father’s age at conception. Although longer LTL is associated with major cancers, shorter LTL is associated with increased propensity to atherosclerosis and diminished survival.
What This Study Adds:
The high variability, heritability, and influences of gender and father’s age on LTL are already present in newborns. Understanding factors that determine LTL at birth contributes to explaining associations of LTL with major cancers, atherosclerosis, and longevity in adults.
Telomeres are fundamental to replicative aging in somatic cells; cell division results in shortening of telomere length (TL), and very short telomeres trigger replicative senescence. TL is proportional across cell types within an individual but is highly variable across individuals.1–4 Thus, individuals who have long (or short) TL in 1 somatic cell type tend to have long (or short) TL in other cell types.1 Leukocyte telomere length (LTL) is the measure most commonly used to study TL dynamics (TL and its age-dependent shortening) and disease risk in human populations. The ramifications of LTL dynamics for human health are an area of active research. Although short LTL is associated with increased risk of atherosclerotic cardiovascular disease5–7 and diminished longevity,8–10 long LTL is associated with major cancers, including melanoma,11 adenocarcinoma of the lung,12 cancers of the breast13 and pancreas,14 and aggressive cancer of the prostate.15
LTL research has been predominantly performed in adults. LTL is highly familial, with an estimated heritability of 65%.16–20 Some studies also suggest stronger maternal than paternal effects on offspring LTL.19,20 LTL is longer in women than in men17–20 and in African-American subjects than in white subjects (of European ancestry).21–23 Moreover, LTL in adult offspring is positively associated with paternal age at conception (PAC), measured in practice by using paternal age at the time of the offspring’s birth.20,24–26 Recent studies suggest that having a short (or long) LTL may be largely determined during the first 2 decades of life.1,4 The present article tests the hypothesis that the characteristics of LTL, as described for adults, are already present at the time of birth. We build upon the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s study of nulliparous births (NuMoM2b [Nulliparous Pregnancy Outcomes Study Monitoring Mothers-to-be]), a pregnancy cohort with a rich and comprehensive data set, including biologic samples.
Methods
Subjects
The NuMoM2b study is a prospective cohort study of 10 000 nulliparous women with singleton gestations. All participants underwent careful assessments during each trimester of pregnancy. Information collected included interview data, self-administered questionnaires, demographic characteristics, and clinical measurements.
Women were enrolled at 2 clinical sites, the Columbia University Medical Center (New York, NY) and Christiana Care Health Systems (Newark, DE). A total of 1861 women were recruited for the parent study in these sites, of whom 944 (51%) were asked to participate in the ancillary study of TL (Supplemental Figure 3). Our protocol for participation was to initially recruit the mother and ask her permission to contact the father. We requested participation in 2 ways. First, women were asked to participate either during 1 of the prenatal study visits or shortly before delivery (n = 577); of these, 91% agreed and 99% of the fathers agreed. Second, women who had delivered before the start of the study (and a small number who had been missed before delivery) were contacted at home with the request for the father’s participation. Of the 367 women contacted in this way, 45% agreed and 99% of the fathers agreed; the main reason for refusal was that mothers who had already delivered did not want an additional burden. All but 2 fathers donated blood. Thus, overall, 685 newborn–mother–father trios were recruited, representing 73% of those contacted.
Data Collection
The present study used the extensive questionnaire data obtained by the parent study, including demographic and outcome information. Maternal blood samples and umbilical cord blood from the study biorepository were obtained for the measurement of LTL, and a blood sample was collected from the father for the same purpose. Maternal blood specimens used for LTL measurements were collected primarily during the first-trimester (63%) and second-trimester (33%) visits, with the remainder collected at the time of delivery. Age-adjusted maternal LTL did not differ according to trimester of blood sampling (data not shown). All 3 samples (mother, father, and newborn) were available for 610 (89%) of the 685 trios recruited; for the remainder, blood samples were not obtained from the umbilical cord for a variety of reasons (eg, no personnel available at the time of delivery). In addition, 5% of the samples had insufficient quantity of DNA for LTL analysis, resulting in 579 trios available for laboratory analyses.
LTL Measurements
Southern blot analyses of the terminal restriction fragments were performed in duplicate on different gels, as previously described.27 DNA was extracted by using the Gentra Puregene Blood Kit (QIAGEN, Valencia, CA). The interassay coefficients of variation for the duplicate LTL measures were 1.1%.
Assessment of Paternity
Paternity was assessed by using short tandem repeat analysis of 16 polymorphic loci (AmpFℓSTR Identifiler PCR Amplification Kit; Applied Biosystems, Foster City, CA). The absence of all 16 paternal loci in the child's DNA sample was interpreted as nonpaternity. We omitted trios in which there was insufficient evidence of paternity (n = 15), resulting in a sample size of 564 eligible trios (Supplemental Figure 3).
Statistical Analysis
We first conducted simple descriptive analyses such as frequencies, means and SDs, and histograms to examine the sample distributions of the outcome of interest (newborn LTL) and key predictors of interest (PAC, parental LTLs, maternal race and educational attainment, newborn gender and birth weight, and gestation categories). The distribution of these variables was compared between those recruited in prospective mode compared with those recruited retrospectively.
We next examined unadjusted associations between newborn LTL and the key predictors of interest by using Spearman’s rank correlation coefficients, t tests, analysis of variance, or χ2 statistics as appropriate. The correlation between PAC and maternal age at conception (MAC) was also examined, as were the correlations between parental LTLs and parental ages.
Generalized linear models were used to test for differences in maternal, paternal, and newborn LTL. In an unadjusted model, we tested for statistically significant differences in the variance between within-trio LTL measures by using Levene’s test.28
To evaluate which variables were significant predictors of newborn LTL, the nonparametric smoothing splines were first fit by using generalized additive models to assess whether nonlinear models were required to adequately describe the relationships between the newborn’s LTL and PAC and MAC. Because no evidence of nonlinearity was found, repeated measures analysis was performed by using generalized estimating equations to account for duplicate measures of LTL while adjusting the variance of the estimates for within-subject clustering. Missing data on the variables of interest (n = 74) resulted in an analytic sample size of 490 trios (378 prospectively recruited and 112 retrospectively enrolled), which were used in the primary analysis. All analyses were conducted by using SAS version 9.3 (SAS Institute, Inc, Cary, NC).
Results
The mean ± SD age of the mothers who participated was 28.4 ± 5.5 years (Table 1). The mothers were more likely to self-identify as white (56%) than African American (9%), Hispanic (29%), or other (6%) (Table 2); the majority of mothers were college educated (62%). The mean age of fathers was 30.7 ± 6.6 years. The newborns comprised 44% female infants and 56% male infants. The mean birth weight was 3337 ± 534 g, and the mean length of gestation was 39.7 ± 1.93 weeks.
TABLE 1.
LTLs and Descriptive Data for Trios With Complete Data
| Variable | N | Mean | SD | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|
| Newborn LTL | 490 | 9.50 | 0.70 | 7.01 | 9.50 | 11.6 |
| Mother LTL | 490 | 7.92 | 0.67 | 6.19 | 7.89 | 9.81 |
| Father LTL | 490 | 7.70 | 0.71 | 5.83 | 7.66 | 9.88 |
| Maternal age, y | 490 | 28.4 | 5.5 | 17 | 29 | 42 |
| Paternal age, y | 490 | 30.7 | 6.6 | 17 | 30 | 56 |
| Birth weight, g | 490 | 3337 | 534 | 970 | 3340 | 4880 |
| Length of gestation, wka | 462 | 39.7 | 1.93 | 29.6 | 40 | 44.3 |
Any lengths of gestation estimated to be >45 weeks (n = 3) were excluded.
TABLE 2.
Bivariate Associations Between Selected Variables and Newborn LTL Measures in Trios With Complete Data
| Variable | N (%) | Newborn LTL | Pa | |||
|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | |||
| Maternal race | <.01 | |||||
| White | 273 (56) | 9.41 | 0.72 | 7.01 | 11.5 | |
| African American | 45 (9) | 9.75 | 0.75 | 8.15 | 11.6 | |
| Asian/Asian Indian, other | 32 (6) | 9.60 | 0.69 | 8.06 | 11.0 | |
| Hispanic | 140 (29) | 9.57 | 0.61 | 7.78 | 10.8 | |
| Maternal education | .58 | |||||
| Less than high school | 19 (4) | 9.59 | 0.79 | 8.35 | 11.5 | |
| High school graduate/GED | 46 (9) | 9.40 | 0.65 | 8.27 | 10.9 | |
| Some college, no degree | 83 (17) | 9.48 | 0.66 | 7.78 | 10.9 | |
| Associate’s/technical degree | 37 (8) | 9.64 | 0.83 | 7.01 | 11.0 | |
| Bachelor’s degree or higher | 301 (62) | 9.49 | 0.69 | 7.14 | 11.6 | |
| Infant gender | .02 | |||||
| Male | 274 (56) | 9.44 | 0.68 | 7.14 | 11.3 | |
| Female | 216 (44) | 9.58 | 0.71 | 7.01 | 11.6 | |
| Birth weight categories | .70 | |||||
| <2500 g | 20 (4) | 9.56 | 0.71 | 7.67 | 10.5 | |
| ≥2500 g | 470 (96) | 9.50 | 0.70 | 7.01 | 11.6 | |
| Gestational age categories | .94 | |||||
| <37 wk | 29 (6) | 9.48 | 0.72 | 7.67 | 10.8 | |
| ≥37 wk | 433 (89) | 9.50 | 0.70 | 7.01 | 11.6 | |
| Missingb | 28 (5) | 9.46 | 0.66 | 8.52 | 10.9 | |
GED, General Educational Development test; Max, maximum; Min, minimum.
P values from analysis of variance testing for difference in mean newborn LTL within levels of categorical covariates.
Missing preterm information if last menstrual period is unknown, last menstrual period is not reported, or gestation >45 weeks.
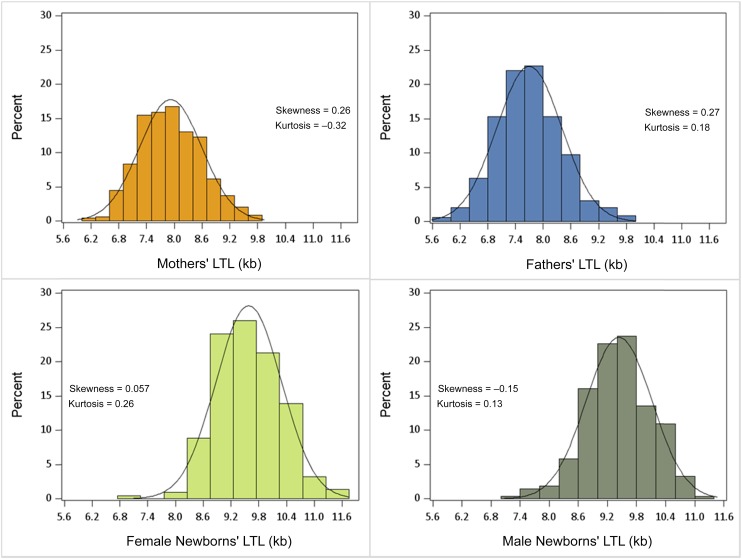
LTLs were normally distributed among the mothers, fathers, and newborns (Table 1, Fig 1). LTL range was 7.01 to 11.6 kb (9.50 ± 0.70 kb) in the newborns, 6.19 to 9.81 kb (7.92 ± 0.67 kb) in the mothers, and 5.83 to 9.88 kb (7.70 ± 0.71 kb) in the fathers. Among female newborns, the LTL range was 7.01 to 9.58 kb (9.58 ± 0.71 kb); among male newborns, the LTL range was 7.14 to 9.42 kb (9.44 ± 0.68 kb). There was no difference in the variance of LTL measures among the newborns, mothers, and fathers (P = .61). LTL was significantly shorter in newborns than in age-adjusted LTL in mothers (P < .0001) and fathers (P < .0001).
FIGURE 1.
Distribution of LTLs in mothers, fathers, and newborns.
The possibility of selection bias was evaluated by comparing the newborn–mother–father trios enrolled in the prospective manner with those enrolled in the retrospective manner (Supplemental Tables 4, 5, and 6). Participation was very high among those enrolled prospectively (91%), and this group comprised most of the study sample. Those enrolled retrospectively were more likely to be Hispanic and African American (P = .0005), were less likely to have a college education (P = .003), and had longer paternal LTL (P = .04). We also regressed paternal LTL on paternal age and found similar associations in the prospectively and retrospectively recruited fathers, regardless of control for other variables. In bivariate analyses, maternal race/ethnicity and newborn LTL were associated only among those recruited prospectively, as was the association between newborn gender and newborn LTL; however, we attributed these anomalies to the small sample size in the retrospectively recruited group.
In unadjusted analyses, newborn LTL was longer by 0.144 kb in girls than boys (P = .02). There was a strong effect of maternal race/ethnicity on the newborn LTL, primarily driven by difference in LTL (0.34 kb) between newborns of white mothers and newborns of African-American mothers (Table 2). No significant differences in newborn LTL were found according to maternal education, low birth weight, or preterm delivery. Correlations between parental LTL and parental age were –0.14 for mothers and –0.18 for fathers (both P < .01). The correlation between PAC and MAC was expectedly high (r = 0.80; P ≤ .0001).
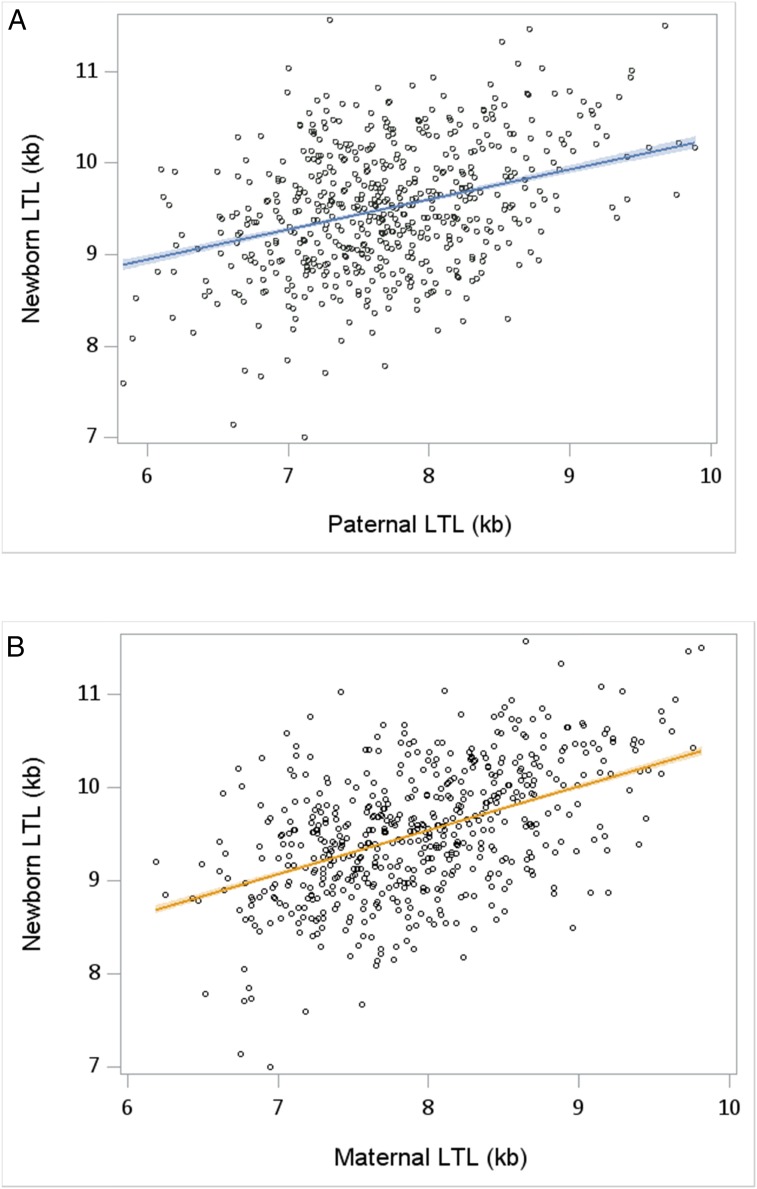
Age-adjusted LTL was longer by 0.175 kb in mothers than in fathers (P < .0001). Age-adjusted LTLs in the mothers and fathers were weakly correlated (r = 0.093; P = .041). Newborn LTL was correlated with parental age–adjusted LTL in the mothers (r = 0.47; P < .01) and fathers (r = 0.36; P < .01) (Fig 2); the difference in the correlations was statistically significant (P = .02). For female newborns, correlations between LTL and age-adjusted maternal LTL and paternal LTL were 0.49 and 0.33, respectively (P = .02, comparing the correlations); for male newborns, they were 0.47 and 0.41 (P = .19, comparing the correlations).
FIGURE 2.
Correlations between age-adjusted LTLs in (A) fathers and (B) mothers versus newborns.
All associations persisted in the generalized estimating equation prediction model, which simultaneously included all variables. Together, all predictors accounted for 40% of the total variance in newborn LTL (Table 3). For each 1-kb increase in mother’s and father’s LTL, newborn’s LTL increased by 0.435 kb (95% confidence interval [CI]: 0.36 to 0.49) and 0.319 kb (95% CI: 0.256 to 0.383), respectively. Female newborns had longer LTL than male newborns (0.181 kb [95% CI: 0.083 to 0.278]). As in the bivariate analyses, newborns of African-American mothers had longer LTL than those of white, Hispanic, or mothers of other race/ethnicity. For each 1-year increase in PAC, we estimated a 0.016-kb (95% CI: 0.04 to 0.28) increase in newborn LTL; for each 1-year increase in MAC, we estimated a 0.005-kb (95% CI: –0.10 to 0.23) increase in newborn LTL.
TABLE 3.
Estimated Associations Between Key Variables and Newborn LTL in Trios With Complete Data
| Variable | β ± SE | 95% CI | P |
|---|---|---|---|
| Mother LTL | 0.425 ± 0.031 | 0.364 to 0.486 | <.0001 |
| Father LTL | 0.319 ± 0.032 | 0.256 to 0.383 | <.0001 |
| Maternal age | 0.005 ± 0.008 | –0.010 to 0.021 | .5213 |
| Paternal age | 0.016 ± 0.006 | 0.004 to 0.028 | .0086 |
| Mother’s race/ethnicity (African American) | 0.162 ± 0.089 | –0.012 to 0.336 | .0682 |
| Mother’s race/ethnicity (Hispanic) | 0.062 ± 0.052 | –0.041 to 0.164 | .2385 |
| Mother’s race/ethnicity (“other”) | 0.065 ± 0.115 | –0.161 to 0.290 | .5748 |
| Female infant gender | 0.181 ± 0.050 | 0.083 to 0.278 | .0003 |
Associations were estimated by using a repeated measures generalized estimating equation model that adjusted for within-subject clustering. Duplicate measures for newborn LTL resulted in a sample size of 979 from the 490 trios (1 trio only had 1 LTL analysis). Overall, 40% of the variance in newborn LTL was explained by this model.
In a sensitivity analysis, a term for mode of recruitment (prospective or retrospective) was included, and the results were essentially unchanged (Supplemental Table 7). The analysis was performed separately for the prospectively recruited trios; in this analysis, results were also essentially unchanged (Supplemental Table 8). In the retrospectively recruited trios, no associations were found between maternal race/ethnicity and infant gender and newborn LTL; the expected associations in the prospectively recruited trios were found. We suspect these differences are due to random variation because the sample size in the retrospectively recruited group was small.
Discussion
This study has 5 key findings. First, the estimated variance of LTL was similar in newborns, mothers, and fathers (Fig 1). Second, parental LTL was associated with newborn LTL (Fig 2), displaying stronger associations with the mothers’ LTL than with the fathers’ LTL. Third, LTL was longer in female newborns than in male newborns and longer in mothers than in fathers, after accounting for parental age. Fourth, LTL in newborns of African-American mothers was longer, although not significantly so, than that of newborns of white mothers and mothers of other races/ethnicities. However, the power was low (African-American subjects, n = 45). Fifth, PAC was associated with newborn LTL. We did not have sufficient numbers of low birth weight infants (ie, <2500 g) or preterm births to evaluate these factors fully.
The LTL variance in newborns is similar not only to that of the parents but also to the variances previously reported in adults.1,3,4,8,21 Our findings also extend to previous observations regarding newborns that the correlation between age-adjusted LTL in adult offspring with the mothers’ LTL is stronger than that with the fathers’ LTL.19,20 The longer LTL in female newborns than in male newborns is also consistent with the longer LTL in women than in men.17–19,22,23 In addition, the magnitude of the PAC effect on the newborn’s LTL (0.016 kb for 1-year increase in PAC) is similar to that reported for adult offspring.24–26 Thus, key characteristics and associations established for LTL in adults are present in newborns.
As we discussed in 2 recent communications,29,30 the prevailing view is that LTL is a “telomeric clock” that captures the aging process, primarily in the form of accumulating burden of inflammation and oxidative stress. This view infers that individuals with short LTL might be biologically older because they experience a higher rate of aging, as displayed in a faster rate of LTL attrition. As shown in the present study, however, the wide interindividual variation in LTL and the major factors that influence LTL are already present at birth. In addition, other studies suggest that individuals who enter adulthood with short (or long) LTL are likely to have short (or long) LTL for their remaining life course.1,4 Longitudinal studies show that although LTL attrition during adulthood varies across individuals within a given population,31 variation in this attrition is probably insufficient to produce a substantial change in LTL percentile ranking in the majority of individuals.4 It is essential, however, to perform such studies during the first 2 decades of life, given that the period of growth and development entails much faster LTL attrition than during adulthood.3,32
The gender difference in LTL at birth is another salient finding that merits further comment. It was proposed that the longer LTL in women compared with men stems from a slower rate of LTL attrition in premenopausal women, presumably because of estrogen-mediated stimulation of telomerase.33 We recently showed in a longitudinal study that the rate of LTL attrition is in fact faster in premenopausal women than in postmenopausal women and that regardless of the menopausal status, age-adjusted LTL of women was longer than that of men.34 Those findings suggested that the gender effect on LTL is established before adulthood. In the present study, we show that the gender effect is present at birth.
In light of these findings and the present research, the concept of LTL as a telomeric clock of human aging is difficult to defend. By proposing that LTL is largely determined before adulthood, and to a large degree at birth,29,30 we have moved away from the paradigm that has dominated the field of telomere epidemiology for more than a decade. We question neither the effects of inflammation and oxidative stress on LTL attrition nor their role in aging and its related diseases, including atherosclerosis. We also acknowledge the effect of the environment on LTL. This effect is shown by the correlation of LTL between spouses, a finding observed before.20 However, the joint effect of these environmental factors on LTL, particularly during adulthood, is likely to be small compared with the effect of the variation of LTL across newborns, which amounts to a range of ∼4 kb.
What is the relevance of LTL at birth to human health? We propose that long telomeres engender more proliferative potential of somatic cells,35 which increases cancer susceptibility due to the accumulation of de novo mutations.36 Short telomeres, in contrast, curtail replicative potential and attenuate cancer susceptibility at the cost of compromised somatic repair, contributing to degenerative diseases, including atherosclerosis. This notion extends to humans the evolutionary role of the cancer–degenerative disease trade-off in shaping different TL across mammalian species.37,38 However, although LTL is a poor index of the individual’s biological age, it seems to serve as a marker of disease susceptibility. We also do not exclude the likelihood that faster LTL attrition due to the heritability of LTL attrition18 or yet unknown factors could be associated with degenerative diseases such as atherosclerosis.
Atherosclerosis, which ultimately afflicts the majority of individuals (provided they live long enough), is among the most common causes of human degenerative disorders. There is now compelling evidence that LTL is relatively short in individuals with atherosclerosis compared with their peers.5 The methodology of the majority of early studies that examined the LTL–cancer connection (with erratic findings) is questionable in our view. These studies suffered from critical shortcomings, including small sample sizes and enrolling subjects retrospectively after chemotherapy and radiotherapy had been initiated, which would shorten LTL.39 However, based on prospective studies, a consensus is now building that major cancers (including sporadic melanoma, adenocarcinoma of the lung, and cancers of the breast, pancreas, and prostate) are associated with an inherently longer LTL.11–15 Our results suggest that the propensity to major cancers associated with long LTL might already be present at birth.
This concept of antecedence (ie, having short or long LTL precedes by decades the manifestation of atherosclerosis or cancer) is consistent with the associations of LTL with these disorders being causal. Further support for causality is provided by the following observations. Genome-wide association studies of LTL have identified single-nucleotide polymorphisms (SNPs) mapped to genetic loci that harbor genes known to maintain telomeres and those whose role in telomere biology is not fully understood.40–43 Jointly, alleles of SNPs that are associated with a short LTL increase the genetic risk for atherosclerosis,40 whereas alleles of the same SNPs that are associated with a long LTL increase the risk for some cancers.44,45 It is reasonable to infer causality when not only LTL but also that gene variants related to LTL are associated with atherosclerosis and cancer.
It is important to underscore both the strengths and limitations of our study. First, we used the high-precision Southern blot method, which provides LTL in absolute units of length,27 rather than the lower precision quantitative polymerase chain reaction method,46,47 which generates telomeric DNA content in relative units. Second, the NuMoM2b study provides a well-defined cohort with meticulous data and biospecimen collection. Third, for those mothers prospectively invited to participate, our response rates were excellent. Fourth, trios with unverified paternity were excluded.
The study is also limited in several ways. Although we had adequate sample size to examine some prenatal determinants of LTL, larger numbers are needed to fully disentangle the effects of PAC and MAC. However, given that TL is longer in sperm of older men,48 it is possible that the offspring’s LTL association with MAC is primarily due to the high correlation between PAC and MAC. In addition, the number of newborns of African-American mothers was small, making it difficult to evaluate the association between race/ethnicity and newborn LTL. The response rate for the retrospectively recruited sample was modest as well, raising the possibility of selection bias in this group.
Conclusions
There is now a body of evidence, culminating in the findings of the present study, challenging the convention that LTL is a telomeric clock of human aging. Understanding the factors that fashion LTL at birth is crucial to gaining insight into the role of telomeres in major human diseases, including the 2 disorders that largely determine the life span of humans in high-income countries: atherosclerosis and cancer. Finally, although we infer that LTL at birth makes a substantial contribution to LTL throughout the life course, the contribution of postnatal LTL attrition during growth and development remains unmeasured and requires longitudinal studies that assess tracking of LTL.
Glossary
- CI
confidence interval
- LTL
leukocyte telomere length
- MAC
maternal age at conception
- PAC
paternal age at conception
- SNP
single-nucleotide polymorphism
- TL
telomere length
Footnotes
Drs Factor-Litvak and Susser participated in designing the study and drafting the manuscript; Ms Kezios and Dr McKeague developed the analytical design and performed all statistical analysis; Dr Kark contributed to the writing of the manuscript; Drs Hoffman and Wapner contributed to study design, oversaw collection of samples, and contributed to the writing of the manuscript; Dr Kimura oversaw all of the measurements of telomeres; Dr Aviv contributed to the study design and the writing of the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the following National Institutes of Health grants: R01 HD071180 from the National Institute of Child Health and Development and R21 ES023582 and 5P30 ES009089 from the National Institute of Environmental Health Sciences. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkad A, Hastings R, Konje JC, Bell SC, Thurston H, Williams B. Telomere length in small-for-gestational-age babies. BJOG. 2006;113(3):318–323 [DOI] [PubMed] [Google Scholar]
- 3.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8(5):e1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benetos A, Kark JD, Susser E, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12(4):615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kark JD, Nassar H, Shaham D, et al. Leukocyte telomere length and coronary artery calcification in Palestinians. Atherosclerosis. 2013;229(2):363–368 [DOI] [PubMed] [Google Scholar]
- 7.Hunt SC, Kimura M, Hopkins PN, et al. Leukocyte telomere length and coronary artery calcium. Am J Cardiol. 2015;116(2):214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzpatrick AL, Kronmal RA, Kimura M, et al. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura M, Hjelmborg JV, Gardner JP, et al. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167(7):799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deelen J, Beekman M, Codd V, et al. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. Int J Epidemiol. 2014;43(3):878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anic GM, Sondak VK, Messina JL, et al. Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer Epidemiol. 2013;37(4):434–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seow WJ, Cawthon RM, Purdue MP, et al. Telomere length in white blood cell DNA and lung cancer: a pooled analysis of 3 prospective cohorts. Cancer Res. 2014;74(15):4090–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu S, Wen W, Shu XO, et al. Association of leukocyte telomere length with breast cancer risk: nested case-control findings from the Shanghai Women’s Health Study. Am J Epidemiol. 2013;177(7):617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch SM, Major JM, Cawthon R, et al. A prospective analysis of telomere length and pancreatic cancer in the alpha-tocopherol beta-carotene cancer (ATBC) prevention study. Int J Cancer. 2013;133(11):2672–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julin B, Shui I, Heaphy CM, et al. Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br J Cancer. 2015;112(4):769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of 3 age groups. Am J Hum Genet. 1994;55(5):876–882 [PMC free article] [PubMed] [Google Scholar]
- 17.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36(2):195–200 [DOI] [PubMed] [Google Scholar]
- 18.Hjelmborg JB, Dalgård C, Möller S, et al. The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52(5):297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363(9408):507–510 [DOI] [PubMed] [Google Scholar]
- 20.Broer L, Codd V, Nyholt DR, et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21(10):1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt SC, Chen W, Gardner JP, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elbers CC, Garcia ME, Kimura M, et al. Comparison between southern blots and qPCR analysis of leukocyte telomere length in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2014;69(5):527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carty CL, Kooperberg C, Liu J, et al. Leukocyte telomere length and risks of incident coronary heart disease and mortality in a racially diverse population of postmenopausal women. Arterioscler Thromb Vasc Biol. 2015;35(10):2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Meyer T, Rietzschel ER, De Buyzere ML, et al. ; Asklepios Investigators . Paternal age at birth is an important determinant of offspring telomere length. Hum Mol Genet. 2007;16(24):3097–3102 [DOI] [PubMed] [Google Scholar]
- 25.Kimura M, Cherkas LF, Kato BS, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hjelmborg JB, Dalgård C, Mangino M, et al. Paternal age and telomere length in twins: the germ stem cell selection paradigm. Aging Cell. 2015;14(4):701–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura M, Stone RC, Hunt SC, et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5(9):1596–1607 [DOI] [PubMed] [Google Scholar]
- 28.Larson MG. Analysis of variance. Circulation. 2008;117(1):115–121 [DOI] [PubMed] [Google Scholar]
- 29.Aviv A, Kark JD, Susser E. Telomeres, atherosclerosis, and human longevity: a causal hypothesis. Epidemiology. 2015;26(3):295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt SC, Kark JD, Aviv A. Association between shortened leukocyte telomere length and cardio-metabolic outcomes. Circ Cardiovasc Genet. 2015;8(1):4–7 [DOI] [PubMed] [Google Scholar]
- 31.Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum—artifact or biology? Nucleic Acids Res. 2013;41(13):e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidorov I, Kimura M, Yashin A, Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol. 2009;37(4):514–524 [DOI] [PubMed] [Google Scholar]
- 33.Bayne S, Jones ME, Li H, Liu JP. Potential roles for estrogen regulation of telomerase activity in aging. Ann N Y Acad Sci. 2007;1114:48–55 [DOI] [PubMed] [Google Scholar]
- 34.Dalgård C, Benetos A, Verhulst S, et al. Leukocyte telomere length dynamics in women and men: menopause vs age effects. Int J Epidemiol. 2015;44(5):1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marión RM, Blasco MA. Telomeres and telomerase in adult stem cells and pluripotent embryonic stem cells. Adv Exp Med Biol. 2010;695:118–131 [DOI] [PubMed] [Google Scholar]
- 36.Luebeck EG, Moolgavkar SH. Multistage carcinogenesis and the incidence of colorectal cancer. Proc Natl Acad Sci U S A. 2002;99(23):15095–15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomes NM, Ryder OA, Houck ML, et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10(5):761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seluanov A, Chen Z, Hine C, et al. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6(1):45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savage SA, Gadalla SM, Chanock SJ. The long and short of telomeres and cancer association studies. J Natl Cancer Inst. 2013;105(7):448–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427, e1–e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangino M, Hwang SJ, Spector TD, et al. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum Mol Genet. 2012;21(24):5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangino M, Christiansen L, Stone R, et al. DCAF4, a novel gene associated with leucocyte telomere length. J Med Genet. 2015;52(3):157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy D, Neuhausen SL, Hunt SC, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107(20):9293–9298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iles MM, Bishop DT, Taylor JC, et al. ; AMFS Investigators; IBD Investigators; QMEGA and QTWIN Investigators; SDH Study Group; GenoMEL Consortium . The effect on melanoma risk of genes previously associated with telomere length. J Natl Cancer Inst. 2014;106(10):dju267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machiela MJ, Hsiung CA, Shu XO, et al. Genetic variants associated with longer telomere length are associated with increased lung cancer risk among never-smoking women in Asia: a report from the female lung cancer consortium in Asia. Int J Cancer. 2015;137(2):311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verhulst S, Susser E, Factor-Litvak PR, et al. Commentary: the reliability of telomere length measurements. Int J Epidemiol. 2015;44(5):1683–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39(20):e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aviv A, Susser E. Leukocyte telomere length and the father’s age enigma: implications for population health and for life course. Int J Epidemiol. 2013;42(2):457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]