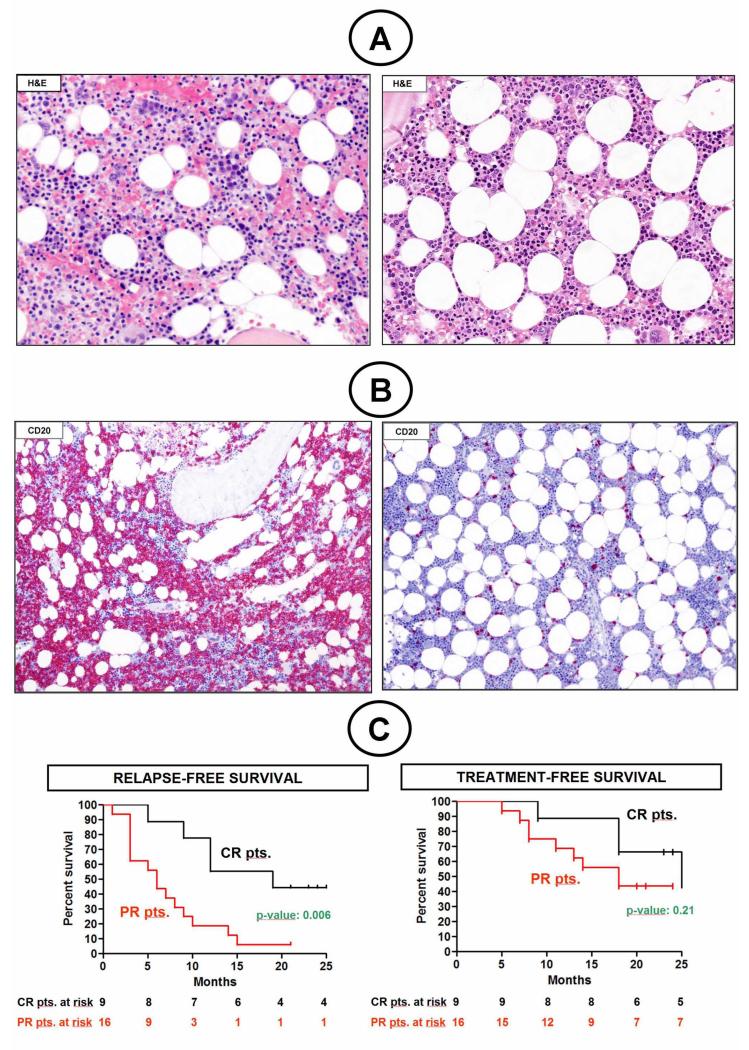
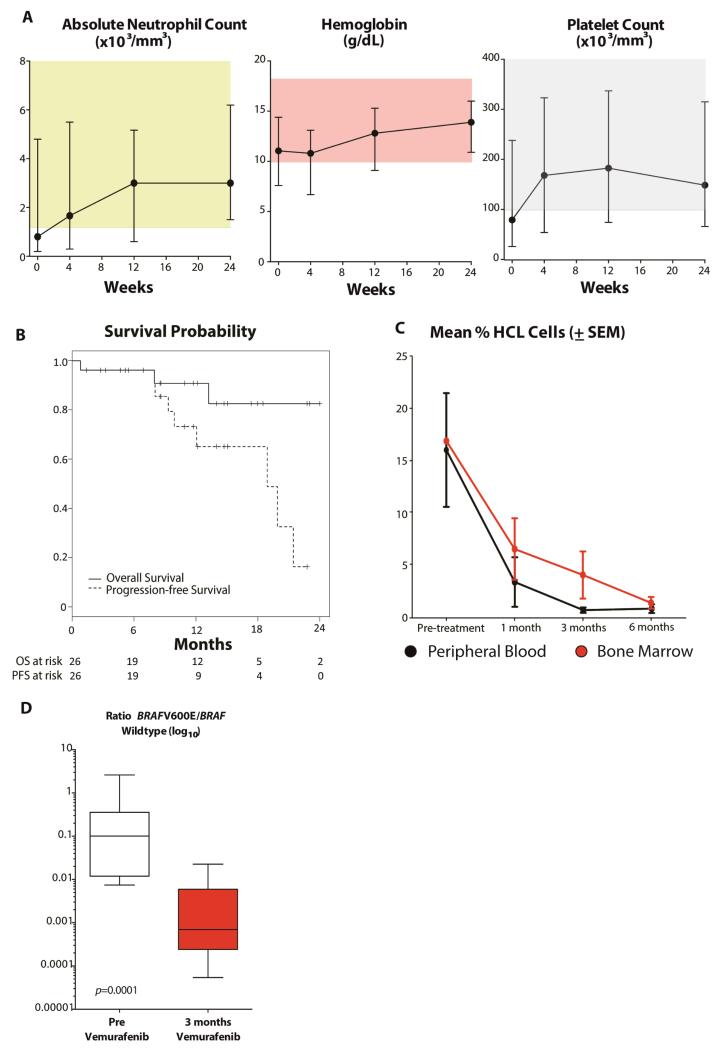
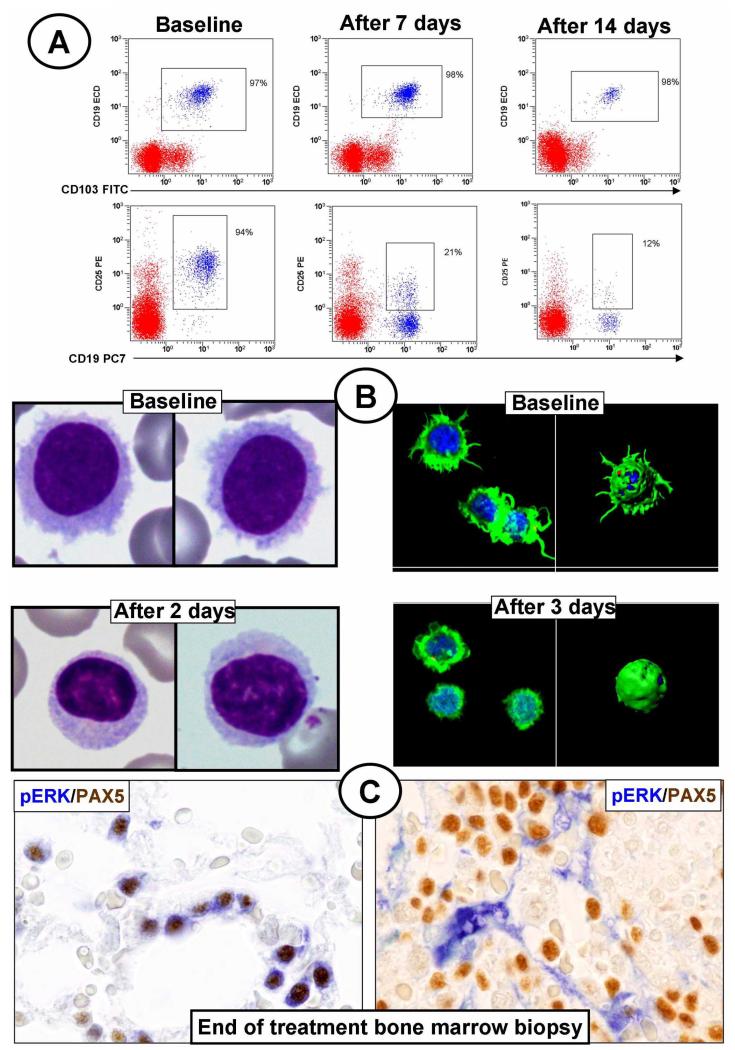
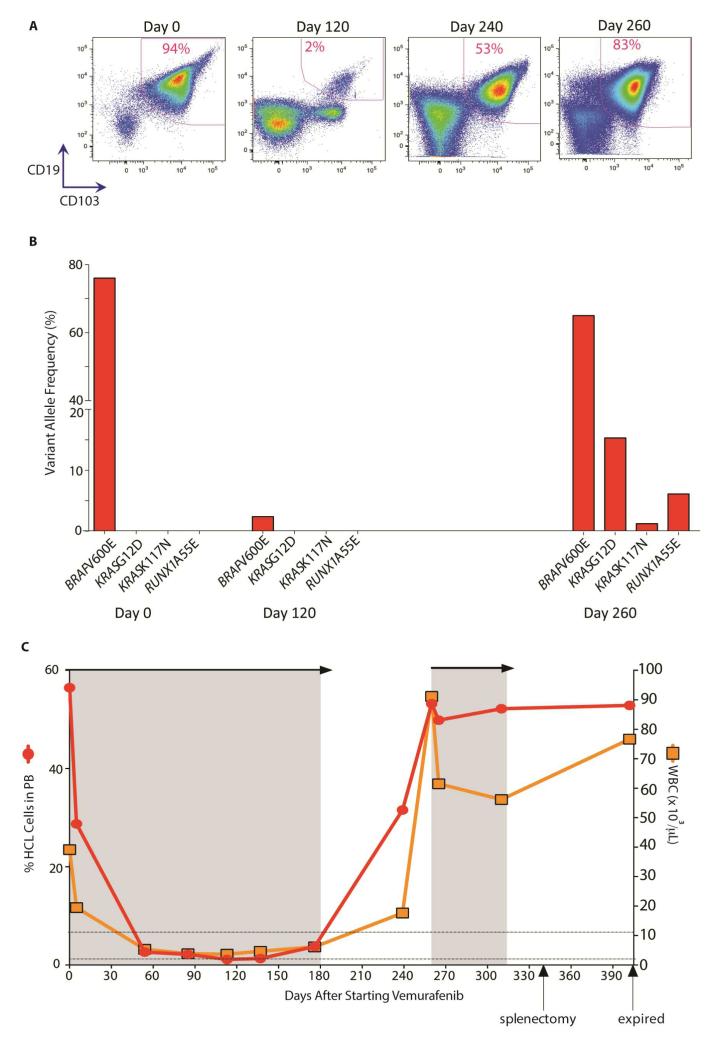
Enrico Tiacci
Enrico Tiacci, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,*,
Jae H Park
Jae H Park, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,*,
Luca De Carolis
Luca De Carolis, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Stephen S Chung
Stephen S Chung, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Alessandro Broccoli
Alessandro Broccoli, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Sasinya Scott
Sasinya Scott, M.P.H.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Francesco Zaja
Francesco Zaja, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Sean Devlin
Sean Devlin, M.S. Ph.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Alessandro Pulsoni
Alessandro Pulsoni, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Young Rock Chung
Young Rock Chung, M.S.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Michele Cimminiello
Michele Cimminiello, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Eunhee Kim
Eunhee Kim, Ph.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Davide Rossi
Davide Rossi, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Richard M Stone
Richard M Stone, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Giovanna Motta
Giovanna Motta, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Alan Saven
Alan Saven, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Marzia Varettoni
Marzia Varettoni, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Jessica K Altman
Jessica K Altman, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Antonella Anastasia
Antonella Anastasia, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Michael R Grever
Michael R Grever, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Achille Ambrosetti
Achille Ambrosetti, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Kanti R Rai
Kanti R Rai, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Vincenzo Fraticelli
Vincenzo Fraticelli, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Mario E Lacouture
Mario E Lacouture, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Angelo Michele Carella
Angelo Michele Carella, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Ross L Levine
Ross L Levine, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Pietro Leoni
Pietro Leoni, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Alessandro Rambaldi
Alessandro Rambaldi, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Franca Falzetti
Franca Falzetti, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Stefano Ascani
Stefano Ascani, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Monia Capponi
Monia Capponi, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Maria Paola Martelli
Maria Paola Martelli, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Christopher Y Park
Christopher Y Park, M.D. Ph.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Stefano Aldo Pileri
Stefano Aldo Pileri, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Neal Rosen
Neal Rosen, M.D. Ph.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Robin Foà
Robin Foà, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Michael F Berger
Michael F Berger, Ph.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Pier Luigi Zinzani
Pier Luigi Zinzani, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,
Omar Abdel-Wahab
Omar Abdel-Wahab, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,^,
Brunangelo Falini
Brunangelo Falini, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,^,°,
Martin S Tallman
Martin S Tallman, M.D.
1Institute of Hematology, Department of Medicine and Center for Hemato-Oncology Research/C.R.E.O, University of Perugia and Ospedale Santa Maria della Misericordia, Perugia, Italy (E.T., L.D.C, S.A., F.F., M.C., M.P.M., B.F.)
2Leukemia Service - Department of Medicine (J.H.P., S.S.C., M.S.T., R.L.L., O.A-W.), Human Oncology and Pathogenesis Program (S.S., Y.R.C., E.K., R.L.L., C.Y.P., M.F.B., O.A-W.), Department of Epidemiology and Biostatistics (S.D.), Department of Pathology (C.Y.P.), Dermatology Service - Department of Medicine (M.E.L.), Department of Pharmacology (N.R.), Center for Molecular Oncology (M.F.B.), Memorial Sloan Kettering Cancer Center, New York, NY, USA
3Department of Experimental, Diagnostic, and Specialty Medicine, Units of Hematology and Hematopathology, Bologna University School of Medicine, Bologna, Italy (A.B., S.P., PL.Z.)
4Hematology Unit - Centro Trapianti e Terapie Cellulari “Carlo Melzi”, DISM, Azienda Ospedaliero-Universitaria, Udine, Italy (F.Z.)
5Hematology, Department of Cellular Biotechnologies and Hematology, Sapienza University, Rome, Italy (A.P., R.F.)
6Hematology and the Bone Marrow Transplant Unit, Azienda Ospedaliera Regionale San Carlo, Potenza, Italy (M.C.)
7Division of Hematology, Department of Translational Medicine, Amedeo Avogadro University of Eastern Piedmont, Novara, Italy (D.R.)
8Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA (R.M.S.)
9Hematology Unit, Ospedale Ferrarotto, Catania, Italy (G.M.)
10Division of Hematology and Oncology, Scripps Clinic, La Jolla, CA, USA (A.S.)
11Department of Onco-Hematology, Fondazione IRCCS - Policlinico San Matteo, Pavia, Italy (M.V.)
12Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USA (J.K.A.)
13Hematology Unit, Spedali Civili, Brescia, Italy (A.A.)
14Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA (M.R.G.)
15Department of Medicine, Section of Hematology, University of Verona, Verona, Italy (A.A.)
16Division of Hematology/Oncology, Hofstra North-Shore-LIJ School of Medicine, New Hyde Park, NY, USA (K.R.R.)
17Onco-hematology Unit, Fondazione di Ricerca e Cura S.S. Giovanni Paolo II, Campobasso, Italy (V.F.)
18Institute of Hematology-1, IRCCS - Azienda Ospedaliero-Universitaria San Martino-IST, Genova, Italy (A.M.C.)
19Department of Hematology, Azienda Ospedaliero-Universitaria “Ospedali Riuniti”, Ancona, Italy (P.L.)
20Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo (A.R.)
1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,^