Abstract
Classical proprioceptors, like Golgi tendon organs and muscle spindles are absent in the extraocular muscles (EOMs) of most mammals. Instead, a nerve end organ was detected in the EOMs of each species including sheep, cats, rabbits, rats, monkeys, and man examined so far: the palisade ending. Until now no evidence appeared that palisade endings are present in canine EOMs. We analyzed dog EOMs by confocal laser scanning microscopy, 3D reconstruction, and transmission electron microscopy. In EOM wholemount preparations stained with antibodies against neurofilament and synaptophysin we found typical palisade endings. Nerve fibers coming from the muscle extended into the tendon. There, the nerve fibers turned 180° and returned to branch into preterminal axons which established nerve terminals around a single muscle fiber tip. Fine structural analyses revealed that each palisade ending in dog EOMs established nerve terminals on the tendon. In some palisade endings we found nerve terminals contacting the muscle fiber as well. Such neuromuscular contacts had a basal lamina in the synaptic cleft thereby resembling motor terminals. By using antibodies against choline acetyltransferase (ChAT) we proved that canine palisade endings are ChAT-immunoreactive. This study shows that palisade endings are present in canine EOMs. In line with prior findings in cat and monkey, palisade endings in dog have a cholinergic phenotype.
Keywords: proprioception, dog, extraocular muscle, palisade endings, choline acetyltransferase
The sense of body position (proprioception) is recognized by specialized receptors in skeletal muscles. These proprioceptors, like Golgi tendon organs (GTOs) or muscle spindles, measure either muscle contraction or muscle stretch and contribute with this information to the cognition of posture. Extraocular muscles (EOMs) take an exceptional position as classical proprioceptors show a broad interspecies variation in their occurrence [21]. Specifically, muscle spindles can only be found in EOMs of mice [20], even-toed ungulates (goat, sheep, pig, camel, cow) [21], monkeys [12], and humans [4, 18, 23]. While muscle spindles in EOMs of ungulates are comparable with those in other skeletal muscles, muscle spindles in monkey and human EOMs exhibit structural particularities. GTOs, on the other hand, can only be demonstrated in EOMs of some monkey species [25] and ungulates [21] and they have a more uniform appearance in these species. In cats, rabbits, guinea pigs, and dogs, neither muscle spindles nor GTOs are present in their EOMs [21]. Until now no explanation was found for the diversity in the endowment of classical proprioceptors within mammalian EOMs.
EOMs however, possess a nerve end organ which cannot be found in any other skeletal muscle, the palisade ending or innervated myotendinous cylinder [1-3, 5, 10, 15, 16, 19, 24]. Palisade endings are located at the proximal and distal muscle tendon junction and were found in cats [1, 2, 15], rabbits [6], sheep [5], rats [11], monkeys [3, 16, 24], and humans [7, 19, 22]. They emanate from axons which enter the muscle at a central nerve entry site, proceed alongside the muscle fibers, enter the tendon, turn 180° in the tendon, and finally branch out and form a cuff of nerve terminals around a muscle fiber tip. In palisade endings of cats [1, 2, 15], sheep [5], monkeys [3, 16, 24], and man [7, 19, 22] nerve terminals establish contacts on the tendon. Additionally, in some palisade endings of these species nerve terminals contact muscle fibers as well. Rabbits [6] and rats [11] are an exception because in palisade endings of these species exclusively neuromuscular contacts were observed [6, 11].
At the moment the function of palisade endings is a matter of debate whether these structures are sensory [1, 2, 8, 11, 24, 29, 30], motor [3, 15, 16, 26] or eventually both [19]. Independent of the function of palisade endings, the question of EOM proprioception is still a contentious issue. To gain more information about the proprioceptive complement in mammalian EOMs and its possible variability it is necessary to analyze a large spectrum of species. So far palisade endings have been found in each species investigated and it is supposed that they are a universal feature in mammalian EOMs [11]. In EOMs of dogs muscle spindles and GTOs are absent and there is no evidence in literature that dog EOMs contain palisade endings [21]. It was the aim of the present study to clarify whether canine EOMs have palisade endings and if present, whether these EOM specific structures have a cholinergic phenotype as demonstrated previously in cats [15]and monkeys [3, 16].
The clinic for ophthalmology of the Veterinary University Vienna provided us with the orbits of a 7 years old male/castrated Magyar Vizsla dog. The animal was euthanized because it suffered from a severe glaucoma in both eyes. The orbits were dissected and all rectus EOMs from both sides including the distal tendon were removed. The muscles were immersion-fixed in 4 % paraformaldehyde in 0.1 M phosphate buffered saline (PBS) pH 7.4 for 4 hours and then rinsed in 4°C in 0.1 M PBS pH 7.4. EOMs were further processed and analyzed by confocal laser scanning microscopy (CLSM), 3D reconstructions, and transmission electron microscopy (TEM).
For CLSM wholemount preparations of the muscle tendon junction were labeled with two different combinations of immunohistochemical triple staining: 1. anti-neurofilament, anti-synaptophysin, phalloidin and 2. anti-choline acetyltransferase (ChAT), anti-synaptophysin, phalloidin. The samples were processed for immunostaining as already described before [3, 15]. To permeabilize the membranes the wholemount preparations were shock-frozen in −80°C cold methylbutan and afterwards thawed in PBS at room-temperature. Then the samples were washed in PBS containing 1 % Triton (PBSTri). The samples were first blocked for 2 hours in 10 % goat serum in PBS and then incubated with the first antibodies for 48 hours. For staining 1: chicken anti-neurofilament antibody (Molecular Probes) dilution 1:2,500 and mouse anti-synaptophysin antibody (Chemicon) dilution 1:200 and for staining 2: mouse anti-synaptophysin (Chemicon) dilution 1:200 and rabbit anti-ChAT antibodies (provided by Prof. Schemann, Munich) dilution 1:250 were used. Then the samples were intensively washed in PBSTri and incubated for 4 hours with one of the second antibodies. For staining 1: goat anti-mouse antibody labeled with Alexa 488 (Molecular Probes) dilution 1:500 and for staining 2: goat anti-mouse labeled with rhodamine (Chemicon) dilution 1:200. The samples were washed again in PBSTri and incubated overnight in a mixture of the other second antibody and phalloidin labeled with Alexa 633 (Molecular Probes) dilution 1:50. The second antibody was for staining 1: goat anti-chicken Alexa 568 labeled (Molecular Probes) dilution 1: 500 and for staining 2: goat anti-rabbit labeled with Alexa 488 (Molecular Probes) dilution 1:500. The wholemounts were analyzed with a Zeiss LSM 510. Images were generated in 3 different fluorescence channels: excitation wave length of 488, 568 and 633 nm. For negative control experiments, primary antibodies were omitted and this resulted in a complete loss of immunostaining.
3D reconstructions were made of palisade endings labeled with, anti-neurofilament anti-synaptophysin and phalloidin. Wholemounts were analyzed by CLSM and series of virtual longitudinal sections were cut through the palisade endings. Each section was photo documented and from these image-stacks the structures of interest were segmented by the use of a dynamic threshold algorithm using Photoshop CS 8.0.1. An individual threshold for one of the structures of interest was set once by the operator in a single sample image and was then automatically adjusted to all other images by a macro. The segmentation procedure resulted in multiple series of binary (black and white) image files, each containing one of the structures of interest (muscle fiber, nerve fiber, or palisade ending). Three-dimensional models of the required structures were generated by recombining the respective sets of binary image files into a single 3D-object using Velocity 4.2 running on a Power Mac G5. The sectional outlines of the respective structures were placed in a z-distance corresponding to the sectional thickness. Polygonal surfaces were then generated using a marching cube algorithm. The final model was visualized as solid object.
For TEM analysis myotendinous junctions of EOMs were immersion fixed in 2 % paraformaldehyde (PFA) and 2.5 % glutardialdehyde (GDA) in 0.1 M PBS. The samples were cut into small strips and post fixed in 1 % osmium tetroxide. Semi-thin cross sections of dehydrated and epon-embedded specimens were cut (starting from the tendinous end) and first analyzed by bright field microscopy. When palisade endings were identified ultra-thin sections were cut and mounted on dioxane formvar-coated (Formvar, SPI, West Chester, PA) copper grids. Sections were stained in 2 % uranyl acetate followed by 0.4 % lead citrate and analyzed by TEM (Zeiss EM 10).
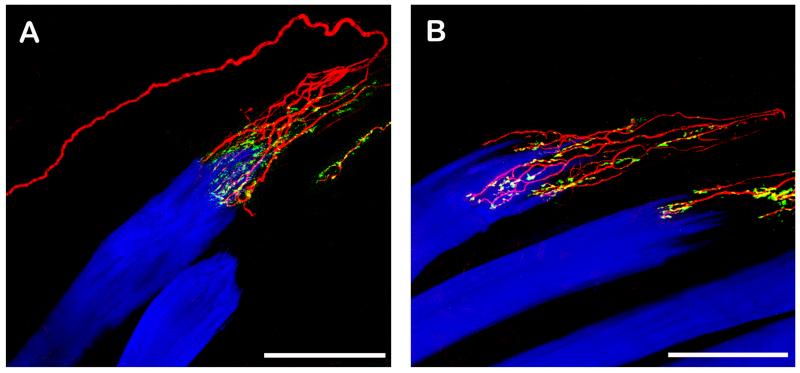
The overall morphology of palisade endings could be best observed in immunolabeled EOM wholemounts. The palisade endings showed the classical design: axons coming from the muscle entered the tendon, formed a u-turn there, approached the muscle fiber and ended in a preterminal arborization surrounding the muscle fiber tip. Preterminal axons established nerve terminals in the tendon as well as around the muscle fiber tip (Figs. 1 – 3). Rarely, we found that a single axon supplied two palisade endings on neighboring muscle fibers (Fig. 3A). We observed numerous palisade endings in each rectus EOM and counted 19 palisade endings in one lateral rectus muscle and 17 and in one medial rectus.
Fig. 1. CLSM images of palisade endings. Nerve fibers are labeled with anti-neurofilament (red), nerve terminals with anti-synaptophysin (green), and muscle fibers with phalloidin (blue). Nerve terminals which exhibit neurofilament and synaptophysin immunoreactivity appear yellow in the overly. The tendon not labeled continues the muscle fiber to the right.
A. A neurofilament positive nerve fiber runs along the muscle fibers into the tendon, forms a 180° turn and ends in an arborization at the muscle fiber tip. The nerve terminals of the palisade ending are synaptophysin positive and are located in the tendon and around the muscle fiber tip. B. Showing another palisade ending. Only the peripheral part of the nerve fiber supplying the palisade ending is shown. Palisade nerve terminals are synaptophysin positive. Scale bars, 100 μm.
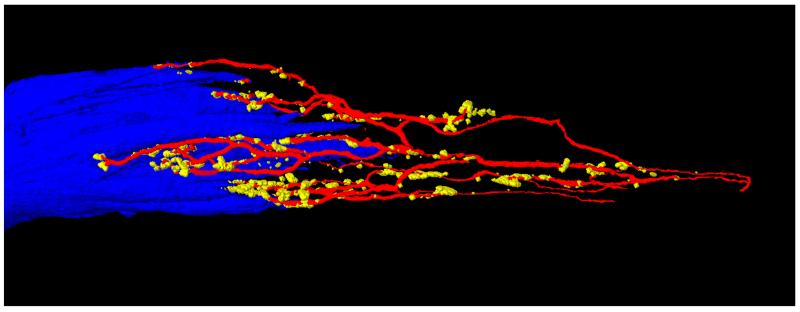
Fig. 2.
3D reconstruction of the palisade ending shown in Figure 1B. The axon (red) forming the palisade ending divides into several preterminal branches at the muscle fiber tip. Preterminal axons establish nerve terminals (yellow) in the tendon and around the muscle fiber tip. The muscle fiber tip is visualized in blue color.
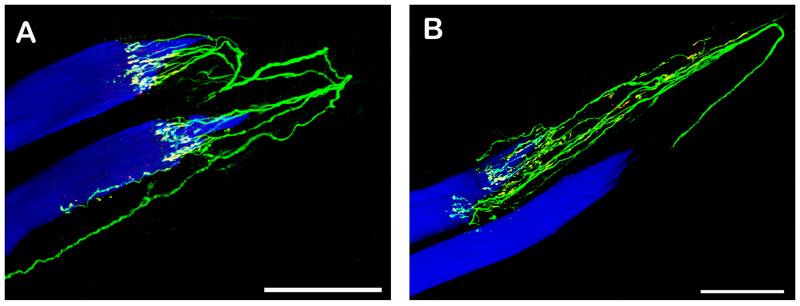
Fig. 3. CLSM images of a palisade endings. Nerve fibers are labeled with anti-ChAT (green), nerve terminals with anti-synaptophysin (red), and muscle fibers with phalloidin (blue). Nerve terminals positive for ChAT and synaptophysin appear yellow in the overlay. The tendon not labeled continues the muscle fiber to the right.
A, B. Nerve fibers supplying palisade endings are ChAT positive. The nerve terminals are double positive for ChAT and synaptophysin. Scale bars, 100 μm.
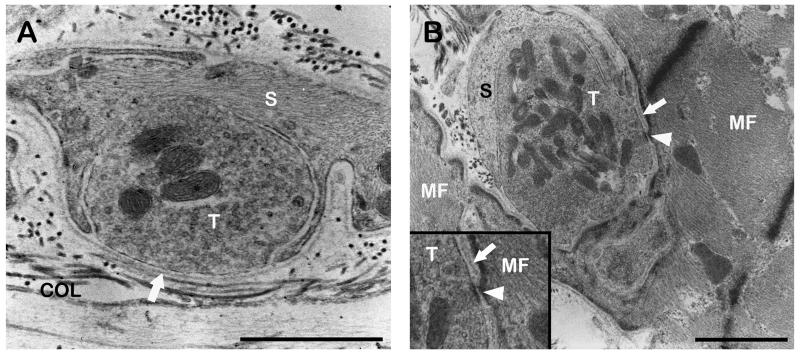
The fine structure of palisade endings was analyzed by TEM. Each palisade ending was enclosed by a connective tissue capsule which extended from the terminal part of muscle fiber into the tendon. Axons forming palisade endings had a myelin sheath and entered the capsule at the tendon level. Inside the capsule, axons lost their myelin sheath and divided into a preterminal axon-ramification. Preterminal axons were completely surrounded by Schwann cells and established nerve terminals to the tendon. Such neurotendinous contacts were partly covered with Schwann cells. At the area of contact a basal lamina separated the axoplasm from the neighboring collagen fibrils. In some palisade endings we observed nerve terminals contacting the muscle fiber as well. The number of such neuromuscular contacts was always significantly lower than the number of neurotendinous contacts. Neuromuscular contacts always had a basal lamina in the synaptic cleft. The basal lamina exhibited interruptions in about one third of the neuromuscular contacts. Neurotendinous and neuromuscular contacts contained mitochondria and numerous clear vesicles (Fig. 4).
Fig. 4.
TEM micrographs of palisade endings. A. High resolution micrograph of a neurotendinous contact in a palisade ending. The nerve terminal (T) contains mitochondria and numerous clear vesicles and is partly covered by a Schwann cell (S). At the contact point only a basal lamina (arrow) separates the nerve terminal and collagen (COL). B. High resolution micrograph of a neuromuscular contact in a palisade ending. A Schwann cell covers part of the nerve terminal which contains mitochondria and clear vesicles. The basal lamina (arrow) between muscle fiber and nerve terminal is in some parts absent (arrowhead). A detail of the nerve terminal is shown in the inset. Scale bars, 1 μm.
Immunolabeling with anti-ChAT/anti-synaptophysin/phalloidin demonstrated that the nerve fibers forming palisade endings were ChAT positive. All nerve terminals of these palisade endings co-localized synaptophysin and ChAT (Fig. 3).
Here, we show that canine EOMs posses palisade endings with a cholinergic phenotype
Palisade endings in dog exhibit a classical shape: axons coming from the muscle extend into the tendon, bend 180 ° and finish in a cuff of nerve terminals around a muscle fiber tip. Palisade endings in dog EOMs are surrounded by a connective tissue capsule which is in line with palisade endings of other species including man [1, 3, 5, 15, 19, 24]. In each palisade ending of dog EOMs we observed nerve terminals that establish contacts on the tendon. Such neurotendinous contacts contain a huge amount of clear vesicles and mitochondria. Neurotendinous contacts full of clear vesicles were also observed in palisade endings of cats [1, 15], sheep [5], monkeys [3, 24], and man [19]. In some palisade endings of dogs we found nerve terminals, likewise full of clear vesicles and mitochondria, contacting the muscle fiber surface as well. Neuromuscular contacts are also present in some palisade endings of cats [1, 15], sheep [19], monkeys [3, 24] and man [19]. By comparing neuromuscular contacts in palisade endings of these species, interspecies variations become apparent. Specifically, neuromuscular contacts in palisade endings of human [19], monkeys [3], and dog (this study) contain a basal lamina in the synaptic cleft thereby exhibiting features of motor terminals [13]. In some neuromuscular contacts in palisade endings of dog the basal lamina was partly dissolved. This feature was also observed in neuromuscular contacts in palisade endings of monkeys [3]. Neuromuscular contacts in palisade endings of cats [1, 15] and sheep [19] are different. They lack a basal lamina in the synaptic cleft thereby resembling sensory nerve terminals in muscle spindles [4, 14, 17]. In conclusion, palisade endings of cats [1, 15], sheep [19], monkeys [3, 24], man [19] and dog (this study) are indistinguishable with respect to neurotendinous contacts whereas neuromuscular contact in palisade endings of this species exhibit structural differences. The reason for this interspecies variation is unclear. It is however hard to believe that interspecies variations represent different types of palisade endings.
Molecular analyses on palisade endings in dog confirm our previous findings on palisade endings in cats [15] and monkeys [3, 24]. Equal to cats [15] and monkeys [3, 24], palisade endings in dog are formed by ChAT-positive nerve fibers. The palisade complex including palisade nerve terminals are ChAT-immunoreactive as well. By labeling palisade endings with antibody against ChAT and antibody against synaptophysin (a marker for sensory as well as motor nerve terminals) we observed that all palisade nerve terminals co-localize ChAT and synaptophysin. We can therefore exclude that palisade endings in dogs receive a double innervation and have two sets of nerve terminals, that is cholinergic and non cholinergic.
So far, palisade endings were observed in each species investigated indicating that they are a universal feature in mammalian EOMs. The present study supports this hypothesis by demonstrating the occurrence of palisade endings in a further species. Moreover, it shows that cholinergicity is a common feature of palisade endings.
Today physiological investigations on palisade endings are missing and their function is still a matter of discussion. Evidence that palisade endings are sensory organs come from morphological and clinical studies [1, 2, 9, 24, 28, 29]. With the exception of rats [11] and rabbits [6], palisade endings in all other species establish nerve terminals on the tendon [1, 3, 5, 15, 19, 24] and such contacts are arguable sensory. Neuromuscular contacts in palisade endings of cat [1] and sheep [5] resemble sensory nerve terminals in muscle spindles. In a single anterograde tracing experiment, neuronal tracers were injected into the sensory trigeminal ganglion of cats and structures resembling palisade endings were identified in EOMs [2]. Indirect evidence for a sensory role of palisade endings comes from clinical experience [28, 29]. Specifically, patients had deficits in spatial perception after strabismus surgery [28, 29]. It was speculated that via this surgical intervention the proprioceptors (palisade endings) at the myotendinous junction were damaged, which in turn resulted in a loss of eye position signals [28, 29].
Other studies indicate that palisade endings are motor structures [3, 15, 16, 19, 26]. For instance, a nerve degeneration experiment demonstrated that lesions of EOM motor nuclei in cat brains lead to degeneration of palisade endings [26]. In addition, molecular analyses of our group proved that palisade endings in EOMs of cats [15] and monkeys [3, 16] exhibit ChAT, choline transporter, and vesicular acetylcholine transporter immunoreactivity. In monkeys [3] and man [19] we showed that neuromuscular contacts when present in palisade endings exhibit features of motor terminals which was confirmed by are α-bungarotoxin binding. Finally, in monkeys nerve fibers forming palisade endings were followed and it was demonstrated in some cases that such nerves establish motor terminals outside the palisade complex [3]. The present study shows that palisade endings in dog are ChAT-immunoreactive and neuromuscular contacts when present in palisade endings exhibit features of motor terminals. These findings complement our prior data on palisade endings and support the theory that palisade endings are effectors.
The function of palisade endings which have effector characteristics is difficult to predict. With the exception of rats [11] and rabbits [6], palisade endings have neurotendinous contacts. Neurotendinous contacts lie in series to the muscle fibers which is analogous to GTOs [27] and Alvarado-Mallart and Pincon Raymond [1] supposed that palisade endings are capable to register muscle fiber contraction. On the other hand, neurotendinous contacts in palisade endings contain ChAT, the synthesizing enzyme of acetylcholine and in fact it would be helpful to know what an effect neurotransmitter release would have on the tendon. At the moment the function of cholinergic neurotendinous contacts is inexplicable and physiological investigations on these EOM specific organs are highly warranted.
Acknowledgements
The authors thank Christiane Hanefl-Krivanek, Regina Mayer, and Marietta Lipowec for their technical assistance. We thank the clinic for ophthalmology of the Veterinary University Vienna for their cooperation. The study was supported by Grant P20881-B09 from the Fonds zur Foerderung der Wissenschaftlichen Forschung (FWF).
References
- 1.Alvarado Mallart RM, Pincon Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979;11:567–84. doi: 10.1016/0040-8166(79)90063-6. [DOI] [PubMed] [Google Scholar]
- 2.Billig I, Buisseret Delmas C, Buisseret P. Identification of nerve endings in cat extraocular muscles. Anat Rec. 1997;248:566–75. doi: 10.1002/(SICI)1097-0185(199708)248:4<566::AID-AR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Blumer R, Konakci KZ, Pomikal C, Wieczorek G, Lukas JR, Streicher J. Palisade endings: cholinergic sensory organs or effector organs? Invest Ophthalmol Vis Sci. 2009;50:1176–86. doi: 10.1167/iovs.08-2748. [DOI] [PubMed] [Google Scholar]
- 4.Blumer R, Lukas JR, Aigner M, Bittner R, Baumgartner I, Mayr R. Fine structural analysis of extraocular muscle spindles of a two-year-old human infant. Invest Ophthalmol Vis Sci. 1999;40:55–64. [PubMed] [Google Scholar]
- 5.Blumer R, Lukas JR, Wasicky R, Mayr R. Presence and structure of innervated myotendinous cylinders in sheep extraocular muscle. Neurosci Lett. 1998;248:49–52. doi: 10.1016/s0304-3940(98)00331-0. [DOI] [PubMed] [Google Scholar]
- 6.Blumer R, Wasicky R, Hotzenecker W, Lukas JR. Presence and structure of innervated myotendinous cylinders in rabbit extraocular muscle. Exp Eye Res. 2001;73:787–96. doi: 10.1006/exer.2001.1085. [DOI] [PubMed] [Google Scholar]
- 7.Bruenech R, Ruskell GL. Myotendinous nerve endings in human infant and adult extraocular muscles. Anat Rec. 2000;260:132–40. doi: 10.1002/1097-0185(20001001)260:2<132::AID-AR30>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 8.Buttner Ennever JA, Eberhorn A, Horn AK. Motor and sensory innervation of extraocular eye muscles. Ann N Y Acad Sci. 2003;1004:40–9. doi: 10.1111/j.1749-6632.2003.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 9.Buttner Ennever JA, Konakci KZ, Blumer R. Sensory control of extraocular muscles. Prog Brain Res. 2005;151:81–93. doi: 10.1016/S0079-6123(05)51003-3. [DOI] [PubMed] [Google Scholar]
- 10.Dogiel A. Die Endigungen der sensiblen Nerven in den Augenmuskeln und deren Sehnen beim Menschen und den Saeugetieren. Arch Mikroskop Anal. 1906;68:501–526. [Google Scholar]
- 11.Eberhorn AC, Horn AK, Eberhorn N, Fischer P, Boergen KP, Buttner Ennever JA. Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity. J Anat. 2005;206:307–15. doi: 10.1111/j.1469-7580.2005.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene T, Jampel R. Muscle spindles in the extraocular muscles of the macaque. J Comp Neurol. 1966;126:547–9. [PubMed] [Google Scholar]
- 13.Harker DW. The structure and innervation of sheep superior rectus and levator palpebrae extraocular muscles. I. Extrafusal muscle fibers. Invest Ophthalmol Vis Sci. 1972;11:956–69. [PubMed] [Google Scholar]
- 14.Harker DW. The structure and innervation of sheep superior rectus and levator palpebrae extraocular muscles. II. Muscle spindles. Invest Ophthalmol Vis Sci. 1972;11:970–9. [PubMed] [Google Scholar]
- 15.Konakci KZ, Streicher J, Hoetzenecker W, Blumer MJ, Lukas JR, Blumer R. Molecular characteristics suggest an effector function of palisade endings in extraocular muscles. Invest Ophthalmol Vis Sci. 2005;46:155–65. doi: 10.1167/iovs.04-1087. [DOI] [PubMed] [Google Scholar]
- 16.Konakci KZ, Streicher J, Hoetzenecker W, Haberl I, Blumer MJ, Wieczorek G, Meingassner JG, Paal SL, Holzinger D, Lukas JR, Blumer R. Palisade endings in extraocular muscles of the monkey are immunoreactive for choline acetyltransferase and vesicular acetylcholine transporter. Invest Ophthalmol Vis Sci. 2005;46:4548–54. doi: 10.1167/iovs.05-0726. [DOI] [PubMed] [Google Scholar]
- 17.Kubota M. Ultrastructural observations on muscle spindles in extraocular muscles of pig. Anat Anz. 1988;165:205–28. [PubMed] [Google Scholar]
- 18.Lukas JR, Aigner M, Blumer R, Heinzl H, Mayr R. Number and distribution of neuromuscular spindles in human extraocular muscles. Invest Ophthalmol Vis Sci. 1994;35:4317–27. [PubMed] [Google Scholar]
- 19.Lukas JR, Blumer R, Denk M, Baumgartner I, Neuhuber W, Mayr R. Innervated myotendinous cylinders in human extraocular muscles. Invest Ophthalmol Vis Sci. 2000;41:2422–31. [PubMed] [Google Scholar]
- 20.Mahran ZY, Sakla FB. The pattern of innervation of the extrinsic ocular muscles and the intra-orbital ganglia of the albino mouse. Anat Rec. 1965;152:173–83. doi: 10.1002/ar.1091520208. [DOI] [PubMed] [Google Scholar]
- 21.Maier A, DeSantis M, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol. 1974;143:397–408. doi: 10.1002/jmor.1051430404. [DOI] [PubMed] [Google Scholar]
- 22.Richmond FJ, Johnston WS, Baker RS, Steinbach MJ. Palisade endings in human extraocular muscles. Invest Ophthalmol Vis Sci. 1984;25:471–6. [PubMed] [Google Scholar]
- 23.Ruskell GL. The fine structure of human extraocular muscle spindles and their potential proprioceptive capacity. J Anat. 1989;167:199–214. [PMC free article] [PubMed] [Google Scholar]
- 24.Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J Neurocytol. 1978;7:693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- 25.Ruskell GL. The incidence and variety of Golgi tendon organs in extraocular muscles of the rhesus monkey. J Neurocytol. 1979;8:639–53. doi: 10.1007/BF01208514. [DOI] [PubMed] [Google Scholar]
- 26.Sas J, Scháb R. Die sogennanten “Palisaden-Endigungen” der Augenmuskeln. Acta Morph Acad Sci Hung. 1952;2:259–266. [Google Scholar]
- 27.Schoultz TW, Swett JE. The fine structure of the Golgi tendon organ. J Neurocytol. 1972;1:1–26. doi: 10.1007/BF01098642. [DOI] [PubMed] [Google Scholar]
- 28.Steinbach MJ, Kirshner EL, Arstikaitis MJ. Recession vs marginal myotomy surgery for strabismus: effects on spatial localization. Invest Ophthalmol Vis Sci. 1987;28:1870–2. [PubMed] [Google Scholar]
- 29.Steinbach MJ, Smith DR. Spatial localization after strabismus surgery: evidence for inflow. Science. 1981;213:1407–9. doi: 10.1126/science.7268444. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex.[see comment] Nat Neurosci. 2007;10:640–6. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]