Abstract
Purpose
To analyze and compare the structural and molecular features of classical proprioceptors like muscle spindles and Golgi tendon organs (GTOs) and putative proprioceptors (palisade endings) in sheep extraocular muscle (EOMs).
Methods
The EOMs of four sheep were analyzed. Frozen sections or whole mount preparations of the samples were immunohistochemically labeled and analyzed by confocal laser scanning microscopy. Triple labeling with different combinations of antibodies against neurofilament, synaptophysin and choline acetyltransferase (ChAT) as well as α-bungarotoxin and phalloidin was performed. Microscopic anatomy of the nerve end organs was analyzed by transmission electron microscopy.
Results
The microscopic anatomy demonstrated that muscle spindles and GTOs had a perineural capsule and palisade endings a connective tissue capsule. Sensory nerve terminals in muscle spindles and GTOs contained only few vesicles whereas palisade nerve terminals were full of clear vesicles. Likewise, motor terminals in the muscle spindles’ polar regions were full of clear vesicles. Immunohistochemistry showed that sensory nerve fibers as well as their sensory nerve terminals in muscle spindles and GTOs were ChAT-negative. Palisade endings were supplied by ChAT-positive nerve fibers and the palisade complexes including palisade nerve terminals were also ChAT-immunoreactive. Motor terminals in muscle spindles were ChAT and α-bungarotoxin -positive.
Conclusions
The present study demonstrated in sheep EOMs that palisade endings are innervated by cholinergic axons exhibiting characteristics typical for motoneurons whereas muscle spindles (except the polar regions) and GTOs are supplied by non-cholinergic axons. These results question whether palisade endings are candidates for proprioceptors in EOMs.
Keywords: sheep, eye movement, proprioception, palisade endings, Golgi tendon organs, muscle spindles, choline acetyltransferase
INTRODUCTION
The sense of self position and movement is generally known as proprioception and is accomplished by sensory receptors located in muscles and related deep tissues. For sensory perception of the eyeball’s position in its socket, extraocular muscles (EOMs) likewise carry proprioceptors which are partly the same as in skeletal muscles such as muscle spindles and Golgi tendon organs (GTOs). 1, 2 However, EOMs are additionally endued with putative proprioceptors which are referred to as myotendinous cylinders or palisade endings. 3-11 Interestingly, the occurrence of the three nerve end organs in EOMs is quite variable from species to species. While muscle spindles were detected in EOMs of mice, 12 even-toed ungulates, 13-20 some monkey species, 21 and humans, 14, 22-25 GTOs on the other hand were just found in even-toed ungulates, 13-15, 20, 26 and very rarely in monkeys. 27 Palisade endings again are present in the EOMs of human, 10, 28 rhesus monkey, 4, 9, 11 cat, 3, 8, 29, 30 rat, 7 rabbit, 6 and sheep. 5
Each of these three nerve end organs has its unique anatomy and structure. In EOMs of even-toed ungulates muscle spindles are composed of a perineural capsule enclosing several intrafusal muscle fibers termed nuclear chain and nuclear bag fibers. In pig EOMs, Friedrich et al 31 show that intrafusal muscle fibers express spindle-special mysion heavy chain isoforms. Intrafusal muscle fibers are innervated by sensory nerve fibers twining around muscle fibers at the equatorial region and by motoneurons terminating on muscle fibers at the muscle spindles’ poles. 13, 15-18 In monkey 21 and human EOMs 22-25 however, most muscle spindles do not contain any nuclear bag fibers. Additionally to nuclear chain fibers, anomalous muscle fibers are regularly present in primate muscle spindles. 22-25 Such anomalous fibers have peripheral myonuclei and are indistinguishable from muscle fibers outside of muscle spindles. 22-25 GTOs have a more uniform appearance among EOMs of different species. 13, 15, 16, 20, 26, 27 They are located at the insertion of muscle fibers into the tendons, comprised of a perineural capsule filled with collagen and are innervated by afferent nerves. Besides collagen bundles, GTOs can contain up to five muscle fibers, which either terminate in the GTO or transverse the organ from pole to pole. 13, 15, 16, 20, 26, 27
Palisade endings are nerve end organs uniquely found in EOMs and were demonstrated to be present in all species so far investigated. 3-11, 14, 28, 29 They are located at the muscle-tendon-junction and are forming a cuff of nerve terminals around the tip of a muscle fiber. Palisade endings arise from myelinated nerve fibers, which enter the muscle at a central nerve entrance spot. Axons proceed alongside the muscle fiber towards its ending and further into the tendon. There the nerves form a 180° curve and return to establish nerve terminals around the muscle fiber tip. 3, 5-11, 14, 28, 29 In most species (cat, 8, 29 sheep, 5 monkey, 4, 11 and man 10) palisade nerve terminals establish contacts to tendons and to muscle fibers. In fact, in cat, 8, 29 sheep, 5 monkey, 4, 11 and man 10 neurotendinous contacts are more frequent and neuromuscular contacts are only observed in a minority of the palisade endings. Palisade endings in rabbits 6 and rats 7 are an exception because exclusively neuromuscular contacts were observed.
Although physiological analyses on palisade endings are missing, the general opinion is that palisade endings are sensory. 1, 3, 7, 11, 28, 29, 32-34 However, in one older study it was demonstrated that nerve fibers supplying palisade endings originate from the EOM motor nuclei (oculomotor, trochlear, and abducens nuclei). 30 Moreover, our prior results demonstrated in cat as well as in monkey EOMs that palisade endings are formed by cholinergic nerve fibers that establish motor terminals outside of the palisade complex, as it was shown in some cases in monkey. 4, 8, 9 In palisade endings of cat 8, monkey 4 and man 10 it was also shown that neuromuscular contacts have features of motor terminals. Nevertheless, the debate about the function of palisade endings is still ongoing whether these structures are sensory, despite their cholinergic properties, motor or both. 10
If palisade endings are in fact cholinergic sensory, could it be that well known sensory nerve structures (muscle spindles and GTOs) are as well cholinergic? The present study seeks to answer this question. For our analysis we chose sheep, because among mammals, sheep is the only species in which muscle spindles, GTOs, and palisade endings are commonly found in EOMs. 5, 17, 20, 26 We investigated by immunohistochemistry different attributes of the nerve fibers and nerve terminals: Neurons were labeled with an antibody against the cytoskeletal structure protein neurofilament and to illustrate synapses an antibody against synaptic vesicle glycoprotein synaptophysin was used. Furthermore, to distinguish cholinergic nerve fibers labeling for ChAT, the enzyme for acetylcholine synthesis, was performed and fluorescently labeled α-bungarotoxin was used to visualize motor endplates. Phalloidin was used to stain muscle fibers. Fine structural analysis of muscle spindles, GTOs and palisade endings were done to complement molecular findings.
MATERIALS AND METHODS
All animals used in this study were treated in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. Four sheep their age varying between 6 month and two years were analyzed in this study. The sheep heads were gratefully provided by a local slaughterhouse. Organic structures were fixed by perfusion. In brief: Perfusion was carried out by catheterizing the internal carotid artery. First, the vascular system of the segregated head was flushed with Ringer solution to remove blood clots and subsequently fixation was performed by perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer (PB pH 7.4). Eye-bulbs with attached EOMs were removed from the head and EOMs including tendons were dissected. The tissue was immersion fixed with 4% paraformaldehyde in PB for 4 hours (for confocal laser scanning microscopy) or alternatively with 4% paraformaldehyde and 2.5% glutaraldehyde in PB (for transmission electron microscopy). EOMs were stored at 4 °C submerged in 0.1 M phosphate-buffered saline (PBS) and further processed for confocal laser scanning microscopy (CLSM) and transmission electron microscopy (TEM).
Confocal Laser Scanning Microscopy
For CLSM, tissue was frozen in cooled (− 80° C) Methylbutan and stored at − 80°C. Fifteen μm thick sections were cut with a crystat microtome (Leica CM1950, Heidelberg, Germany), mounted on gelatine coated slides and immunohistochemically stained. Alternatively, CLSM was performed with wholemount preparations of EOMs that were immuno labeled. Triple labeling of sections and wholemounts was carried out with four different combinations of antibodies and labeling substances (Table 1). For detailed description of the methods performed see our former publications. 8, 9
Table 1.
List of markers and antibodies for immunhistochemical triple staining detection. If not separately indicated the same dilution was used for cryo-sections (CS) and wholemounts (WM).
| Triple Staining | Marker for Muscle Fibers and motor end-plates | Primary Antibodies | Secondary Antibodies |
|---|---|---|---|
| Phalloidin, Neurofilament, Synaptophysin | Alexa Fluor 633 conjugated Phalloidin, CS 1:500 WM: 1:50, Molecular Probes | Chicken anti-Neurofilament, CS: 1:5000 WM 1:2500, Molecular Probes, Mouse anti Synaptophysin, CS: 1:400 WM 1:200, Chemicon | Goat anti-chicken Alexa Fluor 568, 1:500, Molecular Probes, Goat anti-mouse Alexa 488, 1:500, Molecular Probes |
| Phalloidin, Synaptophysin, α-Bungarotoxin | Alexa Fluor 633 conjugated Phalloidin, rhodamine conjugated α-Bungarotoxin CS: 1:500, Molecular Probes | Synaptophysin, CS: 1:400, Chemicon | Goat anti-mouse Alexa Fluor 488, |
| Phalloidin, ChAT, Synaptophysin | Alexa Fluor 633 conjugated Phalloidin | Rabbit anti ChAT, CS: 1:500 WM: 1:250, Prof. Schemann; Mouse anti Synaptophysin, CS: 1:400 WM 1:200 | Goat anti-rabbit Alexa Fluor 488, 1:500, Molecular Probes, Goat anti-mouse rhodamine, 1:200, Chemicon |
| Phalloidin, ChAT, Neurofilament | Alexa Fluor 633 conjugated Phalloidin | Rabbit anti ChAT Chicken anti-Neurofilament | Goat anti-rabbit Alexa Fluor 488, Goat anti-chicken Alexa Fluor 568 |
Triple Labeling of Cryo-Sections
The presence of muscle spindles and GTOs was first verified by light microscopy on frozen EOM sections stained with either haematoxylin/eosin or azan. Once the structures were identified consecutive sections were processed for immunohistochemistry. The sections were first briefly rinsed in PBS and then blocked with 10 % goat serum for one hour. Further on, they were incubated with a combination of two primary antibodies (Table 1) for 48 hours at 4 °C, rinsed (4 times, each 5 minutes) with PBS + 0.1 % Tween and incubated with one of the according secondary antibodies for two hours at 37 °C. After the next washing step the sections were incubated with the other secondary antibody and phalloidin (Table 1) for another two hours. Finally the slides were again rinsed and covered with mounting medium and a cover slip. The labeled sections were first examined by UV-microscopy where the different structures were identified. Later on, the slides were analyzed by CLSM (LSM 510, Carl Zeiss Meditec). Images were generated in 4 different channels: three fluorescence channels (excitation wave length at 488, 568 and 633 nm) and one transmission-light image.
Triple labeling of wholemount preparations
In wholemounts of the EOM myotendinous junction, palisade endings and GTOs were analyzed. Wholemounts of EOMs were frozen and thawed, incubated in PBS containing 1 % Triton (PBSTri) and then blocked with 10 % goat serum in PBSTri for 2 hours. Further on, they were incubated for 48 hours with a combination of two primary antibodies (Table 1) then rinsed (7 times, each 15 minutes) with PBSTri and incubated with one secondary antibody for four hours and after another washing step incubated with the other secondary antibody and phalloidin over night (Table1). At last, the wholemounts were again rinsed and mounted in v/v 60 % glycerine + 40 % PBS. The labeled wholemounts were first examined by UV-microscopy and when the according structures were identified, they were further analyzed by CLSM (LSM 519, Carl Zeiss Meditec). Series of longitudinal virtual CLSM sections of 0.5 – 1 μm thickness were cut through palisade endings and GTOs. Each section was photo documented and three-dimensional projections were formulated on computer using LSM Image Examiner software (Carl Zeiss Meditec). Images were generated in 3 different fluorescence channels: excitation wave length of 488, 568 and 633 nm.
For negative control experiments, primary antibodies were omitted and secondary antibodies were used alone. In all cases the omission of the primary antibodies resulted in a complete lack of immunostaining.
Transmisson Electron Microscopy
After immersion fixation EOMs including tendons were cut longitudinally into small strips. Specimen were postfixed in 1% osmium tetroxide, dehydrated in graded solutions of alcohol and embedded in Epon. Semithin cross sections were cut through the tissue blocks and examined by light microscopy. When the according structures (muscle spindles, GTOs, and palisade endings) were identified, ultrathin sections were cut at appropriate intervals. Sections were mounted on dioxane formvar-coated (Formvar, SPI, West Chester, PA) copper grids and stained in a 2 % uranyl acetate solution followed by 0.4% lead citrate solution. Sections were analyzed by transmission electron microscope (Zeiss EM 10).
RESULTS
Muscle spindles
Structural features
Muscle spindles in sheep EOMs had a fusiform shape with a large subcapsular space in the equatorial region containing acidic mucopolisaccharides. The organ was ensheathed by a perineural capsule that comprised up to eight cell layers. The perineural cells of the capsule were covered with a basal lamina on both sides. The muscle spindle capsules enclosed four to ten intrafusal muscle fibers which had a smaller diameter than the extrafusal muscle fibers outside the spindles. Within the equatorial region of muscle spindles, the intrafusal muscle fibers contained nuclei centrally arranged in a single row or aggregated to clusters, representing the nuclear chain and nuclear bag fibers respectively. The sarcomeres of the nuclear chain fibers exhibited M-lines within the H-bands. Such M-lines were not observed in sarcomeres of nuclear bag fibers. Each muscle spindle contained one to three nuclear bag fibers, the remainder being all nuclear chain fibers (Figs. 1, 2B).
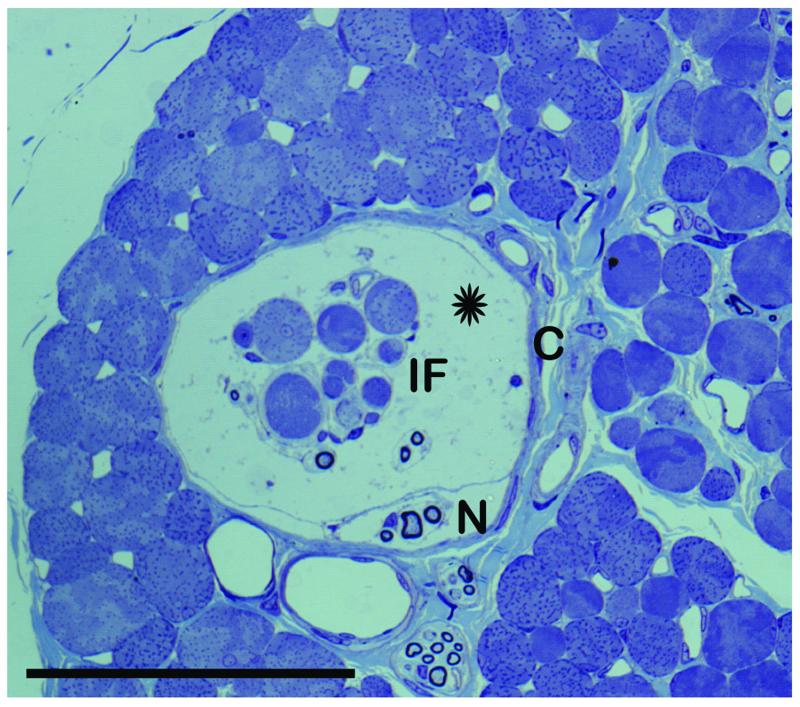
Fig. 1.
Light microscope image of a muscle spindle.
The image shows a cross section through a muscle spindle in the equatorial region. The muscle spindle contains eight intrafusal muscle fibers (IF) which are separated from the capsule by a subcapsular space (asterisk). One thick and several thin nerve fibers (N) are inside the muscle spindle. Capsule (C). Scal bar, 100 μm.
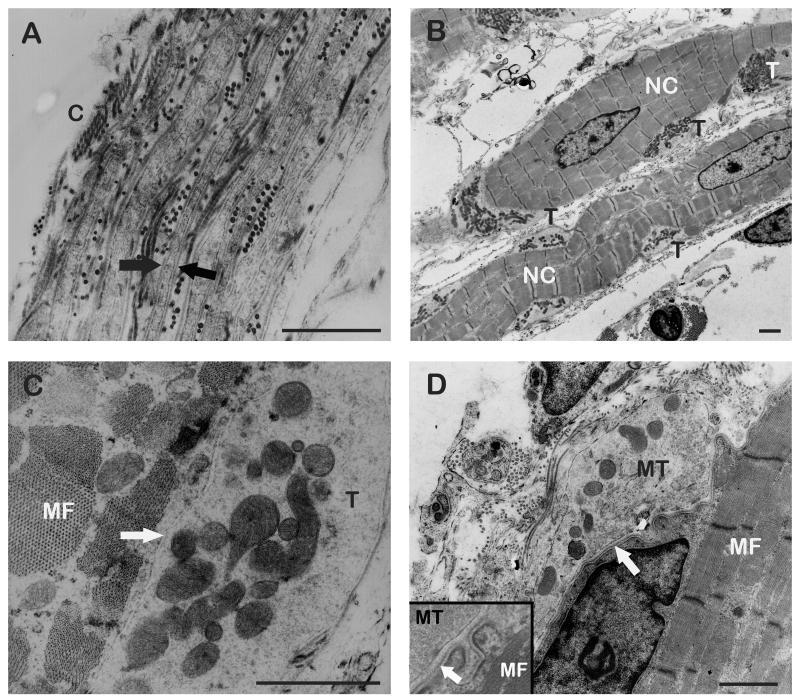
Fig. 2.
TEM micrographs of muscle spindles.
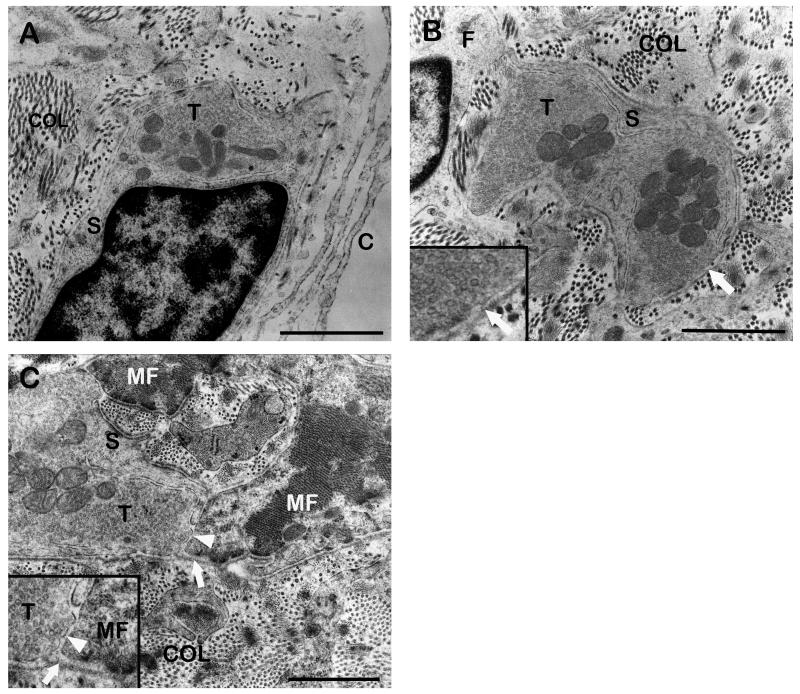
(A) Cross section through the muscle spindle’s capsule (C). The capsule consists of perineural cells which are covered on both sides with a basal lamina (arrows). (B) Longitudinal section through two nuclear chain fibers (NC) being richly endowed with sensory nerve terminals (T). (C) Detail of a sensory nerve terminal (T). The nerve terminal contains mitochondria and the synaptic cleft (arrow) is free from a basal lamina. Muscle fiber (MF). (D) Longitudinal section through a motor terminal (MT). The nerve terminal contains mitochondria and aggregations of clear vesicles. A basal lamina fills the synaptic cleft (arrow). Inset showing a detail of a motor terminal. Muscle fiber (MF). Scale bars, 1 μm.
Innervation
Morphological characteristics
In the equatorial region of the muscle spindles one thick myelinated axon (8-10 μm in diameter) and several thin myelinated axons (2-4 μm) entered the proprioceptor. The thick axon divided into several branches which enwrapped the nuclear chain and nuclear bag fibers thereby forming typical anulospiral nerve endings. Transmission electron microscopy examination demonstrated that these sensory nerve endings contained many mitochondria and only a few, if any vesicles. The synaptic cleft of sensory contacts measured between 20 and 30 nm and did not exhibit a basal lamina. In the polar regions of muscle spindles, motor nerve terminals were observed on intrafusal fibers. Motor terminals contained mitochondria, clusters of numerous clear vesicles, and a basal lamia always filled the synaptic cleft (Figs. 2B-2D). The width of the synaptic cleft measured between 80 and 100 nm.
Molecular characteristics
In muscle spindles, three combinations of triple labeling were performed: Labeling with 1.) anti-synaptophysin, α-bungarotoxin, and phalloidin, 2.) anti-ChAT, anti-synaptophysin, and phalloidin, and 3.) anti-ChAT, anti-neurofilament, and phalloidin.
Labeling with anti-synaptophysin/α-bungarotoxin/phalloidin showed that sensory nerve terminals in the equatorial region were synaptophysin-positive but α-bungarotoxin-negative. In contrast, motor synapses in the polar regions of muscle spindles stained double positive for synaptophysin and α-bungarotoxin. Motor terminals on muscle fibers outside the muscle spindle were as well synaptophysin/α-bungarotoxin positive (Figs. 3A, 3B). Labeling with anti-ChAT/anti-synapthophysin/phalloidin exhibited ChAT-positive nerve fibers entering at the muscle spindles’ equatorial region. ChAT-positive nerve fibers ran towards the spindles’ poles while nerve fibers supplying the spindles’ equatorial region lacked ChAT-immunostaining. Furthermore, sensory nerve terminals in the spindles’ equatorial regions were synaptophysin positive but ChAT-negative whereas in the polar region motor terminals exhibited synaptophysin/ChAT immunoreactivity (Figs. 3C, 3D). Labeling with anti-ChAT/anti-neurofilament/phalloidin showed that muscle spindles received a double innervation. In the equatorial region, neurofilament-positive nerve fibers as well as ChAT/neurofilament-positive nerve fibers entered the muscle spindles. In the equatorial region, nerve fibers established anulospiral endings at intrafusal muscle fibers. These nerve fibers were solely neurofilament immunoreactive. In the spindles’ polar regions, nerve fibers exhibited ChAT and neurofilament immunoreactivity (Figs. 3E, 3F).
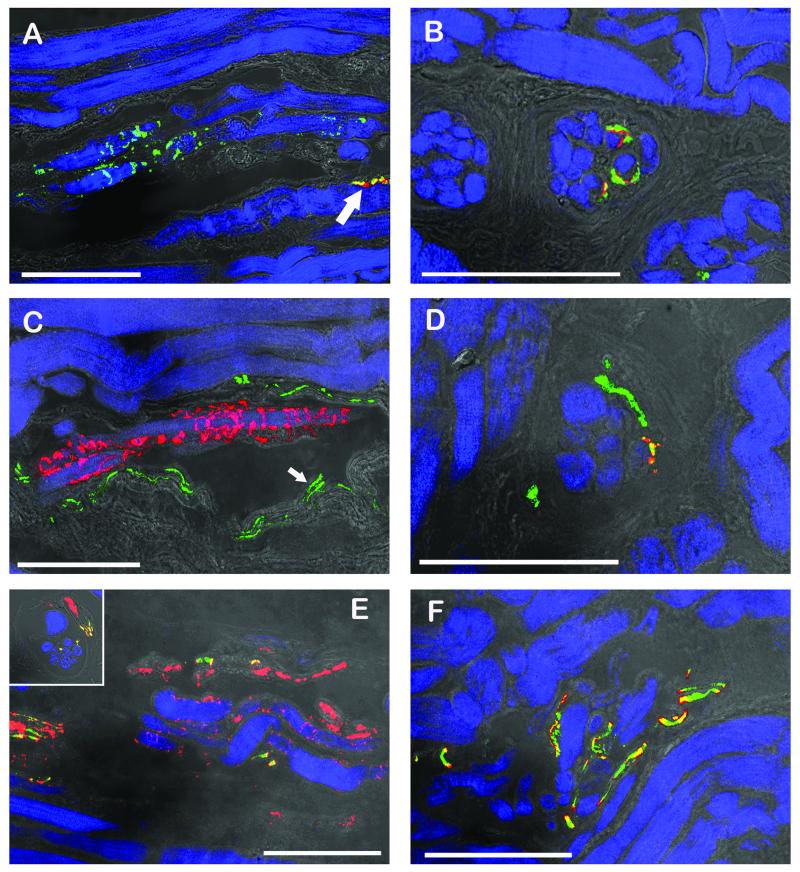
Fig. 3.
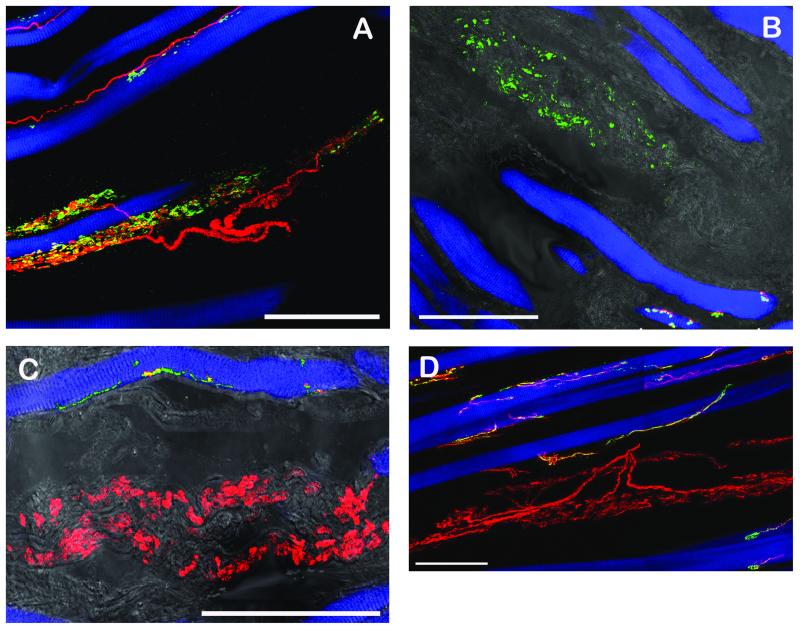
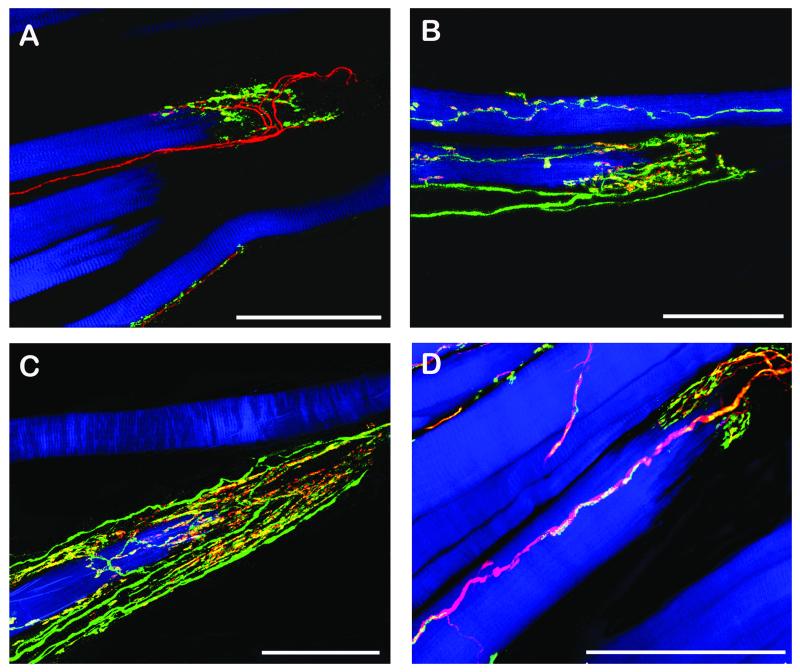
CLSM images of muscle spindles showing the equatorial regions (A, C, E) and the polar regions (B, D, F). The equatorial regions are shown in longitudinal sections whereas the polar regions are shown in cross section (B, D) or oblique section (F) respectively. Triple fluorescent images were overlaid with a transmission light image. (A, B) Labeling with anti-synaptophysin (green), α-bungarotoxin (red) and phalloidin (blue). (A) Sensory nerve endings in the equatorial region are only positive for synaptophysin whereas a motor terminal (arrow) outside the spindle is synaptophysin/α-bungarotoxin-positive. (B) In the muscle spindle’s polar region, motor terminals exhibit synaptophysin/α-bungarotoxin reactivity. (C, D) Labeling with anti-ChAT (green), anti-synaptophysin (red), and phalloidin (blue). (C) Sensory anulospiral nerve endings in the equatorial regions are synaptophysin-positive but ChAT-negative. ChAT-positive nerve fibers are seen alongside the spindle. Two ChAT-positive axons (arrow) running towards the spindle’s pole are visible inside the spindle. (D) In the polar region ChAT-positive nerve fibers establish motor terminals on the intrafusal muscle fibers. Motor terminals co-localize ChAT and synaptophysin. (E, F) Labeling with anti-ChAT (green), anti-neurofilament (red), and phalloidin (blue). (E) In the muscle spindles’ equatorial region nerve fibers solely positive for neurofilament and nerve fibers positive for ChAT/neurofilament are visible. Only neurofilament-positive axons enwrap intrafusal muscle fibers and are thereby forming anulospiral endings in the equatorial region. Inset showing the mixed innervation of a muscle spindle in a cross section. (F) In the polar region axons stain double positive for ChAT and neurofilament. Scale bars, 100 μm.
Golgi tendon organs
Structural features
Golgi tendon organs were observed in each EOM in the distal and proximal tendons. The fusiform receptors were enclosed by a perineural capsule consisting of up to four cell layers. Capsule cells were covered on both sides with a basal lamina. In general, GTOs contained collagen bundles but we also found GTOs comprising collagen bundles and muscle fibers (up to five). These muscle fibers entered the receptor at one pole and either terminated in collagen bundles or passed through. Moreover, typical for GTOs was also a subcapsular gap that surrounded collagen and muscle fibers (when present) and contained acidic mucopolysaccharides. (Fig. 4A, 4B)
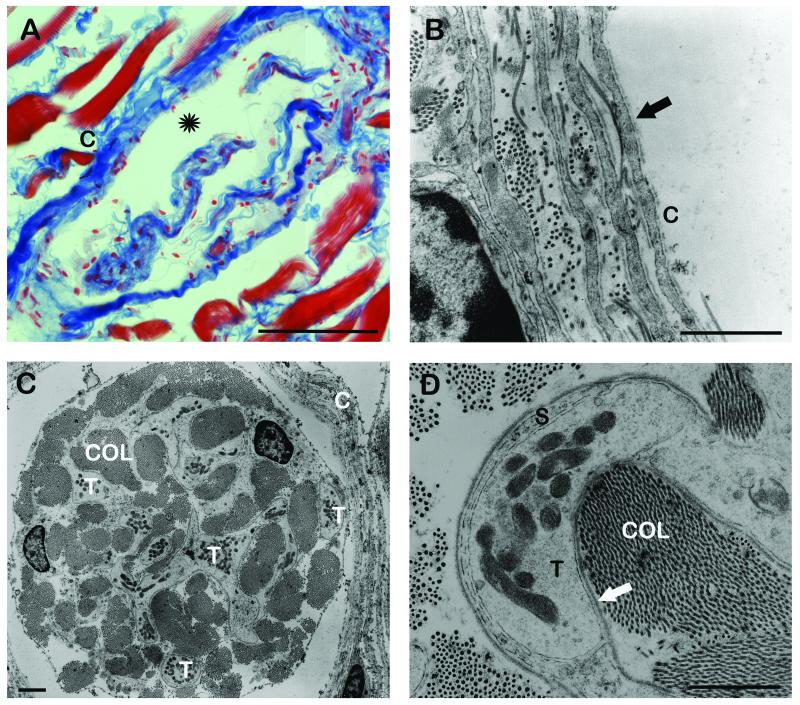
Fig. 4.
Light microscopic image (A) and TEM micrographs (B, C, D) of GTOs (A) Oblique section through a GTO stained with Azan. The GTO is enclosed by a capsule (C). Inside the capsule collagen bundles are visible which are separated from the capsule by a subcapsular space (asterisk). (B) Cross section through the GTO capsule (C) consisting of four perineural cell layers. Perineural cells are ensheathed by a basal lamia (arrow). (C) Cross section through a GTO. Among the collagen bundles (COL) numerous nerve terminals (T) are visible. Capsule (C). (D) High resolution micrograph of a nerve terminal (T) contacting the neighboring collagen fibrils (COL). The nerve terminal is partly covered by a Schwann cell (S) and at the point of contact only a basal lamina (arrow) is interposed between the axolemma and the collagen. Scale bars, (A) 100 μm, (B, C, D) 1 μm.
Innervation
Morphological characteristics
GTOs were innervated by a single axon (8 – 10 μm in diameter) penetrating the GTO capsule at variable points. Inside the organ the axon split into several preterminal branches which finally established nerve terminals at collagen bundles. Nerve terminals were partly covered with Schwann cells and at the point of contact only a basal lamina separated them from the neighboring collagen. Comparable to sensory nerve endings in muscle spindles, nerve endings in GTOs contained mitochondria but hardly any vesicles (Figs. 4C, 4D).
Molecular characteristics
In GTOs, four combinations of triple labeling were performed: Labeling with 1.) anti-synaptophysin, anti-neurofilament, and phalloidin 2.) anti-synaptophysin, α-bungarotoxin and phalloidin, 3.) anti-ChAT, anti-synaptophysin and phalloidin, and 4.) anti-ChAT, anti-neurofilament and phalloidin.
When the first staining was performed, neurofilament labeled nerve fibers entering GTOs could be observed. Nerve terminals contacting collagen bundles inside the GTO were synaptophysin-immunoreactive. Muscle fibers, when present in GTOs, were labeled with phalloidin (Fig. 5A). Labeling with anti-synaptophysin/α-bungarotoxin/phalloidin exhibited that synapses in GTOs were positive for synaptophysin but not for α-bungarotoxin. However, motor end plates outside of the GTO were double positive for synaptophysin and α-bungarotoxin (Fig. 5B). Labeling with anti-ChAT/anti-neurofilament/phalloidin and anti-ChAT/anti-synaptophysin/phalloidin showed that nerve fibers innervating GTOs were neurofilament-positive but ChAT-negative. Nerve terminals within GTOs were positive for synaptophysin but not for ChAT (Figs. 5C, 5D).
Fig. 5.
CLSM images of GTOs in whole mount preparations (A, D) and longitudinal frozen sections (B, C). In frozen sections the triple fluorescent images were overlaid with a transmission light image.
(A) Labeling with anti-neurofilament (red), anti-synaptophysin (green) and phalloidin (blue). A single neurofilament-positive nerve fiber enters a GTO containing a muscle fiber. Inside the GTO the axon divides into nerve branches which establish synaptophysin-positive contacts. (B) Labeling with anti-synaptophysin (green), α-bungarotoxin (red) and phalloidin (blue). Nerve terminals inside the GTO are synaptophysin-immunoreactive but α-bungarotoxin-negative. Motor endplates outside the GTOs stain synaptophysin/α-bungarotoxin-positive. (C) Labeling with anti-ChAT (green), anti-synaptophysin (red), and phalloidin (blue). GTO-nerve terminals are synaptophysin positive but ChAT-negative. Motor nerve terminals contacting muscle fibers outside the GTO co-localize ChAT/synaptophysin. (D) Labeling with anti-ChAT (green), anti-neurofilament (red), and phalloidin (blue). In this staining combination the nerve fiber innervating the GTO exhibits solely neurofilament reactivity. Motor nerve fibers establishing neuromuscular contacts exhibit ChAT/neurofilament immunoreactivity. Scale bars, 100 μm.
Palisade nerve endings
Structural features
Palisade endings were observed in each rectus EOM and were ensheathed by a thin capsule consisting of two to three cell layers. Capsule cells had the appearance of fibrocytes and lacked a basal lamina (Figs. 6A). The typical structure of palisade nerve endings could be observed best in EOM wholemount preparations of the myotendinous intersections. We observed thin nerve fibers that came from the muscle and extended into the tendon. Within the tendon, nerve fibers turned back 180° and divided into a terminal arborization around a single muscle fiber tip (Fig. 7). We did not find differences in palisade endings of the six months and two-year-old sheep.
Fig. 6.
TEM micrographs of palisade nerve endings
(A) Cross section through the capsule of a palisade ending. The capsule (C) consists of fibroctyes which lack a basal lamina. Inside the capsule, a palisade nerve terminal (T) contacting the collagen fibrils is visible. (B) High resolution micrograph of a neurotendinous contact containing mitochondria and a dense aggregation of clear vesicles. The nerve terminal is partly covered with a Schwann cell (S) and at the collagen contact site only a basal lamina (arrow) covers the terminal. Inset: Detail of the contact. (C) High resolution image of a palisade nerve terminal (T) contacting the muscle fiber (MF) which is covered with a basal lamina (arrow). The neuromuscular contacts contain mitochondria and clear vesicles. In the synaptic cleft (arrowhead) a basal lamia is absent. Inset: Detail of the contact. Scale bars, 1μm.
Fig. 7.
CLSM images of palisade endings in whole mount preparations. The unlabeled tendon continues the muscle fibers to the right.
(A) Labeling with anti-neurofilament (red), anti-synaptophysin (green) and phalloidin (blue). A neurofilament-positive nerve fiber forms a palisade ending at a muscle fiber tip. Palisade nerve terminals are synaptophysin-immunoreactive. (B, C) Labeling with anti-ChAT (green), anti-synaptophysin (red), and phalloidin (blue). ChAT-positive nerve fibers supplying palisade endings at the muscle fiber tips. All palisade nerve terminals co-localize ChAT/synaptophysin. (D) Labeling with anti-ChAT (green), anti-neurofilament (red), and phalloidin (blue). The nerve fiber forming a palisade ending exhibits ChAT/neurofilament immunoreactivity. Scale bars, 100 μm.
Innervation
Morphological characteristics
Myelinated axons forming palisade endings had a diameter of 2 to 3 μm and penetrated the capsule of the palisade ending at tendon level. Inside the capsule, axons lost their myelin sheath and divided into preterminal axons. These preterminal axons established nerve terminals at collagen fibrils. Nerve terminals contacting collagen were only partly covered with Schwann cells. At the point of contact, a basal lamina separated nerve terminals from neighboring collagen. Such neurotendinous were observed in each palisade ending and they contained mitochondria and a lot of clear vesicles (Figs. 6A, 6B). In few palisade endings we additionally observed nerve terminals contacting muscle fiber tips. Such neuromuscular contacts contained mitochondria and clear vesicles. In the synaptic cleft a basal lamina was absent (Fig. 6C).
Molecular characteristics
For molecular evaluation three combination of triple labeling were performed in EOM whole mount preparations: Labeling with 1.) anti-synaptophysin, anti-neurofilament, and phalloidin 2.) anti-ChAT, anti-synaptophysin and phalloidin, and 3.) anti-ChAT, anti-neurofilament and phalloidin.
In the first staining combination, neurofilament-positive nerve fibers were observed to form palisade endings at single muscle fiber tips. Palisade nerve terminals exhibited synaptophysin immunoreactivity (Fig. 7A). Additionally, labeling with anti-ChAT/anti-synaptophysin/phalloidin showed that nerve fibers supplying palisade endings were ChAT-positive. In all palisade nerve terminals co-localization of ChAT/synaptophysin was detectable (Figs. 7B, 7C). Moreover, labeling with anti-ChAT/anti-neurofilament/phalloidin brought to light that all nerve fibers forming palisade endings stained double-positive for ChAT and neurofilament (Fig. 7D).
The structural and molecular characteristics of nerve terminals in muscle spindles, GTOs and palisade endings are summarized in Table 2. It is important to note that the material for our ultrastructural investigations was obtained from one single sheep. Moreover the tissue preparation for the fine structural analysis of muscle spindles, Golgi tendon organs and palisade endings was identical. Therefore, differences of nerve terminals in muscle spindles, GTOs, and palisade endings with respect to vesicle content are no signs of tissue artefacts or individual variations.
Table 2.
Morphological and molecular characteristics of nerve terminals in muscle spindles, Golgi tendon organs (GTOs), and palisade endings
| Vesicles in nerve terminals | Basal lamina in the synaptic cleft | Choline acetyltransferase immunoreactivity of nerve terminals | |
|---|---|---|---|
| Sensory nerve terminals in muscle spindles | < * | − | − |
| Sensory nerve terminals in GTOs | < * | + † | − |
| Neurotendinous contact in palisade endings | + | + † | + |
| Neuromuscular contacts in palisade ednings | + | − | + |
| Motor terminals in muscle spindles | + | + | + |
Few if any vesicles are found
Sensory nerve terminals in GTOs and neurotendinous contacts in palisade endings are covered with a basal lamina at the area of contact with the collagen
DISCUSSION
Muscle spindles and GTOs are classical proprioceptors whereas the function of palisade endings is unclear whether these structures are sensory (proprioceptive), 1, 3, 7, 11, 28, 29, 32-34 motor 4, 8, 9, 30 or both. 10 Among mammals sheep is the only species in which these three nerve end organs are observed in common in EOMs. 5, 17, 20, 26 Therefore we consider sheep to be an ideal species to analyze and compare the structural and molecular characteristics of classical proprioceptors and putative proprioceptors (palisade endings). The structure of muscle spindles, GTOs, and palisade endings in sheep EOMs was thoroughly described in previous studies. 5, 17, 20, 26 We re-analyzed morphological features to complement our new data on molecular characteristics of muscle spindles, GTOs, and palisade endings the EOMs of this species
Our morphological findings on muscle spindles, GTOs, and palisade endings in sheep EOMs are in accordance with prior studies. 5, 17, 20, 26 Structural differences become apparent when classical proprioceptors and palisade endings are directly compared. These differences concern cellular components of capsules ensheathing the organs and vesicle content of sensory nerve terminals in muscle spindles and GTOs, as well as nerve terminals in palisade endings. Specifically, muscle spindles and GTOs comprise a capsule of perineural cells which are enclosed by a basal lamina. In contrast, palisade endings have a connective tissue capsule composed of fibrocytes which lack a basal lamina. Sensory nerve terminals contacting intrafusal muscle fibers in muscle spindles’ equatorial region and collagen fibrils in GTOs contain mitochondria but only a few if any vesicles. Sensory nerve terminals on intrafusal muscle fibers lack a basal lamina in the synaptic cleft which is typical in muscle spindles of skeletal muscle including EOMs. 17, 18, 22, 25 In each palisade ending, nerve terminals contacting the tendon are observed. In few palisade endings we also find nerve terminals contacting the muscle fiber surface and without a basal lamina in the synaptic cleft. Neurotendinous contacts and neuromuscular contacts (when present) in palisade endings contain mitochondria and are always full of clear vesicles. Aggregations of clear vesicles are also found in motor terminals on intrafusal muscle fibers in the muscle spindles’ polar regions and in motor terminals on extrafusal muscle fibers.
By immunohistochemistry we have determined the molecular characteristics of muscle spindles, GTOs, and palisade endings. With different combinations of triple labeling we distinguish between non-cholinergic and cholinergic nerve fibers and terminals. We have used α-bungarotoxin and antibody against synaptophysin to identify sensory and motor terminals in muscle spindles and GTOs. It is not sensible to apply this staining for palisade endings because to identify palisade endings it is crucial to label the nerve fibers.
We demonstrate that sensory nerve fibers in muscle spindles’ equatorial region and GTOs are neurofilament-positive but ChAT-negative. Anulospiral endings in muscle spindles’ equator and nerve terminals in GTOs are synaptophysin positive but ChAT and α-bungarotoxin-negative. Muscle spindles receive motor nerve fibers as an additional set of nerve fibers in their polar regions. Such nerve fibers stain positive for ChAT and their nerve terminals positive for ChAT and α-bungarotoxin. In line with our previous findings in cat 8, 14 and monkey 4, 9 we show in sheep that palisade endings have a cholinergic phenotype. Specifically, palisade endings in sheep EOMs are supplied by ChAT/neurofilament positive nerve fibers. The palisade complexes are ChAT/neurofilament immunoreactive as well and all palisade nerve terminals co-localize ChAT and synaptophysin.
Summing up, the present study clearly shows that classical proprioceptors and palisade endings differ with respect to structural and molecular characteristics. These differences with respect to function are discussed below.
The presence of a perineural capsule and a large subcapsular gap is a typical feature of muscle spindles and GTOs both in mammalian EOMs and other skeletal muscles. 16, 24, 35-38 Typically, in skeletal muscle including EOMs the capsular space of muscle spindles and GTOs is filled with perineural liquid containing acidic mucopolysaccharides. 16, 24, 35 It is supposed that this viscous liquid has a damping function and protects the sensory nerve terminals in muscle spindles and GTOs from aberrant activation by mechanical interference coming from outside the organs. 22, 26, 35 Palisade endings in mammals, including man, have a connective tissue capsule without a subcapsular space. 4, 5, 8, 10, 11, 29 Furthermore, in palisade endings of humans Lukas et al. 10 did not find acidic mucopolysaccharides inside their capsule indicating that perineural liquid is lacking. It is therefore a critical question why sensory nerve terminals in classical proprioceptors of skeletal muscle including mammalian EOMs are environed by perineural liqiud and nerve terminals in palisade endings are not. At least, the absence of perineural liquid indicate that nerve terminals in palisade endings are less protected against mechanical stimuli.
Clear vesicles are organelles which are commonly observed in sensory nerve terminals of muscle spindles and GTOs and likewise in nerve terminals of palisade endings. 4, 5, 8, 11, 18, 25, 29, 39 However, with the exception of developing muscle spindles and GTOs, the number of such vesicles is always very low in sensory nerve terminals of classical proprioceptors. 17, 20, 22, 25, 37-40 Only in palisade nerve terminals, a high amount of clear vesicles is usually present. 4, 5, 8-11, 29 The presence of vesicles in palisade endings could indicate that maturation of palisade endings is delayed and that these organs mature at a later point of time. In sheep (this study) and cat 8 animals of different age (between 6 months and two years in sheep and between one and 16 years in cat) were analyzed. No differences in palisade endings were detected in young and older animals. It is therefore extremely unlikely that clear vesicles in palisade nerve terminals are a sign of ongoing maturation.
By immunohistochemistry we determined the molecular features of clear vesicles. We show that vesicles in sensory nerve terminals of muscle spindles and GTOs and palisade nerve terminals differ from each other. In particular, vesicles in sensory nerve terminals of classical proprioceptors are non-cholinergic. Vesicles in palisade nerve terminals of sheep are cholinergic which is in line with our prior findings in palisade endings of cat 8, 14 and monkey. 4, 9 Cholinergic vesicles are also found in motor terminals on intrafusal muscle fibers and extrafusal muscle fibers in sheep EOMs and cholinergic vesicles are a general feature of all kind of motor terminals including en plaque and en qrappe motor terminals. 4, 41 ChAT is the synthesizing enzyme for the acetylcholine which is the neurotransmitter usually found in motor terminals. The present study confirms in another animal species that palisade endings contain acetylcholine and further shows that perceptive synapses in classical proprioceptors do almost certainly not contain acetylcholine. In fact this difference between classical proprioceptors and palisade endings complements our recent molecular findings on palisade endings 4, 8, 9, 14 and support the assumption that palisade endings are effectors and most likely not sensory structures.
Clear evidence that palisade endings are effectors comes from α-bungarotoxin labeling and a nerve degeneration experiment. 4, 8, 10, 30 In cat, 8 monkey, 4and man 10 neuromuscular contacts when present in palisade endings are endowed with nicotinic acetylcholine receptors as demonstrated by staining with α-bungarotoxin. In monkey it was also detected in some cases that nerve fibers supplying palisade endings establish motor contacts outside the palisade complex which was confirmed by α-bungarotoxin binding. 4 Further, in a nerve degeneration experiment Sas and Schảb 30 found that the perikarya of nerve fibers forming palisade endings lie in the EOM motor nuclei. Specifically, lesions of the EOM motor nuclei caused degeneration of motor terminals and additional loss of palisade endings in the EOMs. 30 Nevertheless, there are also arguments which are in favor of a sensory role of palisade endings. With the exception of rabbit 6 and rat, 7 palisade endings in all other species (sheep, 5 cat, 8, 29monkey, 4, 11 and man 10 ) have nerve terminals contacting the tendon. Analogous to GTOs it is legitimate to ague that such neurotendinous contacts are sensory despite their cholinergic phenotype. The strongest argument that palisade endings are sensory comes from a single neuronal tracing experiment. By injecting neuronal tracer into the sensory trigeminal ganglion of cats Billig et al. 3 found labeled nerve endings in the EOMs, one of these resembling palisade endings.
Since there are arguments for a motor as well as a sensory role for palisade endings could it be that this EOM specific structures receive a double innervation from motor and sensory nerve fibers? The answer to this question is no. Specifically, in cat, 8 monkey 9 and now in sheep, labeling of nerve fibers with a general marker for neurons (anti-neurofilment) and with a marker for cholinergic nerve fibers (anti-ChAT) shows that all nerve fibers supplying palisade endings exhibit neurofilament/ChAT-immunoreactivity. Likewise, labeling nerve terminals with a general marker for nerve terminals (anti-synaptophysin) and anti-ChAT demonstrates that all palisade nerve terminals co-localize synaptophysin and ChAT. 8, 9
Functional Considerations
Muscle spindles and GTOs in EOMs of even-toed ungulates exhibit a similar morphology as their counterparts in mammalian limb muscles. It is broadly assumed that these two receptors function similar in EOMs and limb muscles. In fact, electrophysiological studies from Manni et al. 42, 43 in even-toed ungulates demonstrated that muscle spindles in EOMs are sensitive to muscle stretch. GTOs in EOMs of event-toed ungulates are supposed to register muscle fiber contraction. In analogy to GTOs in limb muscles it is assumed that contraction of EOM muscle fibers attached to GTOs tightens the collagen bundles. 26, 36, 39, 44 Nerve terminals in between the collagen are squeezed and distorted thereby generating an action potential which is conducted centrally. 26, 36, 39, 44 In EOMs GTOs with traversing muscle fibers are observed. It is supposed that such muscle fibers could regulate the sensitivity of the organ. 26 Specifically, by contraction of traversing muscle fibers GTOs collagen bundles would relax and more power from external attached muscle fibers would be required to tighten GTO collagen bundles and excite GTO nerve terminals. 26, 45
The function of palisade endings is speculative because physiological evidence is still lacking. Analogous to GTOs, palisade endings have nerve terminals that establish intimate contacts to the collagen fibrils. From this point of view it is legitimate to conclude that neurotendinous contacts in palisade endings are sensory. Due to their location in series with muscle fibers, palisade neurotendinous contacts would register muscle fiber contraction. If this assumption is correct GTOs and palisade endings would exert the same function. Our molecular analyses however, demonstrate that palisade nerve terminals contain ChAT which is a feature more common with excitatory synapses. Upon activation neurotendinous contacts in palisade endings release acetylcholine that would diffuse into the tendon. To date we do not know anything about an acetylcholine receptor site in the tendon and the effect of neurotransmitter release on collagen is difficult to predict. Miledi et al 46 found acetylcholine receptors in the membrane of frog muscle fibers at their attachment with the tendon. If this is also true for palisade endings, acetylcholine released from neurotendinous contacts would diffuse a long distance through the collagen before reaching the target receptors. In some palisade endings of sheep, 5 cat, 8, 14, 29 monkey, 4, 11and man 10 neuromuscular contacts are observed in addition to neurotendinous contacts. In man 10 and recently in monkey 4 it was shown that neuromuscular contacts have characteristics of motor terminals which was confirmed by α-bungarotoxin staining. On activation such contacts would elicit a local contraction of the palisade muscle fiber tip.
There is evidence that proprioceptive input from EOMs reaches several regions of the central nervous system. 47 Interestingly classical proprioceptors are absent in the EOMs of most mammalian species and are all together only found in EOMs of even-toed ungulates. 13-19, 26 The reason for this interspecies variation is unclear. Palisade endings are present in each species so far investigated. For those species lacking muscle spindles and GTOs it was supposed that the palisade endings represent an alternative receptor. 3, 7, 11, 29, 32, 34 In the present study we confirm in sheep that palisade endings are cholinergic and the comparison with muscle spindles and GTOs exhibit that sensory nerve terminals in these organs are non-cholinergic. Further, between well-known proprioceptors and palisade endings differences in the capsular arrangement are also detected. All this evidence suggests that palisade endings are different from classical proprioceptors and put into question whether palisade endings are sensory structures.
Acknowledgements
The authors kindly thank Arslan Hamyat, the head of the local slaughterhouse for the kind provision of animal material.
The study was supported by Grant P20881-B09 from the Fonds zur Foerderung der Wissenschaftlichen Forschung (FWF).
References
- 1.Donaldson IM. The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci. 2000;355(1404):1685–754. doi: 10.1098/rstb.2000.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruskell GL. Extraocular muscle proprioceptors and proprioception. Prog Retin Eye Res. 1999;18(3):269–91. doi: 10.1016/s1350-9462(98)00029-9. [DOI] [PubMed] [Google Scholar]
- 3.Billig I, Buisseret Delmas C, Buisseret P. Identification of nerve endings in cat extraocular muscles. Anat Rec. 1997;248(4):566–75. doi: 10.1002/(SICI)1097-0185(199708)248:4<566::AID-AR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Blumer R, Konakci KZ, Pomikal C, et al. Palisade endings: cholinergic sensory organs or effector organs? Invest Ophthalmol Vis Sci. 2009;50(3):1176–86. doi: 10.1167/iovs.08-2748. [DOI] [PubMed] [Google Scholar]
- 5.Blumer R, Lukas JR, Wasicky R, Mayr R. Presence and structure of innervated myotendinous cylinders in sheep extraocular muscle. Neurosci Lett. 1998;248(1):49–52. doi: 10.1016/s0304-3940(98)00331-0. [DOI] [PubMed] [Google Scholar]
- 6.Blumer R, Wasicky R, Hotzenecker W, Lukas JR. Presence and structure of innervated myotendinous cylinders in rabbit extraocular muscle. Exp Eye Res. 2001;73(6):787–96. doi: 10.1006/exer.2001.1085. [DOI] [PubMed] [Google Scholar]
- 7.Eberhorn AC, Horn AK, Eberhorn N, et al. Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity. J Anat. 2005;206(3):307–15. doi: 10.1111/j.1469-7580.2005.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konakci KZ, Streicher J, Hoetzenecker W, et al. Molecular characteristics suggest an effector function of palisade endings in extraocular muscles. Invest Ophthalmol Vis Sci. 2005;46(1):155–65. doi: 10.1167/iovs.04-1087. [DOI] [PubMed] [Google Scholar]
- 9.Konakci KZ, Streicher J, Hoetzenecker W, et al. Palisade endings in extraocular muscles of the monkey are immunoreactive for choline acetyltransferase and vesicular acetylcholine transporter. Invest Ophthalmol Vis Sci. 2005;46(12):4548–54. doi: 10.1167/iovs.05-0726. [DOI] [PubMed] [Google Scholar]
- 10.Lukas JR, Blumer R, Denk M, et al. Innervated myotendinous cylinders in human extraocular muscles. Invest Ophthalmol Vis Sci. 2000;41(9):2422–31. [PubMed] [Google Scholar]
- 11.Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J Neurocytol. 1978;7(6):693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- 12.Mahran ZY, Sakla FB. The pattern of innervation of the extrinsic ocular muscles and the intra-orbital ganglia of the albino mouse. Anat Rec. 1965;152(2):173–83. doi: 10.1002/ar.1091520208. [DOI] [PubMed] [Google Scholar]
- 13.Abuel Atta AA, DeSantis M, Wong A. Encapsulated sensory receptors within intraorbital skeletal muscles of a camel. Anat Rec. 1997;247(2):189–98. doi: 10.1002/(SICI)1097-0185(199702)247:2<189::AID-AR5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 14.Blumer R, Konacki KZ, Streicher J, et al. Proprioception in the extraocular muscles of mammals and man. Strabismus. 2006;14(2):101–6. doi: 10.1080/09273970600701192. [DOI] [PubMed] [Google Scholar]
- 15.Blumer R, Konakci KZ, Brugger PC, et al. Muscle spindles and Golgi tendon organs in bovine calf extraocular muscle studied by means of double fluorescent labelling, electron microscopy and three-dimensional reconstruction. Exp Eye Res. 2003;77:447–62. doi: 10.1016/s0014-4835(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 16.Blumer R, Wasicky R, Brugger PC, et al. Number, distribution, and morphologic particularities of encapsulated proprioceptors in pig extraocular muscles. Invest Ophthalmol Vis Sci. 2001;42(13):3085–94. [PubMed] [Google Scholar]
- 17.Harker DW. The structure and innervation of sheep superior rectus and levator palpebrae extraocular muscles. II. Muscle spindles. Invest Ophthalmol Vis Sci. 1972;11(12):970–9. [PubMed] [Google Scholar]
- 18.Kubota M. Ultrastructural observations on muscle spindles in extraocular muscles of pig. Anat Anz. 1988;165(2-3):205–28. [PubMed] [Google Scholar]
- 19.Maier A, DeSantis M, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol. 1974;143(4):397–408. doi: 10.1002/jmor.1051430404. [DOI] [PubMed] [Google Scholar]
- 20.Ruskell GL. Golgi tendon organs in the proximal tendon of sheep extraocular muscles. Anat Rec. 1990;227(1):25–31. doi: 10.1002/ar.1092270104. [DOI] [PubMed] [Google Scholar]
- 21.Greene T, Jampel R. Muscle spindles in the extraocular muscles of the macaque. J Comp Neurol. 1966;126(4):547–9. [PubMed] [Google Scholar]
- 22.Blumer R, Lukas JR, Aigner M, et al. Fine structural analysis of extraocular muscle spindles of a two-year-old human infant. Invest Ophthalmol Vis Sci. 1999;40(1):55–64. [PubMed] [Google Scholar]
- 23.Bruenech JR, Ruskell GL. Muscle spindles in extraocular muscles of human infants. Cell Tiss Org. 2001;169(4):388–94. doi: 10.1159/000047906. [DOI] [PubMed] [Google Scholar]
- 24.Lukas JR, Aigner M, Blumer R, et al. Number and distribution of neuromuscular spindles in human extraocular muscles. Invest Ophthalmol Vis Sci. 1994;35(13):4317–27. [PubMed] [Google Scholar]
- 25.Ruskell GL. The fine structure of human extraocular muscle spindles and their potential proprioceptive capacity. J Anat. 1989;167:199–214. [PMC free article] [PubMed] [Google Scholar]
- 26.Blumer R, Lukas JR, Wasicky R, Mayr R. Presence and morphological variability of Golgi tendon organs in the distal portion of sheep extraocular muscle. Anat Rec. 2000;258(4):359–68. doi: 10.1002/(SICI)1097-0185(20000401)258:4<359::AID-AR4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Ruskell GL. The incidence and variety of Golgi tendon organs in extraocular muscles of the rhesus monkey. J Neurocytol. 1979;8(5):639–53. doi: 10.1007/BF01208514. [DOI] [PubMed] [Google Scholar]
- 28.Richmond FJ, Johnston WS, Baker RS, Steinbach MJ. Palisade endings in human extraocular muscles. Invest Ophthalmol Vis Sci. 1984;25(4):471–6. [PubMed] [Google Scholar]
- 29.Alvarado Mallart RM, Pincon Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tiss Cell. 1979;11(3):567–84. doi: 10.1016/0040-8166(79)90063-6. [DOI] [PubMed] [Google Scholar]
- 30.Sas J, Scháb R. Die sogennanten “Palisaden-Endigungen” der Augenmuskeln. Acta Morph Acad Sci Hung. 1952;2:259–66. [Google Scholar]
- 31.Friedrich C, Lemm B, Soukup T, Asmussen G. Determination of slow-tonic MyHC immunoreactivity is an important step in the evaluation of muscle spindles in porcine extraocular muscles. Exp Eye Res. 2007;85(1):54–64. doi: 10.1016/j.exer.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Buttner Ennever JA, Eberhorn A, Horn AK. Motor and sensory innervation of extraocular eye muscles. Ann N Y Acad Sci. 2003;1004:40–9. doi: 10.1111/j.1749-6632.2003.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 33.Steinbach MJ, Smith DR. Spatial localization after strabismus surgery: evidence for inflow. Science. 1981;213(4514):1407–9. doi: 10.1126/science.7268444. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex.[see comment] Nat Neurosci. 2007;10(5):640–6. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
- 35.Brzezinski D,K. Untersuchungen zur Histochemie der Muskelspindeln. Acta histochem. 1961;12:277–88. [Google Scholar]
- 36.Schoultz TW, Swett JE. The fine structure of the Golgi tendon organ. J Neurocytol. 1972;1(1):1–26. doi: 10.1007/BF01098642. [DOI] [PubMed] [Google Scholar]
- 37.Zelena J. Transient sensory neuromuscular junctions in developing rats. Neuroscience. 1979;4(6):811–6. doi: 10.1016/0306-4522(79)90009-5. [DOI] [PubMed] [Google Scholar]
- 38.Zelena J, Soukup T. The development of Golgi tendon organs. J Neurocytol. 1977;6(2):171–94. doi: 10.1007/BF01261504. [DOI] [PubMed] [Google Scholar]
- 39.Schoultz TW, Swett JE. Ultrastructural organization of the sensory fibers innervating the Golgi tendon organ. Anat Rec. 1974;179(2):147–62. doi: 10.1002/ar.1091790202. [DOI] [PubMed] [Google Scholar]
- 40.Landon DN. The fine structure of the equatorial regions of developing muscle spindles in the rat. J Neurocytol. 1972;1(2):189–210. doi: 10.1007/BF01099184. [DOI] [PubMed] [Google Scholar]
- 41.Kimura H, McGeer PL, Peng F, McGeer EG. Choline acetyltransferase-containing neurons in rodent brain demonstrated by immunohistochemistry. Science. 1980;208(4447):1057–9. doi: 10.1126/science.6990490. [DOI] [PubMed] [Google Scholar]
- 42.Manni E, Bortolami R, Desole C. Eye muscle proprioception and the semilunar ganglion. Exp Neurol. 1966;16(2):226–36. doi: 10.1016/0014-4886(66)90101-4. [DOI] [PubMed] [Google Scholar]
- 43.Manni E, Bortolami R, Desole C. Peripheral pathway of eye muscle proprioception. Exp Neurol. 1968;22(1):1–12. doi: 10.1016/0014-4886(68)90015-0. [DOI] [PubMed] [Google Scholar]
- 44.Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev. 1992;72(3):623–66. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- 45.Zelena J, Soukup T. The in-series and in-parallel components in rat hindlimb tendon organs. Neuroscience. 1983;9(4):899–910. doi: 10.1016/0306-4522(83)90278-6. [DOI] [PubMed] [Google Scholar]
- 46.Miledi R, Reiser G, Uchitel OD. Characteristics of membrane channels induced by acetylcholine at frog muscle-tendon junctions. J Physiol. 1984;350:269–77. doi: 10.1113/jphysiol.1984.sp015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buisseret P. Influence of extraocular muscle proprioception on vision. Physiol Rev. 1995;75(2):323–38. doi: 10.1152/physrev.1995.75.2.323. [DOI] [PubMed] [Google Scholar]