Abstract
Objective
Individual differences in sensitivity to cyclical changes in ovarian steroids estradiol (E2) and progesterone (P4) have been implicated in the pathophysiology of menstrually related mood disorder (MRMD). However, no prospective studies have investigated psychosocial risk factors for sensitivity to hormone effects on mood in MRMD. Using a repeated measures approach and multilevel models, we tested the hypothesis that a history of abuse provides a context in which within-person elevations of E2 and P4 prospectively predict daily symptoms.
Method
66 women with prospectively-confirmed MRMD recruited for a trial of oral contraceptives provided 1 month of baseline hormone and mood data prior to randomization. Lifetime physical and sexual abuse experiences were assessed. Across one cycle, women completed daily measures of symptoms and provided blood samples on 5 days across the menstrual cycle. Current E2 and P4 were centered within person (CWP) such that higher values represented cyclical elevations in hormones.
Results
Rates of physical (27%) and sexual (29%) abuse were high, consistent with previous work documenting a link between trauma and MRMD. In women with a history of physical abuse, cyclical increases in P4 predicted greater mood and interpersonal symptoms on the three days following that sample. In women with a history of sexual abuse, cyclical increases in E2 predicted greater anxiety symptoms on the three days following that sample.
Conclusions
Results inform further inquiry into the role of severe life stressors and stress response systems in MRMD. We discuss areas for future research on the psychosocial and physiological pathways through which abuse may influence the link between hormones and symptoms.
Keywords: Ovarian steroid hormones, Estradiol, Progesterone, Abuse, Premenstrual dysphoric disorder
1. Introduction
Premenstrual dysphoric disorder (PMDD) affects about 1–6% of women in their reproductive years (Cohen et al., 2002) and can result in luteal phase functional impairment equivalent to that of major depression, panic disorder, and PTSD (Halbreich et al., 2003). However, the restrictive nature of the DSM-5 PMDD diagnostic criteria, particularly the requirement of an arbitrary 5 symptoms, is controversial (Freeman, 2003). The prevalence of clinically significant premenstrual symptoms that are characterized by cyclical distress, impairment, and treatment seeking, but do not meet the five symptom criterion, is estimated at 13–19% (Epperson et al., 2012). The burden of these menstrually related mood disorders (MRMDs) is high, with 4.5 million disability adjusted life years lost/year in the U.S. (Halbreich et al., 2003).
Both observational and experimental studies implicate changes in the ovarian steroids estradiol (E2) and progesterone (P4) in the pathophysiology of MRMDs. However, the effects of E2/P4 on MRMD symptoms do not appear to be due to abnormal levels of E2/P4 or abnormal cyclical patterns of E2/P4 in women with MRMDs; rather, the best available evidence indicates that MRMD symptoms emerge due to an abnormal sensitivity to cyclical changes in E2 and P4 (Schmidt et al., 1998; Halbreich et al., 1986; Redei and Freeman, 1995; Epperson et al., 2012; Wang et al., 2013). In further support of the idea that MRMDs are caused by individual differences in sensitivity to cyclical hormonal changes, experimental suppression of ovarian steroids using GnRH agonists effectively eliminates symptoms among most women with MRMD (Muse et al., 1984; Brown et al., 1994; Schmidt et al., 1998; Hammarbäck and Bäckström, 2009). Further, addback of luteal phase levels of either E2 or P4 (vs. placebo) causes a re-emergence of symptoms not found in non-MRMD women (Schmidt et al., 1998). In sum, while there is no consistent evidence that women with MRMD show altered levels of, or altered cyclical changes in, ovarian steroids, there is strong evidence that MRMD symptoms are generally linked to abnormal sensitivity to normal cyclical changes in ovarian steroids (Schmidt et al., 1998).
Within the population of women with MRMDs, there exists significant between-person variability in the strength of the within-person links between cyclical steroid changes and daily symptoms (Redei and Freeman, 1995). At present, little is known about the psychosocial correlates of hormonal sensitivity in MRMD. The present study addresses this gap by examining histories of abuse as a psychosocial predictor of the strength of the within-person link between cyclical changes in E2 and P4 and symptom expression in MRMD. There were several reasons for choosing abuse history as a candidate predictor of hormone sensitivity. Because ovarian steroids modulate the hypothalamic-pituitary-adrenal (HPA) axis response to stress (Roca et al., 2003), dysregulation of which has been consistently implicated in affective psychopathology (Heim et al., 2008), including MRMDs (Girdler et al., 2007), we hypothesized that a history of severe stress exposure may modulate affective sensitivity to normal cyclical elevations in ovarian steroids. In support of this hypothesis, a number of studies have linked traumatic experiences to greater odds of MRMD (Perkonigg et al., 2004; Pilver et al., 2011; Bertone-Johnson et al., 2014). Moreover, those with MRMD and histories of abuse show unique alterations in various stress-responsive physiological systems that are not seen in women without a MRMD who have similar abuse histories, including the hypothalamic-pituitary-thyroid axis (Girdler et al., 2004; Bunevicius et al., 2012) and the sympathetic nervous system (Girdler, 2003; Girdler et al., 2007). Finally, we recently found that cyclical increases in P4 were associated with greater susceptibility to mood symptoms and interpersonal problems only among women high in borderline personality features (Eisenlohr-Moul et al., 2015), traits which often develop as adaptations to abuse (Bandelow et al., 2005).
Based on the evidence that traumatic experiences sensitize stress response systems (Ehlert, 2013; McLaughlin et al., 2015), and that these systems (e.g., the HPA axis and sympathetic nervous system) are regulated by ovarian steroids (Patchev et al., 1994; Patchev and Almeida, 1996; Weiser and Handa, 2009; Liu et al., 2012), we hypothesize that sensitization in stress response systems represents a pathway through which women with MRMD develop abnormal mood reactions to normal cyclical changes in ovarian steroids. If this is the case, then histories of traumatic stress should play a role in predicting the links between hormone change and mood symptoms in MRMD (Schmidt et al., 1998).
In a sample of 66 women with prospectively-confirmed MRMD, we sought to test the following predictions:
-
1)
Consistent with evidence that relative elevations in E2 and P4 precipitate symptoms in women with MRMD (Schmidt et al., 1998), we predict that within-person elevations in E2 or P4 (i.e., higher-than-usual relative to one's mean) will be associated with greater symptom severity over three subsequent days among all women with MRMD.
-
2)
Consistent with evidence that stressful life events are correlated with MRMD, we predict that, within a sample of women with prospectively-confirmed MRMD, lifetime presence of either physical or sexual abuse will predict higher negative mood following normal cyclical elevations of ovarian steroids.
2. Method
2.1. Participants
66 participants enrolled in a randomized controlled trial of oral contraceptives (the results of which have not yet been reported; NCT00927095) for the treatment of MRMD were assessed daily (for symptoms) and across five time points (for ovarian steroids) in one baseline menstrual cycle prior to randomization. Descriptive information can be found in Table 1. All women were in good health, reporting no current chronic medical conditions (including any disorder of the reproductive system, such as polycystic ovarian syndrome) and no current Axis I psychiatric disorders. None of the participants self-reported any use of prescription medication for the past 3 months. Participants were paid $150 for their participation in the baseline portion of the study. All procedures were approved by the local IRB and all participants provided informed consent.
Table 1.
Sample descriptive information by abuse history.
| Variable | Full sample (n = 66) | MRMD + no abuse (n = 39) | MRMD + sexual abuse (n = 19) | MRMD + physical abuse (n =18) |
|---|---|---|---|---|
| Age | 30.34 (6.75) | 30.12 (7.32) | 32.00 (5.42) | 31.11 (4.45) |
| Body mass index (BMI) | 26.38 (6.06) | 26.34 (5.86) | 26.40 (6.24) | 25.47 (6.46) |
| Race | ||||
| White | 36 (54%) | 21 (53%) | 16 (84%) | 13 (72%) |
| Black | 16 (24%) | 8 (21%) | 1 (5%) | 1 (5%) |
| Latina | 5 (7%) | 4 (10%) | 1 (5%) | 1 (5%) |
| Asian | 9 (13%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Mixed or other | 1 (2%) | 6 (16%) | 0 (0%) | 3 (17%) |
| Education level | ||||
| High school | 1 (2%) | 1 (3%) | 0 (0%) | 0 (0%) |
| Trade school | 10 (15%) | 3 (7%) | 7 (36%) | 7 (38%) |
| Some college | 21 (32%) | 12 (31%) | 6 (32%) | 3 (17%) |
| College Grad. | 10 (15%) | 7 (18%) | 3 (16%) | 3 (17%) |
| Graduate/professional | 24 (36%) | 16 (40%) | 3 (16%) | 5 (28%) |
| Marital status | ||||
| Married | 36 (54%) | 28 (71%) | 5 (26%) | 5 (28%) |
| Unmarried | 30 (46%) | 11 (29%) | 14 (74%) | 13 (72%) |
| Cycle length in days | 28.36 (4.50) | 28.14 (4.92) | 28.99 (5.23) | 28.47 (5.38) |
| Age at first abuse (Years) | - | - | 8.48 (6.89) | 8.97 (7.29) |
| Histories of abuse | ||||
| Physical abuse | 18 (27%) | - | - | - |
| Sexual abuse | 19 (29%) | - | - | - |
| Both types of abuse | 10 (15%) | - | - | - |
| Psychiatric histories | ||||
| DSM-IV depressive disorder | 29 (43%) | 11 (29%) | 10 (52%) | 8 (44%) |
| DSM-IV anxiety disorder | 6 (9%) | 2 (5%) | 2 (10%) | 2 (11%) |
Note: Standard deviations and within-group percentages in parentheses.
2.2. Procedure
First, MRMD diagnosis was prospectively confirmed using daily ratings across 2–4 menstrual cycles. Second, participants meeting criteria for MRMD were assessed for Axis I psychiatric disorders using the MINI (Sheehan et al., 1998) and for abuse history using a validated interview (Leserman et al., 1997). Participants then began data collection for the present study, which began with daily completion of the daily record of severity of problems (DRSP) each evening for an entire menstrual cycle. During the same baseline cycle, a phlebotomist visited participants on five occasions to collect blood samples twice in the follicular phase (days 2 and 5 following the menstrual onset), and three times in the luteal phase (days 17, 21, and 25 following menstrual onset); time of day was constant within a given woman.
2.2.1. Confirmation of MRMD diagnosis
The diagnosis of MRMD was confirmed prospectively using a standardized system for scoring daily ratings on the daily record of severity of problems (DRSP; described below; (Endicott et al., 2006). DRSP forms were completed daily and mailed into the laboratory weekly to discourage retrospective reporting. Each woman completed the DRSP for 2–4 cycles. A diagnosis of MRMD was made based on the following criteria: (1) > = 30% decrease in emotional symptom severity from the seven luteal phase days preceding menses (mean of days –7 to –1, where day –1 is the day prior to menstrual onset) to the follicular phase baseline mean during days 4 to 10 following menstrual onset on day 1 (Premenstrual Mean—Postmenstrual Mean/the Scale Range of 5), (2) a rating of emotional symptoms as at least moderate (i.e., rating > = 4) on at least two premenstrual week days; (3) remission of symptoms shortly after menstrual onset followed by a symptom free period (> = 6 consecutive follicular days where rating < 4), and (4) criteria 1–3 met in at least two menstrual cycles.
2.2.2. Interview assessment of axis I psychopathology and abuse history
Next, psychiatric and trauma histories were assessed at a laboratory session. Participants were assessed for Axis I disorders using the MINI (Sheehan et al., 1998). A history of Axis I disorder (except psychosis or mania) was allowed if the participant had been in remission for 3 years, except in the case of depressive disorders, for which it was required that the participant had been in remission for 1 year. Current psychiatric diagnoses were exclusionary.
Abuse histories were measured using a standardized interview (Leserman et al., 1997). Physical abuse was coded as present if the participant reported ever experiencing either (1) life threat (i.e., physically attacked with the intent to kill or seriously injure), or (2) other physical abuse (i.e., beaten up, hit, burned). Sexual abuse was coded as present if the participant reported ever experiencing the following forced sexual experiences: (1) a perpetrator touching the participant's breasts, pubic area, vagina, or anus with hands, mouth, or objects; (2) making the participant touch the perpetrator's pubic area or anus with hands, mouth or objects; or (3) vaginal or anal intercourse.
2.3. Measures
2.3.1. Estradiol and progesterone
Blood samples were collected by a phlebotomist in the participants’ homes. Serum E2 (pg/mL) and P4 (ng/mL) were determined using radioimmunoassay (RIA; MP Diagnostics, Santa Ana, California, USA). All assays for a given woman were run in the same batch. For the P4 assay, the intra-assay coefficient of variance was 3.2–10.2% and the inter-assay coefficient of variance was 5.8–11.8%. The sensitivity of the P4 assay was .07 ng/ml; the greatest slope of the standard curve was between 1 and 5 ng/ml, with a standard range of .15–.80 ng/ml. For the E2 assay, the intra-assay coefficient of variance was 3.5–16.7% and the inter-assay coefficient of variance was 7.0–16.4%. The sensitivity of the E2 assay was <10 pg/ml; the greatest slope of the standard curve was between 50 and 200 pg/ml, with a standard range of 10–3,000 pg/ml. Luteal phase P4 levels lower than 3 ng/mL were considered evidence of an anovulatory cycle. Two subjects did not show this expected luteal phase increase in P4; therefore, they were considered to have had an anovulatory baseline cycle and were excluded. Neither of these two participants reported abuse.
2.3.2. Daily MRMD symptoms
Daily symptoms were measured using the DRSP (Endicott et al., 2006). Across 24 items representing emotional, physical, and behavioral symptoms, participants indicated “the degree to which the problems have been experienced today”: 1—Not at all, 2—Minimal, 3—Mild, 4—Moderate, 5—Severe, or 6—Extreme. Following Endicott et al. (2006), we utilized three composite variables: (1) a depressive symptoms composite (felt depressed, felt hopeless, felt worthless or guilty, slept more, trouble sleeping, and felt overwhelmed); (2) an interpersonal conflict composite (anger/irritability, conflicts with other people, and interference in interpersonal relationships), and (3) a somatic symptoms composite (breast tenderness, bloating, headache, and joint or muscle pain). In addition, three more single-item measures represented other core emotional symptoms of PMDD as described in the DSM-5: (1) felt anxious, keyed up, or on edge; (2) had mood swings; and (3) was more rejection sensitive or my feelings were easily hurt. Therefore, six daily outcomes were utilized. Reliability of change (Cranford et al., 2006) for a total symptom score was unacceptably low (Rc = .34), indicating that items in a total summed scale did not covary reliably across repeated measures.
E2 and P4 exert both (1) rapid effects (on the order of hours) due to ovarian steroids and their metabolites acting as neuromodulators in the central nervous system (McEwen, 2002) and (2) lagged effects on the order of days due to E2 and P4 acting as traditional steroids (Bless et al., 1997; Schiller et al., 2013). In order to capture these influences of E2 and P4, we chose to use the average scores for each of these six outcomes across the three days following (days ±1, ±2, and ±3) each blood sample (day 0).
2.4. Analytic plan
Data screening and coding
Variables were screened for normality using procedures defined by Tabachnick and Fidell (2001). DRSP ratings were positively skewed and were log transformed. Analyses were carried out in multilevel models using SAS PROC MIXED, with women at level 2 and repeated measures for each woman at level 1. Abuse variables were coded such that 0 = no history of abuse and 1 = history of abuse. Due to small units of measurement, log-transformed outcomes were multiplied by 100 for greater interpretability of coefficients in tables; graphed estimates have been transformed back to the original scale.
Centering method for hormone predictors: centering within person (CWP)
When used as predictors, E2 and P4 were centered within person (CWP; i.e., “centered within cluster” as described in Enders and Tofighi, 2007) in order isolate the within-person variance in steroids (i.e., [P4 Level at This Sample]—[Person's Average P4 Across All Samples]). Therefore, higher values of E2CWP and P4CWP reflect currently increased levels relative to one's own person means1 (Singer and Willett, 2003; see Eisenlohr-Moul et al., 2015 for visual depiction of method).
Multilevel models
Null multilevel models (i.e., no predictors) were used to calculate the intraclass correlation coefficient (ICC) for each outcome, which represents the percentage of variance in the outcome that can be attributed to stable between-person differences. In addition, these models were used to estimate null model intercepts as a proxy for sample means; given dependencies in the data, the null model intercept is a more valid estimate of sample mean (see Singer and Willett, 2003). Multilevel regression models tested study hypotheses. To account for serial correlation of daily mood reports due to the menstrual cycle, visit number (1–5) was specified using a REPEATED statement with an unstructured covariance matrix. Denominator degrees of freedom were determined using the Kenward–Roger method (Kenward and Roger, 1997).
Hypothesis 1, which predicted that higher-than-usual P4 would be associated with greater symptom expression in all women, was tested in two models predicting each outcome from cycle day (as a covariate to account for any cyclical variance in symptoms not attributable to E2 and P4), E2CWP, and P4CWP. Hypothesis 2, which predicted stronger links between E2 or P4 and symptoms among women with histories of abuse, was tested in two sets of models predicting each outcome from the following: cycle day (as a covariate), presence of abuse (either sexual or physical in two different models), current E2CWP and P4CWP, and the interaction of the abuse variable with current E2CWP and P4CWP. To account for the inflated Type 1 error associated with tests on 6 different outcomes, a Bonferroni correction was utilized to adjust the p value (.05/6 = .0083); therefore, p was set at .0083 for all analyses.
3. Results
3.1. Participant flow and selection of final sample
From July 2007 through September 2011, 321 women requesting evaluation for MRMD were prospectively evaluated as described above. Of these, 96 (30%) met MRMD criteria, 109 (34%) did not meet MRMD criteria, 111 (34% withdrew or were lost to follow-up, and 6 (2%) were excluded due to a current Axis I disorder (four with MDD, and 2 with anxiety disorders). Of the 96 with MRMD, four declined to participate in the research study, five did not meet eligibility criteria (one with polycystic ovarian syndrome, three with recent depression, and one with recent anorexia nervosa), and nine were lost to follow-up, yielding 74 women with MRMD who enrolled in the RCT. Of these 78 women, 70 women completed the baseline month of the RCT that provides the data for the present study. Two subjects were eliminated due to anovulatory baseline cycles (see Section 2.3), and two subjects did not complete the trauma interview; therefore, data from 66 women were used in the present analyses.
3.2. Descriptive information
Although current Axis I diagnoses were exclusionary, prevalence of various psychiatric histories among participants were as follows: 34 (51%) women previously met criteria for any disorder. Of these, 29 (43.65%) previously met criteria for major depressive disorder, 1 (1.47%) previously met criteria for dysthymic disorder, 6 (8.82%) previously met criteria for panic disorder, 1 (1.47%) previously met criteria for social phobia, 4 previously met criteria for agoraphobia, 2 (2.94%) previously met criteria for obsessive compulsive disorder, 8 (11.76%) previously met criteria for specific phobia, 5 (7.35%) previously met criteria for post-traumatic stress disorder, 2 (2.94%) previously met criteria for anorexia nervosa, 5 (7.35%) previously met criteria for bulimia nervosa, and 7 (10.29%) previously met criteria for alcohol dependence. Inclusion of psychiatric histories as covariates did not substantively alter the results of the present investigation. Lifetime prevalence of sexual and physical abuse were relatively high in the sample (see Table 1).
Inspection of within-person (repeated measures) variables revealed that 66 women contributed 312 observations. Null model intercepts (NMI; Singer and Willett, 2003) and ICCs for each repeated measures variable are as follows: Estradiol (NMI = 171.73 pg/ml, ICC = .37), Progesterone (NMI = 7.91 ng/ml, ICC = .22), DRSP Depression Composite (NMI = 1.70, ICC = .27), DRSP Anxiety Item (NMI = 2.13, ICC = .15), DRSP Interpersonal Composite (NMI = 1.90, ICC = .18), DRSP Rejection Sensitivity Item (NMI = 2.00, ICC = .13), DRSP Mood Lability Item (NMI = 2.01, ICC = .10), and DRSP Somatic Composite (NMI = 1.56, ICC = .32). Therefore, the preponderance of variability in both DRSP symptoms and ovarian steroids was at the visit level (i.e., within-person) rather than the person level (i.e., between-person).
3.3. Effects of menstrual cycle day on ovarian steroids and MRMD symptoms
In the sample as a whole, cycle day predicted increases and subsequent decreases in E2 across the cycle (γCYCLEDAY = 6.80, SE = 1.61, t(58.20) = 4.22, p < .0001; (γCYCLEDAY × CYCLEDAY = –.20, SE = .052, t(66.7) = 3.84, p < .0001), as would be predicted in a normal menstrual cycle. Similarly, there was an increase in P4 across the cycle in the sample as a whole (γCYCLEDAY = .53, SE = .045, t(72.60) = 11.75, p < .0001). These effects of cycle day were not significantly moderated by either sexual or physical abuse, indicating that abuse was not associated with significant alterations in the pattern of ovarian steroid change across the cycle (all interaction p's > .19). Furthermore, t-tests did not reveal any significant differences between those with and without abuse in mean levels of E2 or P4 or mean levels of E2 or P4 by menstrual cycle phase (all p's > .18). Crucially, these analyses do not suggest significant alterations in the normative levels or cyclical fluctuations in ovarian steroids among women with abuse.
In order to verify the presence of cyclicity in MRMD symptoms, multilevel models predicted each DRSP outcome from cycle day. Results revealed significant cyclicity in all symptoms as indicated by significant effects of cycle day on all outcomes; symptoms increased from the follicular to the luteal phase of the cycle (average effect of cycle day: γCYCLEDAY = .36, SE = .052, t(83.82) = 6.29, p < .0001). Therefore, as expected based on the eligibility criteria, symptoms fluctuated across the cycle in a manner consistent with the diagnosis of MRMD in the sample as a whole.
3.4. Testing hypothesis 1: do higher-than-usual E2 and P4 predict symptoms on subsequent days?
Consistent with evidence that within-person elevations of E2 and P4 are correlated with symptom emergence in women with MRMD, we hypothesized that cyclical increases in E2 and P4 would each be associated with greater symptoms on subsequent days (controlling for cycle day). In models predicting each symptom from cycle day, E2CWP, and P4CWP, relative elevations in E2 predicted lower symptoms on the DRSP Interpersonal Composite (γE2CWP = −.15, SE = .041, t(138) = −2.72, p = .0073) and the Anxiety Item (γE2CWP = −.27, SE = .057, t(154) = −3.50, p = .0006). Surprisingly, there were no significant effects of P4CWP (all p's > .36) on symptoms. Cycle day remained a strong predictor of symptoms. In sum, although there was a strong link between increasing cycle day (i.e., the luteal phase) and greater symptom expression, only relative elevations in E2CWP predicted (lower) symptom severity on subsequent days in the sample as a whole, whereas relative elevations in P4CWP were not associated with symptom severity on subsequent days in the sample as a whole.
3.5. Evidence of individual differences in the slopes of E2 and P4 on symptoms
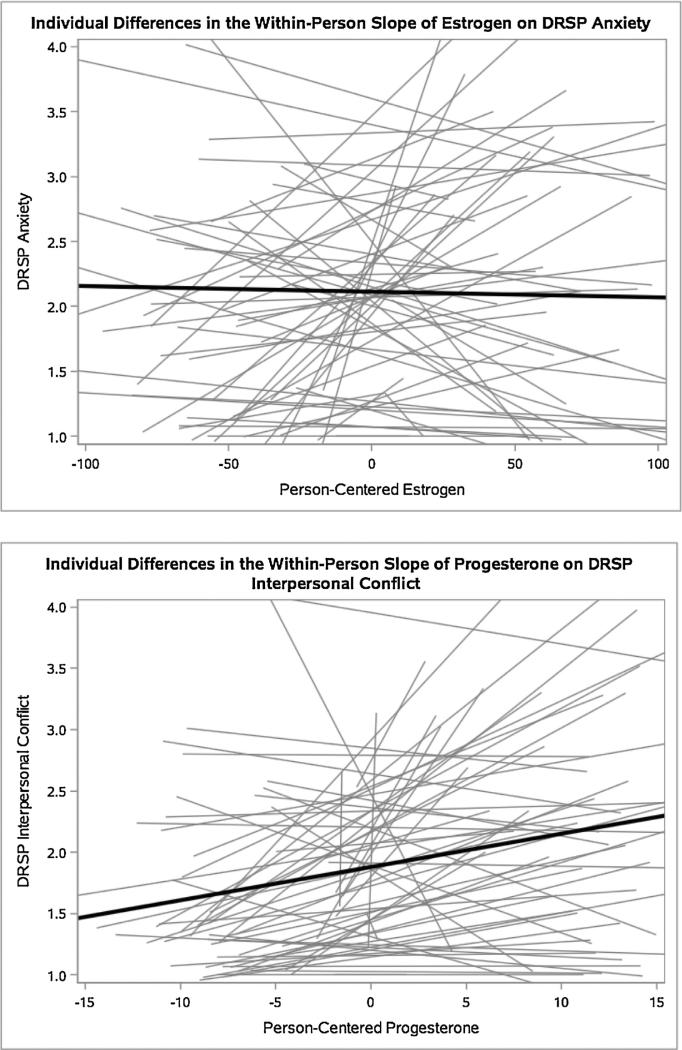
Despite a lack of evidence in the sample as a whole that relative elevations in E2 and P4 predict increase in daily symptoms, inspection of graphs plotting E2CWP and P4CWP against each of the six daily outcomes indicated strong between-person variability in the slopes of both E2CWP and P4CWP on symptom severity on the following three days (see Fig. 1 for illustrative examples). This substantial variability in the extent to which women responded to relative increases in ovarian steroids (E2CWP or P4CWP) with increased symptoms suggest the presence of between-person factors (e.g., experiences of abuse) that result in abnormal mood reactions to cyclical ovarian steroid change.
Fig. 1.
Illustrative graphs of individual differences in the slopes of E2 and P4 on symptoms.
Note. Each gray line represents one subject, and the black lines represent mean within-person effects of hormones on symptoms. These graphs are illustrative of large individual differences in hormone-symptom links across all symptoms.
3.6. Testing hypothesis 2: are abuse histories associated with greater symptoms following higher-than-usual E2 or P4?
3.6.1. Interactive effects of abuse and cycle day on symptoms
Preliminary analyses investigating the moderating role of abuse history on symptom cyclicity revealed that physical abuse, but not sexual abuse, interacted with cycle day to predict each of the six outcomes (Average Interactive Effect of Physical Abuse and Cycle Day Across All Six Outcomes: γPHYSABUSE × CYCLEDAY = 6.59, p < .0001). Women with a history of physical abuse showed large increases in all six symptoms across the cycle, whereas women without a history of physical abuse showed significant but weaker cyclicity of all six symptoms.
3.6.2. Interactive effects of abuse and ovarian steroid hormones on symptoms
It was hypothesized that experiences of physical or sexual abuse would predict stronger associations of ovarian hormones (E2CWP and P4CWP) with symptom expression on subsequent days controlling for cycle day (i.e., controlling for extraneous cycle-related factors). Results of models testing the interactions of physical and sexual abuse with E2CWP and P4CWP are presented in Table 1 (physical and sexual abuse in separate models), Table 2 (physical and sexual abuse in the same model), and Table 3 (both types of abuse and their interaction in the same model). Results were remarkably consistent across models; therefore, the full model presented in Table 3, which controls for the interactive effects of physical and sexual abuse histories, will be described below. In these models, cycle day remained a significant predictor of symptom expression for all outcomes.
Table 2.
Fixed effects of lifetime abuse, person-centered estradiol and progesterone, and their interactions on symptoms.
| Parameter | Outcome (average symptoms across
the three days following the E2/P4 sample) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRSP depression composite | DRSP interpersonal conflict composite | DRSP somatic composite | DRSP mood lability item | DRSP rejection sensitivity item | DRSP anxiety item | |||||||
| Model: sex abuse × hormones | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) |
| Intercept | 13.10 | (5.90) | 8.51 | (6.37) | 13.48 | (5.31) | 11.67 | (7.65) | 10.28 | (7.14) | 10.27 | (7.10) |
| Cycle day | 1.36** | (0.36) | 2.35** | (0.38) | 1.03** | (0.33) | 2.55** | (0.48) | 2.50** | (0.43) | 2.94** | (0.44) |
| Lifetime sexual abuse (SexAb) | 5.23 | (8.72) | 2.27 | (8.38) | −3.54 | (6.52) | 17.15 | (8.93) | 5.91 | (9.93) | 8.11 | (9.70) |
| Current E2CWP | −0.01 | (0.05) | −0.13** | (0.06) | 0.02 | (0.04) | −0.13 | (0.07) | −0.06 | (0.06) | −0.24 | (0.17) |
| Current P4CWP | 0.07 | (0.37) | 0.51 | (0.43) | −0.12 | (0.31) | 0.12 | (0.51) | −0.05 | (0.48) | 0.01 | (0.48) |
| SexAb × Current E2CWP | −0.02 | (0.08) | 0.01 | (0.09) | −0.10 | (0.07) | −0.01 | (0.11) | 0.01 | (0.10) | 0.08 | (0.11) |
| SexAb × Current P4CWP | 0.24 | (0.63) | 0.10 | (0.71) | 0.39 | (0.53) | 0.67 | (0.83) | 0.47 | (0.79) | −0.46 | (0.77) |
| Model: phys abuse × hormones | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) |
| Intercept | 11.57 | (5.86) | 6.56 | (6.22) | 11.17 | (5.29) | 10.27 | (7.44) | 7.10 | (7.20) | 7.11 | (7.01) |
| Cycle day | 1.33** | (0.35) | 2.18** | (0.36) | 1.08** | (0.33) | 2.50** | (0.46) | 2.48** | (0.42) | 2.91** | (0.43) |
| Lifetime physical abuse (PhysAb) | 23.01** | (8.40) | 25.46** | (8.01) | 4.75 | (6.56) | 31.08** | (8.60) | 26.43** | (9.53) | 25.85** | (9.37) |
| Current E2CWP | 0.02 | (0.05) | −0.08 | (0.06) | −0.01 | (0.05) | −0.07 | (0.07) | −0.03 | (0.06) | −0.26** | (0.04) |
| Current P4CWP | −0.37 | (0.39) | 0.02 | (0.44) | −0.05 | (0.33) | −0.59 | (0.51) | −0.56 | (0.48) | −0.16 | (0.48) |
| PhysAb × Current E2CWP | −0.01 | (0.08) | −0.06 | (0.09) | 0.00 | (0.07) | −0.09 | (0.11) | −0.04 | (0.10) | 0.16 | (0.11) |
| PhysAb × Current P4CWP | 1.53** | (0.61) | 2.59** | (0.70) | 0.10 | (0.54) | 2.94** | (0.80) | 2.78** | (0.78) | 0.43 | (0.77) |
Note: Estimates are unstandardized. Results are from lognormal multilevel regression models. Standard errors are in parentheses. CWP = values are centered within participant (i.e., person-centered values). E2 = Estradiol, P4 = Progesterone, SexAb = Lifetime Sexual Abuse, PhysAb = Lifetime Physical Abuse, and DRSP= Daily Record of Severity of Problems. Abuse variables are coded as 1 = Present and 0 = Not Present. Significant fixed effects are shown in bold.
p < .0083, the critical p value based on a bonferroni correction.
Table 3.
Fixed effects of lifetime physical and sexual abuse, person-centered estradiol and progesterone, and their interactions on symptoms.
| Parameter | Outcome (average symptoms across
the three days following the E2/P4 sample) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRSP depression
composite |
DRSP interpersonal conflict
composite |
DRSP somatic composite |
DRSP mood lability item |
DRSP rejection sensitivity
item |
DRSP anxiety item |
|||||||
| Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | |
| Intercept | 12.36 | (6.04) | 7.45 | (6.32) | 12.81 | (5.42) | 9.06 | (7.64) | 7.76 | (7.44) | 7.17 | (7.23) |
| Cycle day | 1.35** | (0.35) | 2.20** | (0.36) | 1.02** | (0.33) | 2.52** | (0.46) | 2.49** | (0.43) | 2.91** | (0.43) |
| Lifetime sexual abuse (SexAb) | −5.28 | (9.31) | −9.08 | (8.91) | −6.21 | (7.19) | 5.69 | (9.60) | −5.20 | (10.62) | −1.49 | (10.37) |
| Lifetime physical abuse (PhysAb) | 25.10** | (9.39) | 29.60** | (9.01) | 7.03 | (7.35) | 28.57** | (9.63) | 28.75** | (10.73) | 26.89** | (10.47) |
| Current E2CWP | 0.02 | (0.06) | −0.09 | (0.06) | 0.02 | (0.05) | −0.09 | (0.07) | −0.04 | (0.07) | −0.28** | (0.08) |
| Current P4CWP | −0.30 | (0.40) | 0.19 | (0.45) | −0.10 | (0.34) | −0.39 | (0.53) | −0.47 | (0.51) | 0.01 | (0.50) |
| SexAb × Current E2CWP | −0.02 | (0.09) | 0.05 | (0.09) | −0.10 | (0.08) | 0.05 | (0.11) | 0.04 | (0.11) | 0.15** | (0.02) |
| SexAb × Current P4CWP | −0.62 | (0.73) | −1.47 | (0.87) | 0.48 | (0.64) | −1.30 | (0.99) | −0.70 | (0.95) | −1.25 | (1.00) |
| PhysAb × Current E2CWP | 0.00 | (0.09) | −0.08 | (0.09) | 0.01 | (0.08) | −0.11 | (0.12) | −0.06 | (0.11) | 0.12 | (0.12) |
| PhysAb × Current P4CWP | 1.89** | (0.71) | 2.80** | (0.87) | −0.13 | (0.64) | 3.71** | (0.98) | 2.34** | (0.95) | 1.22 | (1.01) |
Note: Estimates are unstandardized. Results are from lognormal multilevel regression models. Standard errors are in parentheses. CWP = values are centered within participant (i.e., person-centered values). E2 = Estradiol, P4 = Progesterone, SexAb = Lifetime Sexual Abuse, PhysAb = Lifetime Physical Abuse, and DRSP= Daily Record of Severity of Problems. Abuse variables are coded as 1 = Present and 0 = Not Present. Significant fixed effects are shown in bold.
p < .0083, the critical p value based on a bonferroni correction.
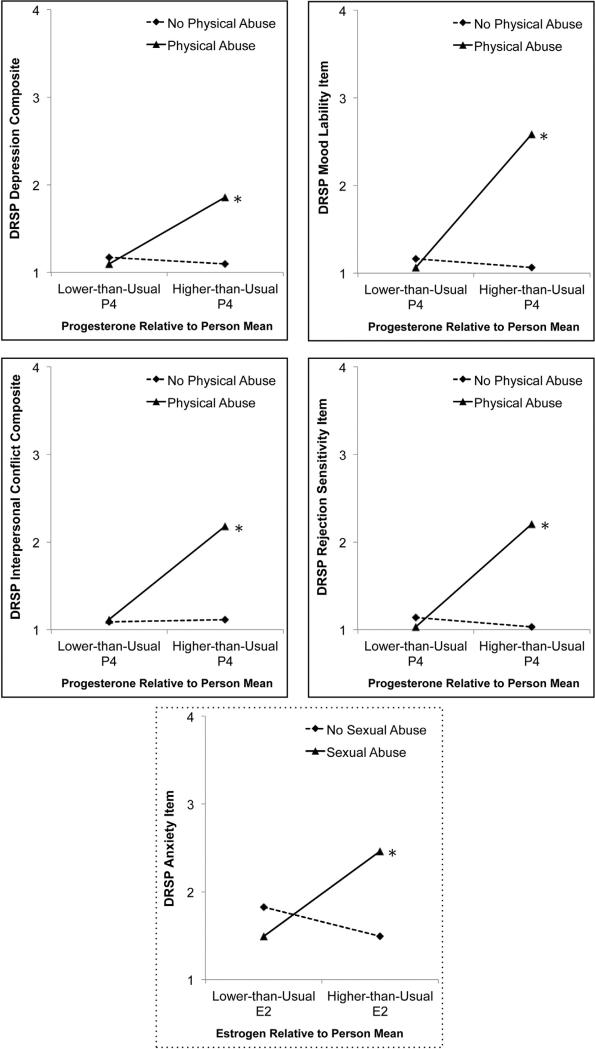
As predicted, history of physical abuse unmasked a positive association between cyclical increases in P4 and mood symptoms as indicated by: the DRSP Depression Composite, Interpersonal Composite, Rejection Sensitivity Item, and Mood Lability item (see top two rows of Fig. 2 for visual depictions). Follow-up analyses revealed that cyclical elevations in P4 were indeed a significant predictor of symptoms among women with a history of physical abuse, but were not a significant predictor of symptoms among women without a history of physical abuse. Additionally, a history of sexual abuse unmasked a positive association between cyclical increases in E2 and anxiety symptoms as indicated by scores on the DRSP Anxiety item (see bottom row of Fig. 2 for visual depiction). Cyclical increases in E2 were a significant predictor of symptoms among women with a history of sexual abuse, and were not a significant predictor of symptoms among women without a history of sexual abuse. There were no significant three-way interactions between physical abuse, sexual abuse, and ovarian steroid elevations (Table 4).
Fig. 2.
Experiences of abuse strengthen within-person links between current person-centered P4 (top rows) and E2 (bottom row) and symptom expression in MRMD.
Table 4.
Simultaneous regression model estimating fixed effects of lifetime physical and sexual abuse, person-centered estradiol and progesterone, and their two- and three-way interactions.
| Parameter | Outcome (average symptoms across
the three days following the E2/P4 sample) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRSP depression
composite |
DRSP interpersonal conflict
composite |
DRSP somatic composite |
DRSP mood lability item |
DRSP rejection sensitivity
item |
DRSP anxiety item |
|||||||
| Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | Est. | (SE) | |
| Intercept | 12.64 | (6.26) | 9.32 | (6.52) | 15.46 | (5.41) | 10.47 | (7.63) | 7.94 | (7.67) | 8.19 | (7.44) |
| Cycle day | 1.35** | (0.35) | 2.11** | (0.36) | 1.01** | (0.33) | 2.53** | (0.45) | 2.49** | (0.43) | 2.89** | (0.43) |
| Lifetime sexual abuse (SexAb) | −8.45 | (12.12) | −13.12 | (11.49) | −17.49 | (8.87) | −7.40 | (12.43) | −7.80 | (13.83) | −9.00 | (13.29) |
| Lifetime physical abuse (PhysAb) | 22.01 | (12.44) | 22.77 | (11.91) | −4.79 | (9.34) | 20.09 | (12.39) | 26.70 | (14.26) | 19.45 | (13.71) |
| Current E2CWP | 0.02 | (0.06) | −0.07 | (0.06) | 0.01 | (0.05) | −0.11 | (0.08) | −0.04 | (0.07) | −0.27** | (0.08) |
| Current P4CWP | −0.35 | (0.42) | 0.14 | (0.47) | −0.02 | (0.36) | −0.45 | (0.53) | −0.49 | (0.53) | −0.03 | (0.51) |
| SexAb × PhysAb | 7.41 | (19.32) | 14.41 | (18.39) | 29.33 | (14.32) | 26.93 | (19.55) | 5.70 | (22.03) | 18.72 | (21.38) |
| SexAb × Current E2CWP | −0.02 | (0.12) | −0.02 | (0.12) | −0.02 | (0.10) | 0.17 | (0.15) | 0.06 | (0.14) | 0.18** | (0.16) |
| PhysAb × Current E2CWP | 0.01 | (0.12) | −0.18 | (0.12) | 0.06 | (0.10) | 0.04 | (0.15) | −0.03 | (0.15) | 0.11 | (0.15) |
| SexAb × PhysAb × Current E2CWP | −0.03 | (0.18) | 0.21 | (0.18) | −0.13 | (0.16) | −0.36 | (0.23) | −0.06 | (0.22) | 0.01 | (0.24) |
| SexAb × Current P4CWP | −0.13 | (1.07) | −1.32 | (1.17) | −0.19 | (0.89) | −0.14 | (1.38) | −0.34 | (1.32) | −0.82 | (1.36) |
| PhysAb × Current P4CWP | 2.29** | (0.90) | 2.64** | (1.18) | −0.80 | (0.86) | 4.82** | (1.27) | 2.76** | (1.29) | 1.74 | (1.43) |
| SexAb × PhysAb × Current P4CWP | −1.03 | (1.48) | 0.19 | (1.74) | 1.52 | (1.31) | −2.88 | (1.96) | −0.90 | (1.93) | −0.97 | (2.05) |
Note: Estimates are unstandardized. Results are from lognormal multilevel regression models. Standard errors are in parentheses. CWP = values are centered within participant (i.e., person-centered values). E2 = Estradiol, P4 = Progesterone, SexAb = Lifetime Sexual Abuse, PhysAb = Lifetime Physical Abuse, and DRSP = Daily Record of Severity of Problems. Abuse variables are coded as 1 = Present and 0 = Not Present. Significant fixed effects are shown in bold.
p < .0083, the critical p value based on a bonferroni correction.
4. Discussion
Experimental (Schmidt et al., 1998) and prospective observational studies (Wang et al., 1996; Halbreich et al., 1986; Redei and Freeman, 1995) suggest that MRMD is characterized by abnormal mood reactions to normal cyclical elevations in E2 and P4. However, there is also evidence for individual differences in sensitivity to normal ovarian steroid changes within the population of women with MRMD (Schmidt et al., 1998; Redei and Freeman, 1995), which were mirrored by the presence of significant variability in the effects of E2 and P4 on symptoms in the present study. The associations presented here provide the first evidence that a between-person psychosocial factor, history of abuse, predicts the strength of associations between cyclical elevations in E2/P4 and the emergence of symptoms in MRMD.
This work demonstrates the importance of considering multilevel interactions between person-level factors (e.g., trauma histories) and time-varying cyclical factors (cycle day, cyclical E2 or P4) in predicting psychiatric symptoms. Psychosocial vulnerabilities may lead to physiological or psychological disturbances that leave abused women vulnerable to cyclical hormone changes. Consistent with this notion of individual differences within MRMD, current E2CWP and P4CWP were generally poor predictors of symptom expression in the sample as a whole, and there was significant between-person variability in the slopes of hormonal predictors on symptoms (Fig. 1). Histories of abuse partially accounted for this variance in the slopes of P4 and E2 on symptoms. A history of physical abuse predicted a stronger association between relative elevations in P4 and symptoms of interpersonal conflict, depression, rejection sensitivity, and mood lability on the following three days. Similarly, a history of sexual abuse predicted a stronger association between relative elevations in E2 and symptoms of anxiety. These results are consistent with the hypothesis that histories of abuse predict greater emotional reactivity to cyclical changes in ovarian steroids among women with MRMD.
These results are partially consistent with previous work in smaller samples (12 < n's < 18 compared with a final n = 66 in the present study) demonstrating sample-wide prospective links between P4 (Wang et al., 1996; Halbreich et al., 1986; Redei and Freeman, 1995) and daily MRMD symptoms. The present findings are also clearly in line with experimental evidence (Schmidt et al., 1998) that not all women with MRMD show a clear link between changes in E2/P4 and symptom expression. Notably, however, there were strong effects of the cycle on symptom expression in the sample as a whole, suggesting that women who do not show strong hormone-symptom links still show expected patterns of premenstrual symptom expression. This highlights the possibility of multiple etiologies of MRMD with distinct mechanisms and contributes to the growing evidence that histories of abuse may identify a clinically distinct subgroup in MRMD (Halbreich et al., 2003; Girdler et al., 2007).
Contrary to previous prospective work, which demonstrated no significant association of E2 with symptom severity (Halbreich et al., 1986; Wang et al., 1996; Redei and Freeman, 1995), we report small yet significant protective main effects of current E2CWP on anxiety symptoms such that higher-than-usual E2 predicted lower symptoms. Notably, these protective effects are qualified by a deleterious effect of cyclical increases in E2 among women with a history of sexual abuse (see Fig. 2), and this deleterious effect is more consistent with experimental evidence regarding the role of E2 in provoking MRMD symptoms (Schmidt et al., 1998). Although these findings appear inconsistent with previous longitudinal studies indicating that changes in E2 are not correlated with symptom expression, previous prospective studies may have been underpowered to detect what was, on average, a small effect of E2CWP.
4.1. Stress sensitivity as a mechanism underlying the correlation between abuse and hormone sensitivity in MRMD
Mechanistic interpretations of the present findings are inappropriate given the correlational nature of the study. However, abuse-related physiological and psychological disturbances could account for the ability of abuse to predict negative emotional reactions to cyclical increases in steroids. Abuse-related alterations in stress response systems represent an especially promising pathway. Symptomatic reactions to cyclical changes in ovarian steroids among abused women may be due to heightened sensitivity of stress response systems to cyclical fluctuations in steroids and/or their GABAergic neurosteroid metabolites (Crowley and Girdler, 2014; Schiller et al., 2014). Consistent with the more compelling pattern of results for P4 in the present paper, experimental work implicates P4—and not E2—in heightened HPA responses to stress. Ovarian estrogens stimulate the HPA-axis through an interaction of estrogen receptors (ERs) via specific estrogen-responsive elements (EREs) in the promoter region of the human CRH gene in animals (Vamvakopoulos, 1993); however, E2 may not play such a direct role in modulating the HPA-axis in human females (Roca et al., 2013). P4 and its GABAergic neurosteroid metabolites (e.g., allopregnanolone) are stress responsive in animals and humans (reviewed in Crowley and Girdler, 2014) and serve as potent, positive allosteric modulators of GABAA receptors via dose-dependent enhancement of the activity of GABA-stimulated Cl-ion channels (Morrow et al., 1987). GABA is the chief inhibitory neurotransmitter in the mammalian central nervous system. The role of GABA in regulating the HPA axis in response to stress by limiting the extent and duration of the stress response is well established (Cullinan et al., 2008). Although P4 is a critical determinant of allopregnanolone, E2 is also likely to positively influence its production through its modulation of the enzymes involved in the conversion of progesterone to allopregnanolone, 5α-reductase and 3α-hydroxysteroid dehydrogenase (Cheng and Karavolas, 1973). In animals, ovarian estrogens also stimulate the HPA axis through an interaction of estrogen receptors (ERs) via specific estrogen-responsive elements (EREs) in the promoter region of the human CRH gene (Vamvakopoulos, 1993). Further, acute stress in animals increases production of E2 in the PVN (Liu et al., 2012) and the presence of E2 in the PVN is associated with an enhanced responsiveness of the HPA axis to stress, mediated in part via E2-induced impairment in glucocorticoid negative feedback (Liu et al., 2012; Weiser and Handa, 2009).
Histories of abuse are associated with sensitization of stress response systems (Heim et al., 2008; Ehlert, 2013), particularly in MRMD (Girdler et al., 2007). Given that normal cyclical changes in ovarian steroids and their metabolites are associated with changes in the regulation of the HPA axis and other stress response systems, especially in MRMD (Schiller et al., 2014; Crowley and Girdler, 2014; Gordon et al., 2015; Roca et al., 2003), this is a particularly promising candidate mechanism. Animal models also implicate sensitization of stress response systems in susceptibility to negative effects of ovarian hormone changes. For example, abrupt changes in E2 and P4 elicit anxious and depressive behavior in rats, but only among animals exposed to chronic social stress (Löfgren et al., 2012). Similarly, another study demonstrated that allopregnanolone fluctuations produced behavioral changes in mice only in the context of a proximal foot-shock stressor (Smith et al., 2006). Finally, among women with prospectively-confirmed MRMD, the extent of dysregulation in HPA responses to a standardized psychosocial stressor predicts greater symptoms (Klatzkin et al., 2014). Therefore, sensitization of stress systems in women with MRMD who have a history of abuse may underlie a heightened sensitivity to normal fluctuations in ovarian steroid hormones and their GABAergic neurosteroid metabolites, which may consequently compromise normal physiological stress regulation.
4.2. Differential effects of physical and sexual abuse on hormone sensitivity
The differential effects of physical and sexual abuse on patterns of symptom reactivity to E2 and P4 suggest hypotheses for future work. First, cyclical elevations in P4 were more strongly associated with mood dysregulation and interpersonal problems in women with a history of physical abuse. Physical abuse results in a cognitive bias in which social cues are perceived as threatening (Bradshaw and Garbarino, 2004); this cognitive vulnerability may interact with P4-related increases in social salience (Maner and Miller, 2014) to elicit greater interpersonal sensitivity and related mood disturbances. Luteal increases in P4 are accompanied by increases in social affiliative motivation (Wirth and Schultheiss, 2006) and increased attention to social cues, especially those signaling threat or opportunity (Maner and Miller, 2014). While such increases in the salience of social stimuli may lead to positive social cognition and behavior when one's historical environment has fostered the development of positive social expectations, histories of physical abuse may cause individuals to respond to increased social salience with negatively-biased social cognition (e.g., expectations of harm and hostility), increased physiological stress responses, and a tendency toward aggressive behavior as a self-protective mechanism (e.g., Bradshaw and Garbarino, 2004). Notably, experiences and traits associated with negative social cognitive bias moderate the effects of oxytocin, another hormone that increases social salience (Bartz et al., 2011), on social cognition (Bartz et al., 2010a) and social behavior (Bartz et al., 2010b). Because physical abuse (compared to sexual abuse) is more often perpetrated by a biologically-related, central attachment figure (U.S. Department of Health and Human Services, 2005), it may be more likely to result in enduring associations between cyclical changes in social salience and cognitive expectations of harm.
Sexual abuse also conferred vulnerability to hormone sensitivity in the present study; however, the pattern of this vulnerability was quite different. Cyclical increases in E2 were more strongly associated with anxious symptoms in women with a history of sexual abuse. Although E2 is somewhat elevated in the luteal phase, its primary peak occurs at midcycle concurrent with ovulation and peak fertility. These midcycle elevations in E2 are also associated with greater sexual interest (Guillermo et al., 2010; Roney and Simmons, 2013), and this greater orientation to sexual cues may activate abuse-related symptoms of anxiety about sexual functioning or revictimization among women with a history of sexual abuse (Neumann et al., 1996; Messman-Moore and Long, 2003). This suggests that women with MRMD who have histories of sexual abuse may be vulnerable to midcycle anxiety. The present study was not designed to test mechanistic hypotheses in which abuse-related cognitive vulnerabilities interact with cyclical changes in social or sexual salience to predict symptoms; however, future work should examine whether disturbances in socially- or sexually-related cognition arising from physical or sexual abuse interact with cyclical changes in E2 and P4 to predict physiological, emotional, and behavioral disturbances.
4.3. Limitations and future directions
Despite the strengths of a large sample, prospective design, and multilevel statistical models, the present study has limitations. First, the sampling protocol (two follicular samples, three luteal samples) was timed to capture luteal increases in P4, but was not timed to capture ovulatory changes in E2. This may have reduced power to detect effects. Second, because the study included only five samples per cycle, it was not possible to identify the hormonal “peak” or to reliably measure acute changes in E2 and P4, which limits our ability to characterize associations between acute changes and lagged hormonal effects on symptoms. Third, although abuse did account for some variance in the slopes of steroids on symptoms, it did not fully explain this variability. Additionally, there may be other pathways by which symptoms become cyclical, including secondary emotional reactions to cyclical somatic symptoms, reproductive disorders causing abnormal steroid profiles, such as polycystic ovarian syndrome, or shame around menstruation. Fourth, the lack of a control group limits the generalizability of our findings to women with prospectively-confirmed MRMD; further work is needed to determine whether an abuse is associated with negative reactions to cyclical steroid change in the general population, or whether this is specific to MRMD. Finally, the present work is of course limited by its correlational nature.
5. Conclusions
The present study provides evidence that histories of abuse predict stronger covariation of ovarian steroids and mood symptoms in women with MRMD. Extending previous studies indicating a link between trauma and MRMD, the present work suggests that abuse is associated with greater emotional reactivity to cyclical elevations in E2 and P4. Clinically, it may be useful to note that histories of abuse are associated with stronger symptom cyclicity and a stronger within-person covariation of ovarian steroids with mood and interpersonal symptoms. Additional prospective and experimental work is needed to investigate whether these effects of abuse on hormone sensitivity are mediated by stress system sensitization, and whether women with histories of abuse show luteal alterations in the ability of GABAergic metabolites of ovarian steroid hormones to effectively regulate stress response systems.
Acknowledgement
None.
Funding
This work was supported by grants from the National Institute of Mental Health (T32MH093315; R01MH081837).
Footnotes
Contributors
David Rubinow and Susan Girdler designed and implemented the study.
Jane Leserman and Crystal Schiller participated in the development of hypotheses.
Jacqueline Johnson assisted with statistical analyses.
Tory Eisenlohr-Moul developed the hypotheses, conducted statistical analyses, and wrote the manuscript.
All authors provided comments to the manuscript and approved the final version.
Given that the sampling method used in the present study generated 2 observations in the follicular phase (very low P4) and 3 observations in the luteal phase (higher P4), identical person-centered models were run using a different measure of central tendency: Maximum value minus minimum value. No substantive differences in model outcomes between the two methods emerged; therefore, we chose to use the traditional within-person centering method described by Enders and Tofighi (2007).
Conflicts of interest
None.
References
- Bandelow B, Krause J, Wedekind D, Broocks A, Hajak G, Rüther E. Early traumatic life events, parental attitudes, family history, and birth risk factors in patients with borderline personality disorder and healthy controls. Psychiatry Res. 2005;134(2):169–179. doi: 10.1016/j.psychres.2003.07.008. http://dx.doi.org/10.1016/j.psychres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc. Cognit. Affect. Neurosci. 2010a:nsq085. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cognit. Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. Effects of oxytocin on recollections of maternal care and closeness. Proc. Natl. Acad. Sci. 2010b;107(50):21371–21375. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Manson JE, Hankinson SE, Rich-Edwards JW. Early life emotional. physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J. Women's Health. 2014 doi: 10.1089/jwh.2013.4674. http://dx.doi.org/10.1089/jwh.2013.4674,140806113637009. [DOI] [PMC free article] [PubMed]
- Bless EP, McGinnis KA, Mitchell AL, Hartwell A, Mitchell JB. The effects of gonadal steroids on brain stimulation reward in female rats. Behav. Brain Res. 1997;82(2):235–244. doi: 10.1016/s0166-4328(96)00129-5. http://dx.doi.org/10.1016/S0166-4328 (96) 00129-5. [DOI] [PubMed] [Google Scholar]
- Bradshaw CP, Garbarino J. Social cognition as a mediator of the influence of family and community violence on adolescent development: implications for intervention. Ann. N. Y. Acad. Sci. 2004;1036(1):85–105. doi: 10.1196/annals.1330.005. [DOI] [PubMed] [Google Scholar]
- Brown CS, Ling FW, Andersen RN, Farmer RG, Arheart KL. Efficacy of depot leuprolide in premenstrual-syndrome—effect of symptom severity and type in a controlled trial. Obstet. Gynecol. 1994;84(5):779–786. [PubMed] [Google Scholar]
- Bunevicius A, Leserman J, Girdler SS. Hypothalamic-pituitary-thyroid axis function in women with a menstrually related mood disorder: association with histories of sexual abuse. Psychosom. Med. 2012;74(8):810–816. doi: 10.1097/PSY.0b013e31826c3397. http://dx.doi.org/10.1097/PSY.0b013e31826c3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Conversion of progesterone to 5–pregnane-3,20-dione and 3–hydroxy-5–pregnan-20-one by rat medial basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93(5):1157–1162. doi: 10.1210/endo-93-5-1157. http://dx.doi.org/10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology. 2014;231(17):3619–3634. doi: 10.1007/s00213-014-3572-8. http://dx.doi.org/10.1007/s00213-014-3572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 2008;213(1-2):63–72. doi: 10.1007/s00429-008-0192-2. http://dx.doi.org/10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013:1–8. doi: 10.1016/j.psyneuen.2013.06.007. http://dx.doi.org/10.1016/j.psyneuen.2013. 06.007. [DOI] [PubMed]
- Eisenlohr-Moul TA, DeWall CN, Girdler SS, Segerstrom SC. Ovarian hormones and borderline personality disorder features: preliminary evidence for interactive effects of estradiol and progesterone. Biol. Psychol. 2015;109:37–52. doi: 10.1016/j.biopsycho.2015.03.016. http://dx.doi.org/10.1016/j.biopsycho.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol. Methods. 2007;12(2):121. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W. Daily record of severity of problems (DRSP): reliability and validity. Arch. Women's Mental Health. 2006;9(1):41–49. doi: 10.1007/s00737-005-0103-y. http://dx.doi.org/10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- Girdler SS. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom. Med. 2003;65(5):849–856. doi: 10.1097/01.psy.0000088593.38201.cd. http://dx.doi.org/10.1097/01.PSY.0000088593.38201.CD. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26(2):201–213. doi: 10.1037/0278-6133.26.2.201. http://dx.doi.org/10.1037/0278-6133.26.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Thompson KS, Light KC, Leserman J, Pedersen CA, Prange AJ. Historical sexual abuse and current thyroid axis profiles in women with premenstrual dysphoric disorder. Psychosom. Med. 2004;66(3):403–410. doi: 10.1097/01.psy.0000127690.38525.ab. [DOI] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am. J. Psychiatry. 2015;172(3):227–236. doi: 10.1176/appi.ajp.2014.14070918. http://dx.doi.org/10.1176/appi.ajp.2014.14070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermo CJ, Manlove HA, Gray PB, Zava DT, Marrs CR. Female social and sexual interest across the menstrual cycle: the roles of pain, sleep and hormones. BMC Women's Health. 2010;10(1):19. doi: 10.1186/1472-6874-10-19. http://dx.doi.org/10.1186/1472-6874-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003;28:1–23. doi: 10.1016/s0306-4530(03)00098-2. http://dx.doi.org/10.1016/S0306-4530(03) 00098-2. [DOI] [PubMed] [Google Scholar]
- Hammarbäck S, Bäckström T. Induced anovulation as treatment of premenstrual tension syndrome: a double-blind cross-over study with GnRH-agonist versus placebo. Acta Obstet. Gynecol. Scand. 2009;67(2):159–166. doi: 10.3109/00016348809004191. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. http://dx.doi.org/10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Bunevicius A, Forneris CA, Girdler S. Menstrual mood disorders are associated with blunted sympathetic reactivity to stress. J. Psychosom. Res. 2014;76(1):46–55. doi: 10.1016/j.jpsychores.2013.11.002. http://dx.doi.org/10.1016/j.jpsychores.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J, Li Z, Drossman DA, Toomey TC, Nachman G, Glogau L. Impact of sexual and physical abuse dimensions on health status: development of an abuse severity measure. Psychosom. Med. 1997;59(2):152–160. doi: 10.1097/00006842-199703000-00007. [DOI] [PubMed] [Google Scholar]
- Liu J, Bisschop PH, Eggels L, Foppen E, Fliers E, Zhou JN, Kalsbeek A. Intrahypothalamic estradiol modulates hypothalamus-pituitary-adrenal-axis activity in female rats. Endocrinology. 2012;153(7):3337–3344. doi: 10.1210/en.2011-2176. http://dx.doi.org/10.1210/en.2011-2176. [DOI] [PubMed] [Google Scholar]
- Maner JK, Miller SL. Hormones and social monitoring: menstrual cycle shifts in progesterone underlie women's sensitivity to social information. Evol. Hum. Behav. 2014;35(1):9–16. [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Messman-Moore TL, Long PJ. The role of childhood sexual abuse sequelae in the sexual revictimization of women: an empirical review and theoretical reformulation. Clin. Psychol. Rev. 2003;23(4):537–571. doi: 10.1016/s0272-7358(02)00203-9. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur. J. Pharmacol. 1987;142(3):483–485. doi: 10.1016/0014-2999(87)90094-x. http://dx.doi.org/10.1016/0014-2999(87) 90094-X. [DOI] [PubMed] [Google Scholar]
- Muse KN, Cetel NS, Futterman LA, Yen SSC. The premenstrual syndrome. N. Engl. J. Med. 1984;311(21):1345–1349. doi: 10.1056/NEJM198411223112104. http://dx.doi.org/10.1056/NEJM198411223112104. [DOI] [PubMed] [Google Scholar]
- Neumann DA, Houskamp BM, Pollock VE, Briere J. The long-term sequelae of childhood sexual abuse in women: a meta-analytic review. Child Maltreat. 1996;1(1):6–16. [Google Scholar]
- Patchev VK, Almeida O. Gonadal steroids exert facilitating and buffering effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J. Neurosci. 1996;16(21):7077–7084. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62(1):265–271. doi: 10.1016/0306-4522(94)90330-1. http://dx.doi.org/10.1016/0306-4522(94) 90330-1. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Yonkers KA, Pfister H, Lieb R, Wittchen HU. Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. J. Clin. Psychiatry. 2004;65(10):1314–1322. doi: 10.4088/jcp.v65n1004. [DOI] [PubMed] [Google Scholar]
- Pilver CE, Levy BR, Libby DJ, Desai RA. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch. Women's Mental Health. 2011;14(5):383–393. doi: 10.1007/s00737-011-0232-4. http://dx.doi.org/10.1007/s00737-011-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei E, Freeman EW. Daily plasma estradiol and progesterone levels over the menstrual cycle and their relation to premenstrual symptoms. Psychoneuroendocrinology. 1995;20(3):259–267. doi: 10.1016/0306-4530(94)00057-h. http://dx.doi.org/10.1016/0306-4530(94) 00057-H. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J. Clin. Endocrinol. Metab. 2003;88(7):3057–3063. doi: 10.1210/jc.2002-021570. http://dx.doi.org/10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Roney JR, Simmons ZL. Hormonal predictors of sexual motivation in natural menstrual cycles. Horm. Behav. 2013;63(4):636–645. doi: 10.1016/j.yhbeh.2013.02.013. http://dx.doi.org/10.1016/j.yhbeh.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Schiller CE, O'Hara MW, Rubinow DR, Johnson AK. Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol. Behav. 2013;119:1–8. doi: 10.1016/j.physbeh.2013.06.009. http://dx.doi.org/10.1016/j.physbeh.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Schmidt PJ, Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology. 2014;231(17):3557–3567. doi: 10.1007/s00213-014-3599-x. http://dx.doi.org/10.1007/s00213-014-3599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N. Engl. J. Med. 1998;338(4):209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; 2003. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Pearson; New York: 2001. [Google Scholar]
- Vamvakopoulos GPC. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J. Clin. Investig. 1993;92(4):1896–1902. doi: 10.1172/JCI116782. http://dx.doi.org/10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–895. doi: 10.1016/j.neuroscience.2008.12.058. http://dx.doi.org/10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Horm. Behav. 2006;50(5):786–795. doi: 10.1016/j.yhbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]