Abstract
Semaphorins are a large family of secreted and membrane-bound proteins. Recently, several roles of semaphorins in the immune system have emerged. Several semaphorins and their receptors are expressed in a variety of lymphoid and myeloid cells and affect immune cell functions, including cell proliferation, differentiation, chemotaxis, and cytokine production. However, the roles of class 3 semaphorins in human myeloid cells are not well known. Here we examined the regulation of expression of class 3 semaphorins and their receptors by inflammatory stimuli and their function in human macrophages. We show that the expression of Sema3A receptors (neuropilin-1 (NRP-1), NRP-2, plexin A1, plexin A2 and plexin A3) significantly increased during M-CSF-mediated differentiation of monocytes into macrophages under conditions that promote an M2 alternatively activated macrophage phenotype. Consistent with increased NRP-1 expression, cell surface binding of Sema3A increased during M2 differentiation. IFN-γ and LPS that promote classical M1 macrophage activation affected expression of NRP-1, NRP-2 and plexin A1. IFN-γ decreased NRP-1 expression and LPS suppressed NRP-2 and plexin A1 expression. Furthermore we show that Sema3A induced apoptosis in monocyte-derived macrophages, and cooperated with anti-Fas CH11 antibody to augment apoptosis. Our results suggest Sema3A plays a role in induction of apoptosis in monocyte-derived macrophages that are resistant to Fas-induced apoptosis and that its function can be modulated in inflammatory conditions.
Keywords: Semaphorin, Neuropilin, Plexin, Macrophages
INTRODUCTION
Semaphorins are a large family of secreted and membrane-bound proteins, characterized by a distinct cysteine-rich domain of about 500 amino acids, called the sema domain. These proteins were originally discovered in the nervous system, where they have been implicated in repulsive axon guidance during the development of the nervous system [1,2]. Class 3 semaphorins are secreted proteins and have 6 members, Sema3A-3F. Class 3 semaphorins bind to signaling receptors of the plexinA family, but also require neuropilins as binding co-receptors. Sema3A binds only neuropilin-1 (NRP-1), while Sema3B, Sema3C, and Sema3F bind both NRP-1 and NRP-2. In contrast to other class 3 semaphorins, Sema3E does not require neuropilins for signaling. Neuropilins have a very short cytoplasmic domain that can not transduce intracellular signals, and plexins form complexes with neuropilins and act as the signal-transducing components of the Semaphorin-neuropilin-plexin ligand-receptor complex [3]. Recently, several roles of semaphorins in the immune system have emerged. Several semaphorins and their receptors are expressed in a variety of lymphoid and myeloid cells and affect immune cell functions, including cell proliferation, differentiation, chemotaxis, and cytokine production [4]. However, the expression and the roles of class 3 semaphorins in human myeloid cells are not well known.
In this report, we examined the expression and function of class 3 semaphorins and their receptors in human peripheral blood monocytes and monocyte derived M2-like macrophages. M2 macrophages are alternatively activated macrophages that produce low amounts of inflammatory cytokins such as TNF or IL-12 production and are involved in immunoregulation and tissue remodeling [5]. We show that expression of NRP-1, NRP-2, plexin A1, plexin A2 and plexin A3 increased during M-CSF-driven differentiation of human monocytes into M2-like macrophages. In monocyte-derived macrophages, Sema3A significantly induced apoptosis. These results describe a new role for class 3 semaphorins in human myeloid cells.
MATERIALS AND METHODS
Cell Isolation and Culture
Monocytes were obtained from peripheral blood mononuclear cells by positive selection, using anti-CD14 magnetic beads, as recommended by the manufacturer (Miltenyi Biotech). Monocytes were used fresh or differentiated to macrophages during 2 days of culture in αMEM medium supplemented with 10% fetal bovine serum and 20 ng/ml macrophage colony-stimulating factor (R&D Systems). THP-1 cells were cultured at 37°C in 5% CO2 using RPMI medium with 10% fetal bovine serum.
Flow Cytometry
Human monocytes, monocyte-derived macrophages and THP-1 cells were incubated with Fc receptor (FcR) blocking reagent (Miltenyi Biotec) and incubated with CD304 (BDCA-4/Neuropilin-1) antibodies (Miltenyi Biotec) for 10 minutes at 4°C, as recommended by the manufacturer. Murine IgG1 mAbs were used as isotype controls. After two washes, the cells were stained with FITC-conjugated anti-IgG1 secondary antibodies for 10 minutes at 4°C. After two washes, the cells were resuspended in PBS/0.5% BSA and analyzed by flow cytometry.
Binding of Sema3A to cells
Human monocytes, monocyte-derived macrophages and THP-1 cells were incubated with 2 μg/mL Sema3A/Fc (R&D Systems) in binding buffer at 4°C for 60 minutes, according to a previous report [6]. After two washes, Sema3A/Fc bound to cells was detected with FITC-conjugated F(ab′)2 goat anti–human IgG Fc (Jackson Immuno-Research Laboratories).
RNA extraction and real-time PCR
Total cellular RNA was isolated using an RNeasy Mini kit (Qiagen), according to the manufacturer’s instructions, and was reverse transcribed with a First Strand cDNA Synthesis kit (Fermentas). Reverse transcription reactions included the reverse transcriptase enzyme (RT) for cDNA, or omitted the enzyme (No-RT) as negative control. The latter therefore amplified only contaminating genomic DNA. Real-time PCR was performed with the iCycler iQ thermal cycler and detection system (Biorad) and the PCR Core Reagents kit (Applied Biosystems). Relative expression was normalized for levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Oligonucleotide primers used are as follows:
GAPDH, ATC AAG AAG GTG GTG AAG CA and GTC GCT GTT GAA GTC AGA GGA;
NRP-1, TGAGCC CTG TGG TTT ATT CC and CGT ACT CCT CTG GCT TCT GG;
NRP-2, GGT CGC CGG CGG GGA TTG G and TCG GTG GGG TAG GGG GTG GTT GTC;
Plexin A1, CTC CCT GCC GTG GCT GCT CAA CAA and ACC ACA GTG CGG CCC CGA TAG TCA;
Plexin A2, CGC TCT CCC CCT GGC CAT CAA GTA and CCA GAA GCG CAG AGG GAG GCA GTT;
Plexin A3, TGG GCG CAG TGA ACC GAG TCT TTA and CTG GGA CCT CGG GGC TGT TGG;
Plexin A4, GCC CCT CTT CTC CCT GTT CTG TGC and CTG GGA CCT CGG GGC TGT TGG;
Sema3A, GGT TGC CCA GCT CCC TTT AC and TAT CTT GTC GTC TTG TGC GTC TCT;
Sema3C, ATC GCA GCG CTG AGA TTC CTT TAC and GAT GCG CTT GTG TCT CCA GTC C;
Sema3F, CGC GCC CAG GCC ACA CCA T and CAT CGG GCG GAG GGC ACC AT.
Phagocytosis Assays
E-IgG were prepared using human erythrocytes that were opsonized with rabbit anti-human red blood cells (RBC) (Rockland Immunochemicals), labeled with CellTrace™ CFSE cell proliferation kit (Molecular Probes). Erythrocytes (Es) or E-IgGs were incubated with adherent macrophages for 1 hour at a ratio of 20:1. Extracellular Es and E-IgGs were lysed using water and intracellular fluorescence of macrophages was measured using flow cytometry. The phagocytosis index was calculated by multiplying the mean fluorescence intensity by the fraction of cells that were phagocytic, as described previously [7].
Detection of apoptosis
Human PB monocyte or monocyte-derived macrophages were stimulated with 200 ng/mL anti-Fas CH11 antibody (Upstate Biotechnology), in the absence and presence of Sema3A-Fc (150 ng/mL) for indicated times. Cell death was assessed by staining using FITC-annexin V (BD Biosciences Pharmingen). Briefly, after washing cells twice with cold PBS, cells were resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. Cells were incubated with 5 μl of Annexin V-FITC and 5 μl of PI for 15 min at RT (25°C) in the dark and then analyzed by flow cytometry within one hour.
Statistical analysis
Results are expressed as means plus or minus SD. Student t test was applied to evaluate group differences; a P value of less than 0.05 was considered significant.
RESULTS
Expression of Class 3 Semaphorins and Their Receptors
Semaphorins are widely expressed in most tissues and organ systems such as the nervous, cardiovascular, musculoskeletal, renal, reproductive, and immune systems [2]. Since there is no previous report about expression of class 3 semaphorins in human monocytes and monocyte-derived macrophages, we examined expression of class 3 semaphorins in human monocytes, monocyte-derived macrophages and THP-1 cells. For excluding the contamination of genomic DNA, PCR product signal was examined using RT and No-RT condition. We examined the amounts of signal from RT and No-RT condition, using real-time RT-PCR. Omission of RT consistently resulted in a decreased signal (higher threshold cycle) (data not shown). Also, PCR products from +RT condition showed a single peak with a characteristic melting temperature on LightCycler melting analysis, and displayed a single product at the appropriate size for Sema3A on agarose gels (Fig. 1A). However, PCR products from No-RT condition did not show any peak with characteristic melt temperature, and displayed no products on agarose gels (Fig. 1A and data not shown). Thus Sema3A mRNA is expressed in human peripheral blood monocytes. Sema3A expression increased when monocytes were differentiated with M-CSF under conditions that promote an M2 phenotype [7] (Fig. 1B). Recent studies did not detect Sema3A mRNA in human peripheral blood monocytes, likely because of lower sensitivity [8,9]. Expression of Sema3C and Sema3F did not increase during macrophage differentiation (Fig. 1C).
Figure 1. Expression of class 3 semaphorins on human peripheral blood monocytes, macrophages and THP-1 cells.
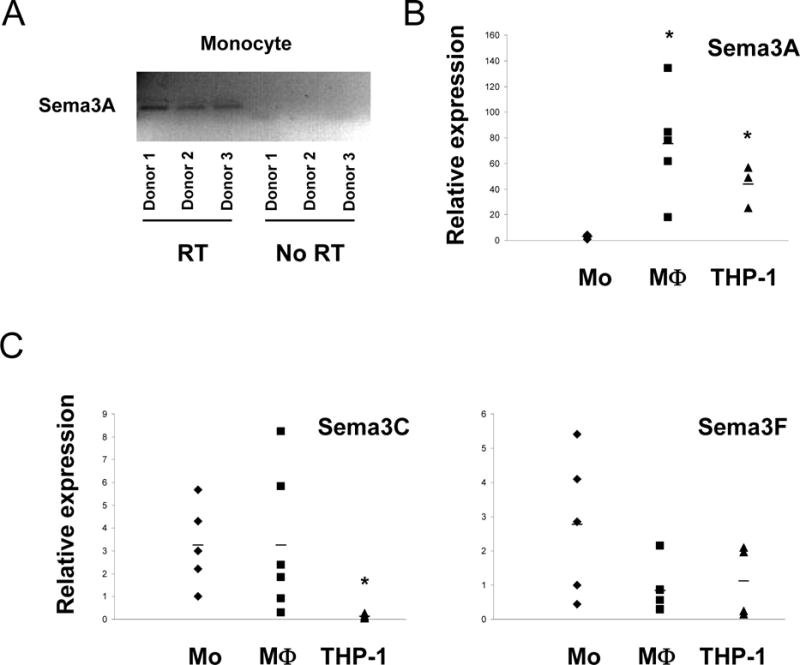
A. Human monocytes were differentiated to macrophages by culturing with 20 ng/ml of M-CSF for 2 days, and then mRNA was isolated. Also, mRNA was isolated from freshly isolated monocytes and THP-1 cells. PCR products from RT and no-RT conditions were displayed on agarose gels. B and C. Gene expression in human monocytes, macrophages and THP-1 cells was analyzed by real-time PCR. mRNA levels were normalized relative to the expression of GAPDH. Horizontal bars are shown as average of more than three independent experiments (* p < 0.05, vs monocytes by Student t-test).
The functions of class 3 semaphorins are mediated by plexins such as plexin A1–A4 and plexin D1 [10]. Also Neuropilins, NRP-1 and NRP-2, are co-receptors for class 3 semaphorins and form complexes with plexin A1–4, and these complexes mediate intracellular signaling by class 3 semaphorins. So we examined the expression of neuropilins and plexins A1–4 during macrophage differentiation. We examined the amounts of signal for Plexin A1, A2, A3 and A4 under RT and No-RT conditions, using real-time RT-PCR. Plexin A1, A2, A3 and A4 were expressed in monocytes. Plexin A1, A2 and A3, but not A4, expression significantly increased during macrophage differentiation (Fig. 2A). Expression of NRP-1 and NRP-2 also increased during macrophage differentiation, although the increase in NRP-2 did not achieve statistical significance (Fig. 2A and 2B). These results show that macrophages acquire expression of many semaphorin receptors during differentiation, suggesting a gain of responsiveness to their semaphorin ligands. The concomitant expression of semaphorins and their receptors suggest a potential autocrine loop.
Figure 2. Expression of Neuropilins and Plexins in human peripheral blood monocytes, macrophages and THP-1 cells.
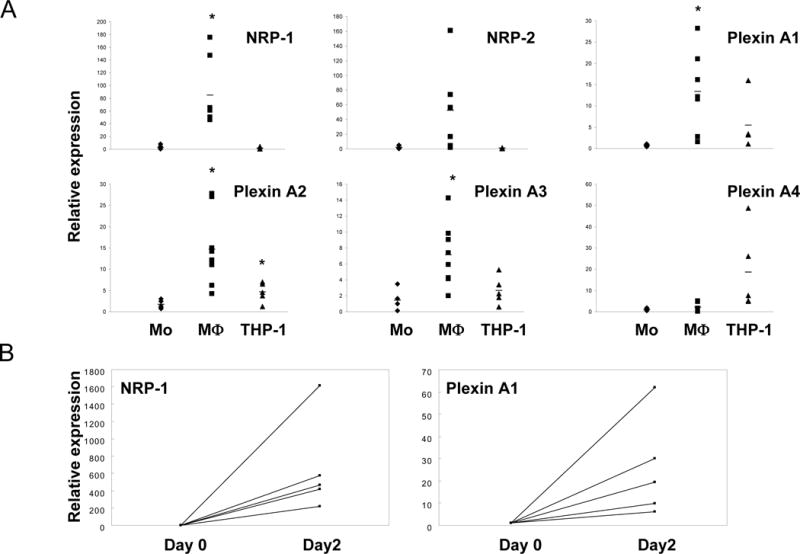
A. Human monocytes were differentiated to macrophages with 20 ng/ml of M-CSF for 2 days, and then mRNA was isolated. Also, mRNA was isolated from freshly isolated monocytes and THP-1 cells. mRNA was analyzed by real-time PCR. mRNA levels were normalized relative to the expression of GAPDH. B. mRNA from macrophages cultured with M-CSF for 2 days (Day 2) was analyzed by real-time PCR and compared to monocytes of same donors (Day 0). Horizontal bars show average of more than three independent donors (* p < 0.05, vs monocytes by Student t-test).
Binding of Sema3A to Macrophages
To corroborate our real time PCR results showing increased semaphorin receptor expression during macrophage differentiation (Figs. 1 and 2), we analyzed cell surface expression of NRP-1 and the ability of a Sema3A-Fc protein to bind to macrophages. Consistent with result of NRP-1 gene expression, expression of cell surface NRP-1 protein significantly increased during macrophage differentiation with M-CSF, as measured using a specific MAb and flow cytometry (Fig. 3A). In addition, we found that binding of Sema3A to cells increased during macrophage differentiation (Fig. 3B). These results that macrophages express semaphorin receptors and bind Sema3A support the notion that macrophages acquire the ability to respond to semaphorins as they differentiate from monocytes.
Figure 3. Surface expression of NRP-1 on human peripheral blood monocytes and macrophages.
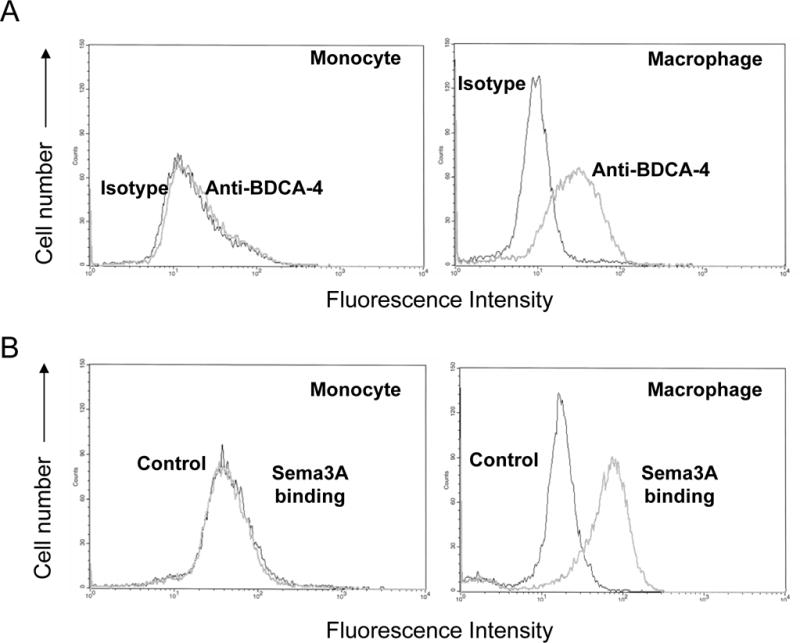
Human monocytes were differentiated to macrophages with 20 ng/ml of M-CSF for 2 days, and macrophages were compared with fresh monocytes for expression of NRP-1. A. Cell surface NRP-1 levels were assessed by flow cytometry using anti-BDCA4 antibodies. B. Sema3A binding to the cell surface was assessed by flow cytometry using Sema3A/Fc. Data are representative of more than three experiments.
Inhibition of NRP and Plexin A1 Expression by IFN-γ and LPS
IFN-γ and LPS are potent activators of macrophages that promote an M1 classically activated inflammatory phenotype that differs from the M2 phenotype promoted by M-CSF [5]. We examined the effects of IFN-γ and lipopolysaccharide (LPS) on class 3 semaphorin receptor expression. Interestingly, IFN-γ significantly decreased NRP-1 expression, and IFN-γ had minimal effect on NRP-2 and plexin A1 expression (Fig. 4). In contrast, LPS did not suppress NRP-1 expression, but did suppress NRP-2 and plexin A1 expression.
Figure 4. Effects of IFNγ and LPS on expression of NRP-1 and Plexin A1.
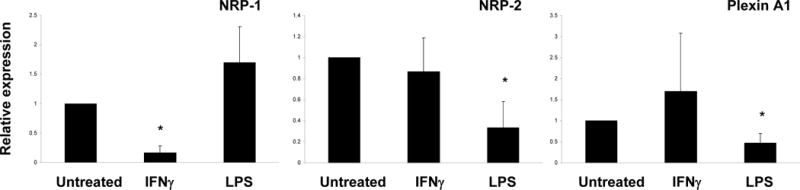
Human monocytes were cultured with M-CSF (20 ng/ml) for 1 day. IFN γ (100 U/ml) or LPS (100 ng/ml) was added at the initiation of cultures. mRNA was measured using real-time PCR. mRNA levels were normalized relative to the expression of GAPDH, and results are shown as mean ± SD of three independent experiments (* p < 0.05, vs untreated). Representative results from at least three independent experiments.
Biological Function of Sema3A on Monocytes-Derived Macrophages
Sema3A inhibits cytoskeleton rearrangement via depolymerization of F-actin and attenuates microtubule dynamics [11]. The cytoskeleton is required for cell migration and phagocytosis. We examined the effect of Sema3A on migration of monocyte-derived macrophages in response to MCP-1, but the migration was not changed by Sema3A (data not shown). Next, we examined the effect of Sema3A on phagocytosis of opsonized human erythrocytes. The phagocytosis of opsonized human erythrocytes by macrophages was not affected by treatment with Sema3A (Fig. 5A). It was reported that Sema3A induces apoptosis in endothelial cells and augments Fas-mediated apoptosis in T cell lines [12,13]. We examined the Sema3A-induced apoptosis in monocyte-derived macrophages that express Sema3A receptors. Sema3A significantly induced apoptosis in human macrophages, consistent with our results showing increased cell surface Sema3A binding and increased expression of its receptors (Fig. 5B). Sema3A augmented apoptosis induced with the anti-Fas CH11 antibody. Consistent with previous report [14], monocyte-derived macrophages were resistant to apoptosis induced by the anti-Fas CH11 antibody. Thus, this result suggests a role for Sema3A in induction of apoptosis in monocyte-derived macrophages that are resistant to Fas-induced apoptosis.
Figure 5. Sema3A-induced apoptosis in human peripheral blood monocyte and macrophage, compared with anti-Fas antibody.
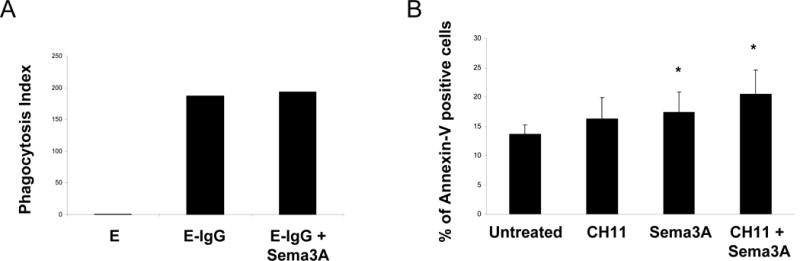
A. Human monocytes were cultured with M-CSF for 2 days, then E-IgGs were added to macrophages. Phagocytosis index was measured using flow cytometry. E, erythrocytes; E-IgG, erythrocytes opsonized with human IgG1. Data are representative of two experiments. B. Human monocytes were cultured with M-CSF for 2 days, then were stimulated with 200 ng/ml CH11 in the presence or absence of Sema3A/Fc (150 ng/ml) for 4 hours. Cell death was assessed by staining with FITC-annexin V. Results are shown as mean ± SD of four independent experiments (* p < 0.05, vs untreated).
DISCUSSION
In this report, we show that the expression of Sema3A receptors (NRP-1, NRP-2, plexin A1, plexin A2 and plexin A3) significantly increased during M-CSF-mediated differentiation of monocytes into macrophages. Consistent with increased expression of NRP-1, cell surface binding of Sema3A increased during differentiation. We first observed that Sema3A and its receptors are co-expressed in human monocyte-derived macrophages during M-CSF-mediated differentiation, and these results suggest that autocrine and/or paracrine activity of Sema3A signaling is induced during M-CSF-mediated differentiation of monocytes.
Immunological properties have been increasingly demonstrated for Semaphorins. Sema3A inhibited migration of monocytic cells in previous report. This report also suggested that this inhibitory effect of migration is not mediated by NRP-1 or NRP-2, because these receptors are not expressed on cell surface [15]. Sema4A binds TIM-2 and stimulates T cells activation and differentiation [16]. Sema4D binds CD72 and enhances the activation of B cells and dendritic cells [17]. Sema6D binds plexin A1-TREM2-DAP12 receptor complex and stimulate dendritic cells [18]. Also Sema7A expressed on activated T cells interacts with α1β1 integrin and plays a crucial role in T cell–mediated macrophage activation at sites of inflammation [19]. Recently, two reports demonstrated that Sema3A inhibits T cell proliferation [9] and augments Fas-mediated apoptosis in Jurkat cells [13]. The findings from these reports demonstrate that several semaphorins have crucial roles in various immune responses, and these semaphorins can be grouped as immune semaphorins. However, the roles of Sema3A have not been well examined in human monocyte-derived macrophages. In the present study, we show that Sema3A and its receptors are co-expressed during M-CSF-mediated differentiation of monocytes into macrophages, and Sema3A induces apoptosis in monocyte-derived macrophages.
Expression of Semaphorins and their receptors is modulated by the inflammatory stimulation and status of cell activation. In a previous report, TGF-β1 increased the cell surface expression of Sema6A on dendritic cells and IFNγ significantly increased Sema6A expression on in vitro-generated Langerhans cells [8]. Dendritic cell maturation induced by both TNFα and IL-1β or by CD40L significantly increases the expression of Sema3A mRNA [9]. Fresh isolated naïve T cells did not express NRP-1, but NRP-1 was expressed on some CD69+ T cells after 24 hours in vitro activation with PMA and ionomycin [20]. In present study, NRP-1 and plexin A1 was induced during M-CSF-mediated differentiation of monocytes into macrophages, and IFNγ significantly inhibits the expression of NRP-1 and LPS inhibits the expression of plexin A1. During monocyte-to-macrophage differentiation, cytokines induce several specialized and polarized phenotypes, including M1 and M2 cells [21]. Classically M1 macrophages are induced by IFNγ alone or in concert with LPS, TNFα or GM-CSF and produce inflammatory cytokines such as TNF and IL-12. In contrast, M2 macrophages result from monocyte exposure to IL-4 or IL-13 and exhibit alternative functions, such as tissue remodeling or host defense against helminths. Recent study suggests that M-CSF-driven differentiation leads per se to the acquisition of M2 properties [5]. Our results show that NRP-1 and plexin A1 are induced in M-CSF-treated cells and expression of these genes is inhibited by IFNγ or LPS. These results suggest that NRP-1 and plexin A1 can work in distinguishing between M1 and M2 cells. M2 polarization is associated with the up-regulation of NRP-1 and plexin A1, and NRP-1 and plexin A1 are decreased in M1 cells.
The binding receptor for Sema3A is NRP-1. This receptor also binds other ligands such as Sema3C and vascular endothelial growth factor-165 (VEGF165) in a competitive manner. Sema3C blocks the binding of Sema3A to NRP-1 and abolishes the repulsive effect of Sema3A in axons expressing NRP-1 [22]. Also VEGF165 competes with Sema3A for binding to NRP-1 in endothelial cells, and blocks Sema3A-induced inhibition of lamellipodia spreading and cord formation in endothelial cells [5]. In our results, Sema3C was expressed in human monocytes and macrophages, at similar levels to Sema3A. This result suggests the possibility that Sema3C can compete with Sema3A for binding to NRP-1 and modulate the biologic effect of Sema3A. It was reported that Sema3C is expressed in the synovial tissue of patients with rheumatoid arthritis [23], Sema3A is induced by both TNFα and IL-1β or by CD40L [9], Sema3A inhibits T cell activation [9], and Sema3C acts as competitive inhibitor of Sema3A [22], suggesting that the activity of inflammatory diseases such as RA can be influenced by the balance of biologic function between Sema3A and Sema3C. In addition to a role as a class 3 semaphorin receptor, NRP-1 acts as a co-receptor for VEGFs by forming complexes with VEGFR2/KDR, and enhances VEGFR2 signaling and function [24]. In the vascular system, NRP-1 is expressed mainly by arterial endothelium, whereas NRP-2 is expressed by venous and lymphatic endothelium [25]. Hypoxic conditions, such as vascular occlusion, induce both NRP-1 and NRP-2, and these NRPs may be involved in vascular differentiation into arteries and veins.
Sema3A is expressed in several different tissues and has various, distinct roles in several biologic functions such as bone, cartilage and heart formation [26], cell death [12,13], platelet adhesion and aggregation [27], cell migration [15, 28], and cytoskeletal reorganization [9,11]. We examined the functions of Sema3A in monocyte-derived macrophages. First, the cytoskeleton is required for cell migration and phagocytosis and cytoskeleton reorganization is inhibited by Sema3A, so we examined the effect of Sema3A on phagocytosis of monocyte-derived macrophages. However Sema3A did not change the efficacy of phagocytosis in macrophages. Second, we examined the effect of Sema3A on apoptosis. In our experiments, expression of NRP-1 and plexin A1 were consistently increased in cells cultured with M-CSF for 2 days, compared with freshly isolated monocytes (Fig 2B). Consistent with this result, Sema3A significantly induced apoptosis in cells cultured with M-CSF for 2 days. In this experiment, we can not use freshly isolated monocytes as control cells, because freshly isolated monocytes undergo substantial spontaneous apoptosis during culture, compared with Sema3A-induced effect. Also in our experiments, IFNγ inhibits NRP-1 expression and LPS inhibits plexin A1 during culture. These results suggest the possibility that these inflammatory molecules can prevent the Sema-3A induced apoptosis of macrophages via down-regulation of its receptors during inflammatory conditions. Recently, it was reported that Sema3C is expressed in RA synovial tissues [23]. So it is possible that Sema3C can inhibit Sema3A-induced apoptosis by competing with Sema3A for binding NRP-1 in RA synovial tissues.
In conclusion, the expression of NRP-1 and plexin A1 on monocyte-derived macrophages suggest that Sema3A may play an important role in these cells. In the present experiments, we found that Sema3A induces apoptosis in macrophages resistant to Fas-mediated apoptosis. This result indicates that Sema3A can work as an alternative apoptosis-inducing agent in conditions resistant to Fas-mediated apoptosis. Also expression of NRP-1 and plexin A1 is inhibited by IFNγ and LPS, respectively. These results suggest that in inflammatory condition, M1 cells escape the effect from Sema3A through down-regulation of Sema3A receptors.
Acknowledgments
This work was supported by grants from the NIH to L. B. I. and the Arthritis Foundation to K.-H. P.-M.
Abbreviations
- NRP-1
Neuropilin-1
- NRP-2
Neuropilin-2
- LPS
lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He Z, Wang KC, Koprivica V, Ming G, Song HJ. Knowing how to navigate: mechanisms of semaphorin signaling in the nervous system. Sci STKE. 2002;2002:RE1. doi: 10.1126/stke.2002.119.re1. [DOI] [PubMed] [Google Scholar]
- 2.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 6.Narazaki M, Tosato G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood. 2006;107:3892–3901. doi: 10.1182/blood-2005-10-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pricop L, Salmon JE, Edberg JC, Beavis AJ. Flow cytometric quantitation of attachment and phagocytosis in phenotypically-defined subpopulations of cells using PKH26-labeled Fc gamma R-specific probes. J Immunol Methods. 1997;205:55–65. doi: 10.1016/s0022-1759(97)00053-7. [DOI] [PubMed] [Google Scholar]
- 8.Gautier G, de Saint-Vis B, Senechal B, Pin JJ, Bates EE, Caux C, et al. The class 6 semaphorin SEMA6A is induced by interferon-gamma and defines an activation status of langerhans cells observed in pathological situations. Am J Pathol. 2006;168:453–465. doi: 10.2353/ajpath.2006.050288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, et al. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006;36:1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- 10.Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005;62:1363–1371. doi: 10.1007/s00018-005-5018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282:26294–26305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- 13.Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, et al. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood. 2008;111:2290–2299. doi: 10.1182/blood-2007-06-096529. [DOI] [PubMed] [Google Scholar]
- 14.Kiener PA, Davis PM, Starling GC, Mehlin C, Klebanoff SJ, Ledbetter JA, et al. Differential induction of apoptosis by Fas-Fas ligand interactions in human monocytes and macrophages. J Exp Med. 1997;185:1511–1516. doi: 10.1084/jem.185.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, et al. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol. 2001;166:4348–4354. doi: 10.4049/jimmunol.166.7.4348. [DOI] [PubMed] [Google Scholar]
- 16.Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch’ng E, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- 17.Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 18.Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 20.Dzionek A, Inagaki Y, Okawa K, Nagafune J, Rock J, Sohma Y, et al. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol. 2002;63:1133–1148. doi: 10.1016/s0198-8859(02)00752-8. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Nakamura F, Jin Z, Kalb RG, Strittmatter SM. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci. 1998;1:487–493. doi: 10.1038/2203. [DOI] [PubMed] [Google Scholar]
- 23.Miller LE, Weidler C, Falk W, Angele P, Schaumburger J, Scholmerich J, et al. Increased prevalence of semaphorin 3C, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1156–1163. doi: 10.1002/art.20110. [DOI] [PubMed] [Google Scholar]
- 24.Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- 25.Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212:237–248. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- 26.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 27.Kashiwagi H, Shiraga M, Kato H, Kamae T, Yamamoto N, Tadokoro S, et al. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106:913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- 28.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]


