Abstract
Excess dietary saturated fatty acids such as palmitic acid (PA) induce peripheral and hypothalamic inflammation. Hypothalamic inflammation, mediated in part by microglial activation, contributes to metabolic dysregulation. In rodents, high fat diet-induced microglial activation results in nuclear translocation of nuclear factor-kappa B (NFκB), and increased central pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). The hypothalamic neuropeptide orexin A (OXA, hypocretin 1) is neuroprotective in brain. In cortex, OXA can also reduce inflammation and neurodegeneration through a microglial-mediated pathway. Whether hypothalamic orexin neuroprotection mechanisms depend upon microglia is unknown. To address this issue, we evaluated effects of OXA and PA on inflammatory response in immortalized murine microglial and hypothalamic neuronal cell lines. We demonstrate for the first time in microglial cells that exposure to PA increases gene expression of orexin-1 receptor but not orexin-2 receptor. Pro-inflammatory markers IL-6, TNF-α, and inducible nitric oxide synthase in microglial cells are increased following PA exposure, but are reduced by pretreatment with OXA. The anti-inflammatory marker arginase-1 is increased by OXA. Finally, we show hypothalamic neurons exposed to conditioned media from PA-challenged microglia have increased cell survival only when microglia were pretreated with OXA. These data support the concept that OXA may act as an immunomodulatory regulator of microglia, reducing pro-inflammatory cytokines and increasing anti-inflammatory factors to promote a favorable neuronal microenvironment.
Keywords: Neuroinflammation, Immunomodulation, Palmitic acid, Neurodegeneration, Cytokine, Hypocretin
Introduction
The orexins (orexins A and B; hypocretin 1 and 2) are hypothalamic peptides produced in lateral hypothalamic neurons and released widely throughout the CNS [1, 2]. Orexin A (OXA) and B (OXB) regulate homeostatic mechanisms of energy balance and metabolism [3] through activation of two G-protein coupled receptors, orexin receptors 1 and 2 (OX1R and OX2R, respectively) [2]. Recent studies have shown that orexin plays a role in neuroprotection [3, 4], in part by reducing lipid peroxidation, apoptosis, and inflammation [5-8]. Data suggest that the neuroprotective effects of orexin could rely on modulation of microglia, the resident immune cells of the brain.
Microglia are initiators of the neuroinflammatory response and are highly reactive to endogenous signaling. Microglia are highly dynamic, transitioning between neurotoxic pro-inflammatory (M1) and neuroprotective (M2) phenotypes. For example, following cerebral ischemic events, microglia are first activated to a neuroprotective M2 phenotype as oxygen levels decrease, and then switch to a pro-inflammatory M1 phenotype, inducing cell death [9]. While inflammation is a component of a normal immune response, chronic activation to M1 pro-inflammatory phenotypes can cause microglia to become refractory and contribute to subsequent neuronal dysfunction [10].
Several lines of evidence suggest a role for orexin in modulation of microglia. In cerebral ischemia models, pretreatment with OXA reduces infarct size through a microglial-mediated pathway [8]. Microglia may also become more sensitive to orexin signaling after activation. The potent pro-inflammatory agonist lipopolysaccharide (LPS) increases tumor necrosis factor alpha (TNF-α) in microglia, but also increases OX1R expression, and OXA treatment prior to LPS exposure reduces TNF-α in microglia [8]. These data indicate that increased microglial OX1R could enhance responsiveness to orexin, thus enhancing capability to counter an inflammatory insult. We are especially interested in how these orexin-microglia dynamics might impact brain health in the context of diet-induced obesity.
Dietary intake influences neuronal function, overall brain health, and cognition [11]. High fat diet increases circulating pro-inflammatory cytokines released from microglial cells, resulting in hypothalamic neuroinflammation and neurodegeneration [12, 13]. Chronic intake of saturated fatty acids (SFA) such as palmitic acid (PA, C16:0), activate microglia to an M1 phenotype, eliciting the release of pro-inflammatory cytokines [14, 15]. High fat diets cause this response by activating nuclear translocation of microglial nuclear factor-kappa B (NFκB), initiating release of pro-inflammatory cytokines such as TNF-α and interleukin-6 (IL-6) [13-15]. Palmitic acid activates microglia through a toll like receptor 4 (TLR-4)-dependent pathway, inducing the release of TNF-α and IL-6 [14]. Further, microglia activated by SFA via TLR-4 induce neuronal cell death [14]. Given the above findings on orexin action in microglia, orexin signaling might promote microglia switching to a protective M2 phenotype, protecting against palmitic acid induced inflammation.
The objective of these studies was to determine if orexin reduces PA-induced neuroinflammation by altering microglial M1/M2 phenotype dynamics. To test whether orexin treatment influences microglial phenotype, we evaluated the effect of OXA on PA-induced release of pro-inflammatory cytokines in an immortalized murine microglial cell line (designated BV2). We first validated that PA activates microglia to an M1 state via TLR-4. In our next set of experiments, we tested whether OXA pretreatment influenced levels of the M1 pro-inflammatory markers IL-6, TNF-α; inducible nitric oxide synthase (iNOS); and the M2 anti-inflammatory marker arginase-1 in microglia. Finally, we performed a series of studies to determine how conditioned media from these prior tests altered hypothalamic neuronal survival.
Materials and Methods
Cell culture and reagents
Immortalized murine microglial cells (BV2) and adult murine hypothalamic cells (mHypoA-1/2, cited elsewhere as CLU172; CELLutions Biosystems) [16-18] were grown in Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal bovine serum and 1% penicillin, streptomycin, and neomycin (Invitrogen) and maintained at 37°C with 5% CO2. Orexin A peptide (American Peptides) was suspended in phosphate buffered saline (PBS, Invitrogen) and diluted to 300 nM in DMEM. Palmitic acid (Sigma Aldrich) was conjugated to fatty acid free bovine serum albumin (BSA) [19] and diluted to 0.1 mM in DMEM. Lipopolysaccharide (Sigma Aldrich) was reconstituted in PBS and diluted to 0.4 μg (100ng/ml) in DMEM. The TLR-4 inhibitor TAK-242 (EMD Millipore) was reconstituted in DMSO and diluted to 100 nM in DMEM.
For microglial experiments with OXA, BV2 cells were seeded in T-25 flasks at 7×105 cells and grown to ∼80% confluency. Concentrations of OXA and PA are based on Xiong et al and Wang et al [8, 14]. For all assays, cells were serum starved for 24 h. The experiment was completed in two stages: a 1 h pre-incubation followed by a 4 h challenge. Pre-incubation used either vehicle (PBS) or OXA (300 nM). For challenge, cells were exposed to vehicle (fatty acid free BSA), PA (0.1 mM), or LPS (0.4 μg; 100 ng/ml). There were a total of 5 treatment groups: vehicle-vehicle (control), vehicle-LPS, vehicle-PA, OXA-vehicle, and OXA-PA. After treatment, supernatant and cells were rapidly collected and stored at -20°C. Supernatant from microglial cultures treated as described here was used as conditioned media for hypothalamic neuronal cultures (described below).
For microglial experiments with TAK-242, cells were seeded in 6 well plates at 3.5×105 cells per well and grown to ∼80% confluency. Concentrations and time points were based on Matsunaga et al and Takashima et al [20, 21]. Cells were serum starved for 24 h. TAK-242 (100 nM) or vehicle was added 20 minutes prior to incubation with PA (0.1mM) or vehicle for 4 h.
Real-time RT-PCR
Total RNA was extracted from BV2 cells with Trizol (Invitrogen) as previously described [5, 22]. Concentrations were determined using spectrophotometric readings at 260 and 280 nm (Nanodrop 8000, Thermo Fisher Scientific) and 2.5 μg RNA was used for each reaction. Primer sequences were generated using MacVector 15 for OX1R (NM_198959), OX2R (NM_198962), IL-6 (NM_031168), iNOS (NM_010927), arginase-1 (NM_007482) and GAPDH (NM_017008). Relative mRNA expression of target genes was determined using SYBR Green detection normalized to GAPDH using the Δ-ΔCT method [23].
Enzyme-linked immunosorbent assay (ELISA)
TNF-α level in culture media was determined using an ELISA kit (BioLegend Inc.). Concentrations were determined using a spectrophotometer (SpectraMax-M5; Molecular Probes). Data are presented as picograms of TNF-α/ml.
Cell Viability Assay for Hypothalamic Cells
mHypoA-1/2 cells were seeded in a 96 well plate at 5×103 cells per well overnight. Amicon Filters (Millipore) were used to remove PA and OXA and concentrate conditioned media (supernatant from microglial cultures described above) [24]. Concentrated conditioned media was used at a six-fold concentration and added to mHypoA-1/2 cells for 24 h. Time points were based on previously described studies [14, 25, 26]. Cell survival was determined using a resazurin-based assay (Presto Blue, Invitrogen) producing a fluorescent signal [5]. Activity was determined using a spectrophotometer (SpectraMax-M5) and presented as percent relative fluorescence units (RFU) change vs. control.
Statistical Methods
Significance differences were determined by unpaired, two-tailed t-tests using Graph Pad Prism 5.
Results
Palmitic acid activates BV2 microglia via TLR-4
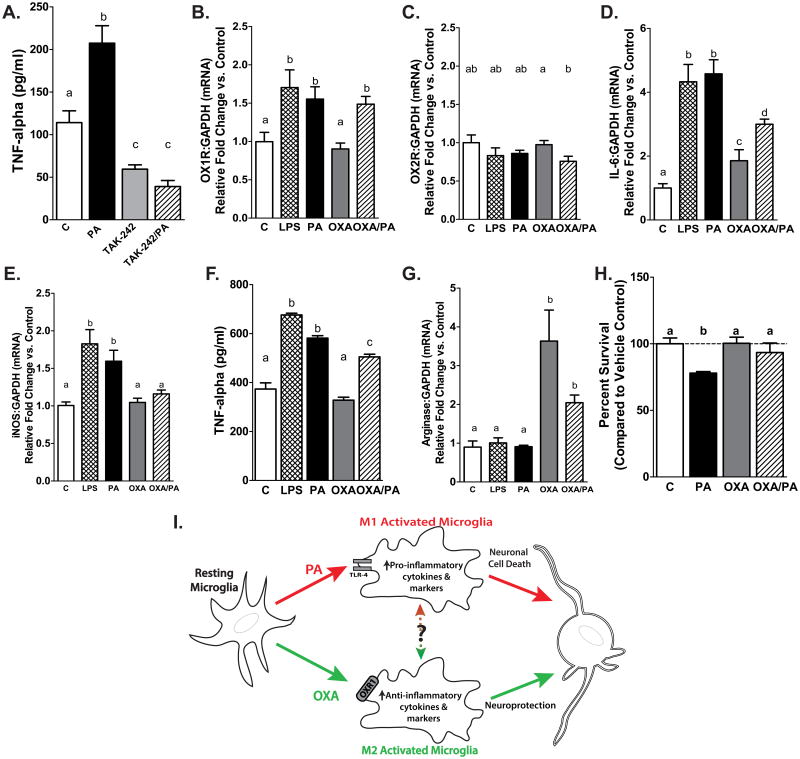
To verify that PA induces pro-inflammatory cytokine release from microglial cells through activation of TLR-4 receptor, BV2 microglial cells were treated with the TLR-4 inhibitor TAK-242 (or vehicle control; C) in the presence or absence of PA for 4 h. As expected, PA increased TNF-α secretion by 90 percent compared to control, by 320 percent vs. TAK-242 only, and by 400 percent vs. TAK-242 plus PA (p<0.001 vs. C, p<0.0001 vs TAK-242 and TAK-242 plus PA). Treatment with TAK-242 significantly reduced endogenous TNF-α production. Further, TAK-242 attenuated PA induced TNF-α secretion. (Fig. 1A)
Figure 1. (A) Palmitic acid activates microglia via TLR-4.
TNF-α secretion from microglial BV2 cells increases following 4 h PA exposure. Inhibiting TLR-4 attenuates PA–induced TNF-α secretion. Microglia treated with TLR-4 inhibitor (TAK-242) have significantly reduced TNF-α secretion (p<0.001 PA vs. vehicle control (C), p<0.0001 PA vs. TAK-242, and TAK-242+PA, p<0.001 C vs. TAK-242 and TAK-242+PA). (B-C) Palmitic acid increases orexin 1 receptor expression. Microglia exposed to PA or LPS show increased OX1R expression (B) but not OX2R expression (C). Pretreatment of microglia with OXA before PA challenge increased OX1R expression and reduced OX2R expression. Different letters above bars represent statistical significance at p<0.05 OXA vs. OXA/PA. (D) Orexin A suppresses pro-inflammatory IL-6 expression in microglial cells. Pro-inflammatory marker IL-6 expression is increased in BV2 microglia following PA and LPS exposure. Pretreatment of microglia with OXA before PA challenge reduces IL-6 expression compared to microglia exposed to PA only, but remains increased relative to vehicle (p<0.05 C vs. OXA, p<0.001 LPS vs. OXA and OXA/PA, p<0.001 PA vs. OXA and OXA/PA p<0.0001 C vs. LPS, PA, and OXA/PA). (E) Orexin A suppresses pro-inflammatory iNOS expression. Palmitic acid and LPS increase microglial iNOS expression, while OXA pretreatment attenuates PA-induced iNOS expression (PA and LPS p<0.001 vs control, p<0.05 vs OXA and OXA/PA). (F) Orexin A reduces TNF-α secretion from BV2 microglial cells. PA and LPS treatment increase TNF-α secretion compared to vehicle– or OXA only–treated microglia. Pretreatment of microglia with OXA before PA challenge reduces TNF-α secretion compared to PA only– but not vehicle–treated microglia. Different letters above bars represent statistical significance at p<0.0001. (G) Orexin A increases M2 marker arginase-1 gene expression. Pretreatment with OXA prior to PA challenge increases arginase-1 gene expression (OXA/PA p<0.001 vs. control, p <0.05 vs. PA and LPS). OXA alone increases arginase-1 expression in microglial cells (p <0.001 OXA vs. control, PA, and LPS). (H) Orexin A attenuates hypothalamic neuronal cell death in microglial-conditioned media. Adult hypothalamic cells have increased cell death following 24 h exposure to conditioned media from microglia stimulated with PA. Hypothalamic cells exposed to conditioned media from microglia pretreated with OXA before PA challenge, or vehicle-treated microglia, have reduced cell death compared to those exposed to media from PA-challenged microglia. p<0.0001 Vehicle vs. PA, p<0.001 PA vs. OXA/PA, p<0.0001 PA vs. OXA (I) Hypothesized Orexin A microglial immunomodulation pathway. We hypothesize that OXA reduces M1 microglial activation and increase M2 microglial activation to maintain neuronal survival. Saturated fatty acid challenge (PA) induces an M1 microglial phenotype and the release of pro-inflammatory cytokines, contributing to neurodegeneration. Orexin A may influence proportion of M1 microglia by reducing rate of activation or potentially by aiding in conversion between M1 and M2 states.
Palmitic acid increases expression of microglial orexin 1 receptor
To determine if PA increases microglial OX1R expression in our model, we pretreated BV2 microglia for 1 h with either OXA or vehicle control. Following pretreatment, microglia were exposed to PA, LPS, or vehicle for 4 h. Palmitic acid and LPS increased expression of OX1R (Fig. 1B; p<0.05 vs. control and OXA only) by 55 and 75 percent respectively, but not OX2R (Fig. 1C) in microglial cells following 4 h exposure.
Orexin A suppresses pro-inflammatory markers and increases expression of anti-inflammatory M2 marker arginase-1arginase-1 in microglial cells
Gene expression of the pro-inflammatory cytokine IL-6 is increased by 3.5 fold following PA and LPS exposure (Fig. 1D; p<0.0001 vs. control, p<0.001 vs. OXA only). Further, OXA pretreatment prior to PA challenge reduced IL-6 expression by 50 percent compared to PA only (Fig. 1D; p<0.0001 vs. control, p<0.001 vs. OXA only). Expression of pro-inflammatory marker iNOS is also increased following PA and LPS exposure by 60 and 75 percent respectively (Fig. 1E; p<0.001 vs control, p<0.05 vs OXA and OXA/PA). Conversely, OXA pretreatment attenuated PA–induced increase of iNOS expression (Fig. 1E). To test whether OXA influences TNF-α secretion in microglia, BV2 cells were pretreated with OXA or vehicle and challenged with PA, LPS, or vehicle as described above. An ELISA for TNF-α in the supernatant showed that PA and LPS treatment increased TNF-α secretion by 90 percent compared to vehicle control (p<0.0001) or OXA treatment only (p<0.0001) (Fig. 1F). Microglia treated with OXA plus PA reduced TNF-α secretion by 20 percent compared to PA only, but not to vehicle control (Fig. 1F; p<0.0001 vs. control and OXA only). As shown in Fig. 1G, OXA increased arginase-1 gene expression by 250 percent (p<0.001 vs. control, p <0.05 vs. PA and LPS). Notably, OXA pretreatment prior to PA challenge also increased arginase-1 expression by 100 percent (p<0.001 vs. control, PA, and LPS).
Orexin A attenuates hypothalamic neuronal cell death in microglial-conditioned media
Exposure to conditioned media from PA–treated microglia induces neuronal cell death due to the release of pro-inflammatory cytokines and other inflammatory factors [14]. We tested whether OXA treatment could affect inflammatory properties of conditioned media from PA-challenged microglia. Hypothalamic cells exposed to conditioned media from microglia treated with OXA only or pretreated with OXA and challenged with PA showed increased viability compared to hypothalamic cells exposed to conditioned media from PA–activated microglia (Fig. 1H; p<0.0006 vs. OXA, p<0.05 vs. OXA/PA). Further, cells exposed to conditioned media from PA–activated microglia had increased cell death compared to vehicle control (Fig. 1H; p=0.0005).
Discussion
Microglia are vital to neuronal health by maintaining a favorable microenvironment within the CNS [27, 28]. Communication between neurons, microglia, and other CNS cells is highly dynamic and responsive to environmental stimuli. This balance can be disturbed if microglial activation state tips toward a chronic inflammatory phenotype, as is observed in obesity [15, 29, 30]. An important unanswered question is whether it is the high fat diet or obesity itself that directly induces microglial activation. High fat diets and obesity, either independently or synergistically, have the same consequences: microglial activation and prolonged circulation of pro-inflammatory cytokines [15, 29, 30]. Our data confirm prior reports indicating PA induces microglial cytokine production through a TLR-4 dependent pathway (Fig. 1A) [14]. We have shown that OX1R expression is increased in microglia challenged with PA or LPS (Fig. 1B). To the best of our knowledge, this is the first report demonstrating that PA increases OX1R expression in microglia. Our data also suggest that orexin can alter the activation state of microglia, reducing microglial M1 pro-inflammatory state by promoting the conversion to M2 phenotype, as OXA pretreatment reduced microglial M1 pro-inflammatory response during PA challenge (Fig. 1D-F). Consistent with previous reports [8], these data support that OXA can act as an immunomodulatory regulator of microglia. Most importantly, we show for the first time both that hypothalamic cells exposed to conditioned media from OXA and OXA/PA treated microglia have increased cell survival compared to those exposed to media from PA-activated microglia without orexin, and that OXA pretreatment may shift microglia to an M2 protective phenotype (Fig. 1G-H). Our results demonstrate OXA modulates microglial activation states in response to PA exposure by reducing pro-inflammatory cytokine release and increasing anti-inflammatory markers.
In microglia activated by PA, addition of OXA significantly reduced iNOS expression. Nobunaga et al. demonstrated that microglial derived iNOS contributed to a loss of orexin producing neurons and subsequent metabolic dysfunction in mice fed a high fat diet [31]. Reduction of iNOS expression due to OXA modulation in our study could therefore help in maintaining a favorable environment for surrounding cells. Likewise, conditioned media from PA-activated microglia did not induce hypothalamic cell death when microglia were pretreated with OXA prior to PA challenge. It is plausible that increasing the pretreatment time with OXA prior to PA exposure could further reduce pro-inflammatory cytokine release.
Our findings that PA increases OX1R expression in microglial cells are consistent with previous findings indicating that the pro-inflammatory stimuli LPS also increases OX1R expression [8]. Increased microglial OX1R expression in response to a TLR-4 mediated pro-inflammatory stimulus could represent a compensatory response to reduce the release of inflammatory cytokines. Others have demonstrated that astrocytes, a subset of glial support cells, are responsive to OXA through OX1R by increasing migration following OXA exposure, further indicating OXA is modulatory not only through neuronal cells but also glial cells [32]. Until recently, microglia were thought to be passive support cells, but are now understood to contribute to fine-tuning neural-glial circuitry through cultivating synapses and altering plasticity in healthy and diseased brains [33, 34]. Additionally, microglia maintain the brain microenvironment through phagocytizing debris, pathogens, dead cells, and misfolded proteins. In neurological disorders, microglia can be chronically activated to M1 phenotypes, resulting in an unfavorable environment for neuronal networks [35, 36]. Here we lay the groundwork for understanding how OXA can modulate microglia responses to promote neuronal survival. Performing a more complete profile of other pro– and anti–inflammatory cytokines in future studies could provide insight into how priming microglia with OXA can provide a favorable brain microenvironment and maintain neuronal health. While we do not yet fully know how OXA modulates microglia, one mechanism may be through shifting microglia towards an M2 phenotype, or at least slowing rate of conversion to an M1 state. In other studies of acute neuroinflammatory responses, microglia activated to an M2 protective phenotype, including increased arginase-1 expression, showed reduction in neuronal injury and inflammation [37-40]. Promotion of M2 microglial function has been demonstrated to either support or enhance neural-glial cross talk via increases in the microglial CX3CR1 (fractalkine) receptor [41]. Fractalkine receptor increase has not been evaluated with respect to OXA stimulation, but merits further research.
In summary, these data support the hypothesis that orexin influences the M1/M2 activation state of microglia during challenge with SFA, reducing pro-inflammatory cytokine release to maintain neuronal survival in the surrounding microenvironment (Fig. 1). Future work will examine the implications of orexin on microglial dynamics in the context of diet and obesity. In other inflammatory diseases, microglia have been profiled to gain a better understanding of disease status [42]. Chronic microglial activation in response to high fat diet and overnutrition has yet to be fully characterized. Delineating microglial phenotypes in the hypothalamus in response to chronic high fat diet could be useful in appreciating the neuropathology and the development of obesity. Understanding the long and short term effects of PA-activated microglia and OXA-mediated immunomodulation could lead to therapies for maintaining neuronal networks involved in regulating healthy metabolism.
Highlights.
Palmitic acid (PA) increases microglial orexin receptor 1 expression.
PA increases microglial pro-inflammatory cytokine release.
Orexin A reduces microglial pro-inflammatory cytokine release.
Orexin A attenuates hypothalamic cell death induced by PA-activated microglia.
Orexin A may function as an immunomodulatory regulator of microglia.
Acknowledgments
We thank Dr. Dongsheng Cai (Albert Einstein College of Medicine) for his insightful comments on a prior version of this manuscript. We thank Drs. Philippe Marambaud (Feinstein Institute for Medical Research, Manhasset, NY) and Weihua Zhao (Methodist Hospital, Houston, TX) for providing the BV-2 cell line. Funding provided by the Department of Veterans Affairs BLR&D BX001686 (TAB) and RR&D RX000441 (CMK), NIDDK R01 DK100281 (CMK), and the University of Minnesota Healthy Foods, Healthy Lives Institute (CMD).
Abbreviations
- PA
palmitic acid
- TNF-α
tumor necrosis factor alpha
- NFκB
nuclear factor-kappa B
- OXA
orexin A/hypocretin 1
- OX1R
orexin receptor 1
- OX2R
orexin receptor 2
- IL-6
interleukin 6
- LPS
lipopolysaccharide
- TLR-4
toll like receptor 4
- RT-PCR
real time polymerase chain reaction
- RFU
relative fluorescence units
- ELISA
enzyme-linked immunosorbent assay
- BSA
bovine serum albumin
References
- 1.de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Butterick TA, et al. Orexin: Pathways to obesity resistance? Rev Endocr Metab Disord. 2013;14(4):357–64. doi: 10.1007/s11154-013-9259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotz C, et al. Brain orexin promotes obesity resistance. Ann N Y Acad Sci. 2012;1264:72–86. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butterick TA, et al. Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neurosci Lett. 2012;524(1):30–4. doi: 10.1016/j.neulet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokolowska P, et al. Orexins protect neuronal cell cultures against hypoxic stress: an involvement of Akt signaling. J Mol Neurosci. 2014;52(1):48–55. doi: 10.1007/s12031-013-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokolowska P, et al. Orexins promote survival of rat cortical neurons. Neurosci Lett. 2012;506(2):303–6. doi: 10.1016/j.neulet.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Xiong X, et al. Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke. 2013;44(3):764–70. doi: 10.1161/STROKEAHA.112.681700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–70. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 10.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217–24. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9(7):568–78. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab. 2013;24(1):40–7. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–62. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, et al. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signalling. Br J Nutr. 2012;107(2):229–41. doi: 10.1017/S0007114511002868. [DOI] [PubMed] [Google Scholar]
- 15.Valdearcos M, et al. Microglia Dictate the Impact of Saturated Fat Consumption on Hypothalamic Inflammation and Neuronal Function. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasi E, et al. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. Journal of neuroimmunology. 1990;27(2-3):229–37. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 17.Belsham DD, et al. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J. 2009;23(12):4256–65. doi: 10.1096/fj.09-133454. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Feng P. OX2R activation induces PKC-mediated ERK and CREB phosphorylation. Exp Cell Res. 2012;318(16):2004–13. doi: 10.1016/j.yexcr.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pike LS, et al. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807(6):726–34. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga N, et al. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79(1):34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 21.Takashima K, et al. Analysis of binding site for the novel small-molecule TLR4 signal transduction inhibitor TAK-242 and its therapeutic effect on mouse sepsis model. Br J Pharmacol. 2009;157(7):1250–62. doi: 10.1111/j.1476-5381.2009.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15(3):532–4. 536–7. [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Colella M, et al. Palmitic acid is associated with halorhodopsin as a free fatty acid. Radiolabeling of halorhodopsin with 3H-palmitic acid and chemical analysis of the reaction products of purified halorhodopsin with thiols and NaBH4. Biochim Biophys Acta. 1998;1370(2):273–9. doi: 10.1016/s0005-2736(97)00276-9. [DOI] [PubMed] [Google Scholar]
- 25.Taylor DL, et al. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci. 2005;25(11):2952–64. doi: 10.1523/JNEUROSCI.4456-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pais TF, et al. Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflammation. 2008;5:43. doi: 10.1186/1742-2094-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wake H, et al. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Purkayastha S, Cai D. Hypothalamic microinflammation: a common basis of metabolic syndrome and aging. Trends Neurosci. 2015;38(1):36–44. doi: 10.1016/j.tins.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nobunaga M, et al. High fat diet induces specific pathological changes in hypothalamic orexin neurons in mice. Neurochem Int. 2014;78:61–6. doi: 10.1016/j.neuint.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Shu Q, et al. Orexin-A promotes cell migration in cultured rat astrocytes via Ca2+-dependent PKCalpha and ERK1/2 signals. PLoS One. 2014;9(4):e95259. doi: 10.1371/journal.pone.0095259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 34.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 35.Nolan YM, Sullivan AM, Toulouse A. Parkinson's disease in the nuclear age of neuroinflammation. Trends Mol Med. 2013;19(3):187–96. doi: 10.1016/j.molmed.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Olson EE, McKeon RJ. Characterization of cellular and neurological damage following unilateral hypoxia/ischemia. J Neurol Sci. 2004;227(1):7–19. doi: 10.1016/j.jns.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Guerrero AR, et al. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9:40. doi: 10.1186/1742-2094-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-de Puig I, et al. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J Cereb Blood Flow Metab. 2013;33(12):1955–66. doi: 10.1038/jcbfm.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chhor V, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain, behavior, and immunity. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2015 doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilcock DM. Neuroinflammatory Phenotypes and Their Roles in Alzheimer's Disease. Neuro-degenerative diseases. 2013 doi: 10.1159/000354228. [DOI] [PubMed] [Google Scholar]