Abstract
Objective
Obesity-associated reduction in testosterone is based on the effect of high estradiol from an increase in aromatase activity present in the abundant adipose tissue. The resulting hyperestrogenemia suppresses the hypothalamic-pituitary-gonadal unit resulting in low testosterone production. Although weight loss has been found to be effective in reducing estradiol and raising testosterone levels in studies of younger men, its effect in frail obese older men is understudied. Thus, objective of this study was to determine the effect of lifestyle intervention on hormone levels in frail obese older men.
Design
Randomized controlled study of lifestyle intervention in frail, obese older men (≥65 yo) for 52 weeks.
Setting
University hospital.
Methods
Forty frail, obese elder men were randomized, for a 52-week (12 months) study, to any of the following treatment groups: (1) control group, (2) diet-induced weight loss group (diet group), (3) ET group (exercise group), and (4) diet-induced weight loss and ET group (diet–exercise group). The objective was to achieve a ~10 % weight loss at 6 months and maintain this weight for an additional 6 months. Physical function was assessed by the modified physical performance testing (modified PPT). Estradiol was measured by radioimmunoassay, testosterone by automated immunoassay, and sex hormone-binding globulin by enzyme-linked immunoassay.
Results
After 12 months of intervention, diet alone resulted in a weight loss of −10.1 ± 1.9 kg (8.5%) in the diet alone group and −9.1 ± 0.9 kg (9.2%) in the diet-exercise group. This resulted in a significant decrease (both p<0.05) in total estradiol compared to baseline among subjects in the diet (−2.5 ± 1.3 pg/ml) and diet-exercise group (−2.2 ± 4.0 pg/ml). Free estradiol also significantly decreased (both p <0.05) in both diet (−0.39 ± 0.14 pmol/nmol) and diet-exercise (−0.52 ± 0.12 pmol/nmol). Total testosterone significantly increased (p<0.05) among the diet (71.0 ± 21.0 ng/dl) and diet-exercise (49.9 ± 15.5 pg/ml) resulting in values of 287.0 ± 28.1 ng/dl in the diet and 317.6 ± 33.1 ng/dl in the diet-exercise group. However, because there was a significant increase in sex hormone-binding globulin in both diet and diet-exercise, free testosterone index and the changes in free testosterone index were not significant compared to baseline. Regardless of changes in hormonal levels, patients in the diet, exercise, and diet-exercise groups experienced significant improvement in the modified PPT from baseline.
Conclusion
Weight loss from lifestyle intervention resulted in significant decrease in total and free estradiol in frail obese older men, but this did not result in clinically important increase in total testosterone nor a significant increase in free testosterone. Thus, alternative forms of treatment in addition to lifestyle intervention may be necessary to improve the hormonal profile among these patients. However, whether further improvement in hormonal profile would result in better physical performance than what can be achieved by lifestyle alone in these subjects, remains uncertain.
Keywords: Obesity, testosterone, diet, exercise
INTRODUCTION
Obesity is an increasing problem worldwide and is one of the major contributors to burgeoning health care cost in the US (1). Obesity is associated with a host of complications mostly impacting mortality such as the metabolic syndrome, diabetes mellitus (and its complications), hypertension, heart attacks and strokes (2). As a result, the primary focus of medical care in these patients is the prevention and treatment of most of the above conditions, yet a great majority of these patients also have obesity-associated problems that may affect quality of life such as osteoarthritis, frailty which impacts independence (2;3), and hypogonadism (4;5).
The mechanism for obesity-associated hypogonadism is based on the increase in aromatase activity in the expanded adipose tissue volume resulting in increased conversion of androgen precursors to estrogens leading to hyperstrogenemia (6;7). In turn the high levels of estrogen suppress the hypothalamic pituitary unit resulting in lower levels of gonadotropins, hence less production of testosterone from the testes (5;8). Given that the main mechanism of low testosterone production is not because of testicular failure but the presence of excess adipose tissues (5;8;9), it follows that reduction in fat mass should be the primary approach of improving the hormonal status of these men (10).
Several studies have shown an improvement in hormonal profile by weight loss from lifestyle intervention in obese men (10-14). Weight loss interventions in these studies vary from study to study with some using very low calorie diet only while others combined with exercise and most with no pre-specified target weight loss resulting in great success in certain studies compared to others (10). However, most of these studies were done in relatively healthier younger men. Very little is known as to whether weight loss would also result in improvement in hormonal profile in frail elderly obese who may have limited capacity to exercise. Therefore, the objective of this study was to evaluate the effect of supervised lifestyle intervention on the hormonal levels of frail obese older men participating in a lifestyle intervention study (15). This study was designed to promote 10% of loss of body weight in the first six months and weight maintenance in the succeeding six months.
METHODS
Study population
This study was a secondary analysis of data on the male participants from a previous study on the effect of lifestyle intervention on physical function in obese older adults (15). This study was done in accordance with the guidelines in the Declaration of Helsinki for the ethical treatment of human subjects. It was conducted at Washington University School of medicine and was approved by the Institutional Review Board. The participants were recruited through advertisements and written informed consent was obtained from each subject. Eligibility criteria included: 1) older age (≥65 years), 2) obese (BMI ≥ 30 kg/m2), 3) sedentary lifestyle (regular exercise <1 h/wk or <2 x/wk for the last 6 months), 4) stable body weight (±2 kg) over the past year, and 5) on stable medications for 6 months before enrollment. In addition, all participants were required to have mild-moderate frailty determined by meeting at least two operational criteria: modified physical performance test (PPT) score of 18–32, peak oxygen consumption (VO2peak) of 11–18 ml ml/kg, or difficulty in performing two instrumental activities of daily living (ADL) or one basic ADL (3). The effects of diet, exercise and combination of diet and exercise (diet-exercise) on measures of frailty and physical function, body composition and bone mineral density, and cognition and quality of life on these subjects were reported previously (15-18). The present study reports the effects of diet, exercise and diet-exercise on the changes in serum hormone levels among male participants in the study.
Study design
The participants were randomized, for a 52-week study, to any of the following treatment groups: (1) control group, (2) diet-induced weight loss group (diet group), (3) ET group (exercise group), and (4) diet-induced weight loss and ET group (diet–exercise group). Details regarding the intervention strategies have been described previously (15). Briefly, subjects in the control group were advised to continue their usual dietary or activity habits. The diet group was recommended a diet that would provide an energy deficit of 500 to 750 kcal/day from their daily energy requirement and asked to meet weekly as a group for adjustments of calorie intake and behavioral therapy with a dietitian. Their food diaries were reviewed and new goals were set based on diary reports. The objective was to achieve a ~10 % weight loss at 6 months and maintain this weight for an additional 6 months. The subjects in the exercise group were asked to maintain a weight-stable diet while participating in a multicomponent ET program supervised by a physical therapist. Each session was approximately 90 min in duration: 15 min of flexibility exercises, 30 min of aerobic exercises, 30 min of progressive resistance training, and 15 min of balance exercises. Subjects in the diet–exercise group participated in both weight management and ET programs described above. All subjects were provided supplements to ensure an intake of ~1,500 mg of calcium/day and ~1,000 IU vitamin D/day.
Biochemical measurements
Blood samples were obtained in the morning after subjects fasted for at least 12-hours. Serum samples were extracted and stored at −80°C until analysis. Enzyme-linked immunosorbent assay kits were used to measure sex-hormone binding globulin (SHBG) (Alpco Diagnostics, Salem, NH). Radioimmunoassay kits were used to measure serum estradiol (Ultra-sensitive estradiol DSL-4800; Diagnostic Systems Laboratories Inc., Webster, Tex), while serum total testosterone was measured by automated immunoassay (VITROS® 5600, Raritan, NJ). The coefficient of variation for these assays were <10%. The free estradiol index was calculated as the molar ratio of total estradiol to SHBG (pmol/nmol) (19). The free testosterone index was calculated by using the formula previously described by Sowers et al. (20): Free testosterone index = 100 × testosterone (ng/dl)/28.84 × SHBG (20)
Statistical Analyses
The outcomes for this report were the changes in testosterone, estradiol and sex hormone binding globulin from baseline at months 6 and 12. Intention-to-treat analyses were performed with inclusion of all participants who provided any data after baseline. Baseline characteristics were compared using analyses of variance or Fisher's exact tests. A three-factor, repeated measures analysis of variance, adjusting for baseline values, was used to determine the effects of diet and exercise on the outcome variables, as well as to determine any interaction between diet and exercise. Analyses testing for within-group changes also were performed using mixed-model repeated-measures analyses of variance. Pearson's correlation was used to examine relationships among changes in selected variables. Data are presented as mean ± SE. P≤0.05 was considered statistically significant. All data were analyzed using SAS software, version 9.3.
RESULTS
Our study population consisted of 40 frail obese older men with a mean age of 69.3 ± 4.1 years old, mean BMI of 36.8 ± 4.6 kg/m2 and mean physical performance score of 27.9 ± 2.7. Table I shows the age, baseline BMI, weight, waist circumference and physical function of our study population and the changes at 6 months and 1 year. There were no significant differences in these baseline characteristics among the groups. Over the one year period, the diet intervention resulted in significant decreases in body weight and waist circumference whereas the exercise intervention had no effect. Thus, within the intervention groups, there were also significant decreases in body weight and waist circumference in the diet and diet-exercise groups but not in the exercise and control groups. On the other hand, both the diet intervention and the exercise intervention resulted in a significant increase in the physical performance test score without an interaction effect between diet and exercise. Therefore, within the intervention groups, there was also a significant increase in the PPT score in the diet, exercise, and diet-exercise groups but not in the control group.
Table 1.
Baseline Characteristics and Changes in Weight and Physical Function with Lifestyle Therapy
| Control (n = 9) | Diet (n = 9) | Exercise (n = 10) | Diet-exercise (n = 12) | Effect of Exercise | P Value Effect of Diet | Interaction | |
|---|---|---|---|---|---|---|---|
| Age (years) | 68.7 ± 1.1 | 68.3 ± 1.3 | 71.5 ± 1.6 | 68.7 ± 1.3 | |||
| Body Mass Index (kg/m2) | 37.5 ± 1.9 | 39.1 ± 1.5 | 35.9 ± 0.9 | 35.1 ± 1.5 | |||
| Body Weight (kg) | |||||||
| Baseline | 112.1 ± 5.2 | 118.8 ± 3.7 | 109.7 ± 3.3 | 104.2 ± 6.1 | |||
| 6 months | 109.2 ± 5.4 | 108.1± 5.4* | 109.9 ± 3.0 | 96.8 ± 6.6* | |||
| 1 year | 112.7 ± 5.8 | 108.6 ± 5.8* | 109.9 ± 3.3 | 95.9 ± 5.7* | |||
| Δ 1 year | 0.6 ± 1.6 | −10.1 ± 1.9 | −0.2 ± 1.4 | −9.1 ± 0.9 | 0.20 | <0.001 | 0.59 |
| Waist Circumference (cm) | |||||||
| Baseline | 121.5 ± 3.2 | 125.0 ± 2.1 | 121.3 ± 2.5 | 120.0 ± 5.0 | |||
| 6 months | 121.6 ± 3.6 | 118.0 ± 2.0* | 121.7 ± 3.0 | 114.7 ± 6.1 | |||
| 1 year | 122.7 ± 4.2 | 116.2 ± 2.4* | 121.0 ± 2.0 | 110.1 ± 5.9* | |||
| Δ 1 year | 1.2 ± 2.3 | −8.8 ± 1.5 | 0.7 ± 1.3 | −9.9 ± 2.6 | 0.71 | 0.01 | 0.84 |
| Physical Performance Test | |||||||
| Baseline | 27.1 ± 1.1 | 29.0 ± 2.2 | 27.5 ± 1.0 | 28.3 ± 0.8 | |||
| 6 months | 27.8 ± 1.1 | 31.1 ± 0.8* | 30.7 ± 1.1* | 31.9 ± 1.2* | |||
| 1 year | 27.3 ± 1.0 | 32.0 ± 06* | 31.4 ± 1.1* | 33.4 ± 1.0* | |||
| Δ 1 year | 0.2 ± 0.7 | 3.0 ± 0.4 | 3.9 ± 0.6 | 5.1 ± 0.7 | 0.00 | 0.03 | 0.57 |
Values are mean ± SE
P<0.05 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analyses of variance.
Mean testosterone levels at baseline were below 300 ng/dl level set by the Endocrine Society as cut-off for diagnosis of hypogonadism in all the groups (21). Over the one year period, the diet intervention resulted in a significant increase in testosterone level and a significant decrease in estradiol level, whereas exercise had no effect on these two hormones (Table 2 and Figure 1). Accordingly, within the intervention groups, there was also a significant increase in testosterone level and a significant decrease in estradiol level in the diet and diet-exercise groups but not in the exercise and control groups (Table 2). However, because the increases in testosterone levels were modest, the mean levels of testosterone in the diet and diet-exercise groups remained below the below 300 ng/dl (i.e. 287.0 ± 28.1 ng/dl) in the diet and in the low 300s in the diet-exercise group (i.e. 317.6 ± 33.1 ng/dl).
Table 2.
Baseline, Follow-up, and Changes in Testosterone and Estradiol with Lifestyle Therapy
| Control (n = 9) | Diet (n =9) | Exercise (n =10) | Diet-exercise (n=12) | Effect of Exercise | P Value Effect of Diet | Interaction | |
|---|---|---|---|---|---|---|---|
| Testosterone (ng/dL) | |||||||
| Baseline | 271.3 ± 32.8 | 211.1 ± 47.1 | 273.8 ± 45.4 | 267.7 ± 36.1 | |||
| 6 months | 278.6 ± 36.6 | 261.4 ± 26.5* | 284.0 ± 57.3 | 319.1 ± 50.5* | |||
| 1 year | 229.0 ± 27.3 | 287.0 ± 28.1* | 257.1 ± 52.7 | 317.6 ± 33.1* | |||
| Δ 1 year | −42.7 ± 11.9 | 71.0 ± 21.0 | −22.6 ± 44.2 | 49.9 ± 15.5 | 0.96 | 0.01 | 0.64 |
| Estradiol (pg/mL) | |||||||
| Baseline | 17.8 ± 1.8 | 15.6 ± 1.2 | 16.3 ± 1.6 | 17.5 ± 0.9 | |||
| 6 months | 17.3 ± 1.8 | 13.7 ± 0.9* | 16.1 ± 1.7 | 13.5 ± 1.2* | |||
| 1 year | 17.2 ± 2.0 | 13.1 ± 1.1* | 14.9 ± 1.7 | 14.8 ± 1.2* | |||
| Δ 1 year | −0.5 ± 1.5 | −2.5 ± 1.3 | −0.7 ± 1.1 | −2.2 ± 4.0 | 0.51 | 0.04 | 0.46 |
| SHBG (nmol/L) | |||||||
| Baseline | 49.7 ± 11.1 | 38.8 ± 4.8 | 43.9 ± 5.2 | 46.6 ± 24.9 | |||
| 6 months | 51.4 ± 12.3 | 45.4 ± 8.2 | 46.8 ± 6.3 | 43.9 ± 22.5 | |||
| 1 year | 50.9 ± 12.1 | 45.9 ± 6.6 | 46.9 ± 7.5 | 57.9 ± 31.6 | |||
| Δ 1 year | 2.2 ± 1.5 | 7.1 ± 3.7 | 2.5 ± 2.5 | 7.5 ± 8.2 | 0.97 | 0.03 | 0.27 |
| Free Testosterone Index | |||||||
| Baseline | 24.5 ± 3.2 | 20.5 ± 2.7 | 23.2 ± 4.1 | 24.1 ± 4.0 | |||
| 6 months | 31.0 ± 10.7 | 20.7 ± 4.2 | 23.5 ± 5.5 | 25.2 ± 4.6 | |||
| 1 year | 20.6 ± 3.2 | 23.5 ± 6.4 | 21.7 ± 4.4 | 22.9 ± 3.8 | |||
| Δ 1 year | −3.9 ± 1.0 | 3.3 ± 2.3 | −2.4 ± 1.0 | −1.2 ± 2.6 | 0.59 | 0.43 | 0.52 |
| Free Estradiol Index | |||||||
| Baseline | 1.66 ± 0.24 | 1.64 ± 0.29 | 1.54 ± 0.25 | 2.01 ± 0.52 | |||
| 6 months | 1.94 ± 0.60 | 1.36 ± 0.26* | 1.48 ± 0.27 | 1.18 ± 0.14* | |||
| 1 year | 1.66 ± 0.36 | 1.25 ± 0.27* | 1.32 ± 0.22 | 1.13 ± 0.19* | |||
| Δ 1 year | 0.00 ± 0.15 | −0.39 ± 0.14 | 0.06 ± 0.07 | −0.52 ± 0.12 | 0.41 | 0.04 | 0.65 |
Values are mean ± SE; SHBG = Sex-Hormone Binding Globulin
P<0.05 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analyses of variance.
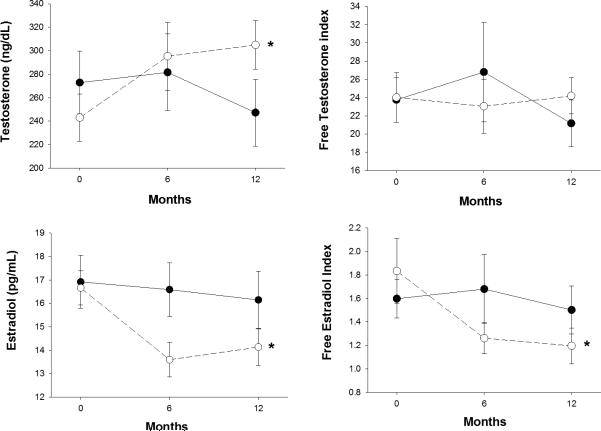
Figure 1.
Changes in testosterone, free testosterone index, estradiol, and free estradiol index according to the presence or absence of diet. Open circles (diet or diet-exercise group). Closed circles (exercise or control group). *Significant effect of diet; P <0.05. Values are mean ± SE.
The diet intervention resulted in a significant increase in SHBG whereas the exercise intervention had no effect on SHBG (Table 2). Consequently, adjustment for the changes in SHBG showed that the diet intervention had no effect on the free testosterone index (Table 2 and Figure 2) similar to the exercise intervention. By contrast, with adjustment for the changes in SHBG, the diet intervention significantly decreased levels of the free estradiol index (Figure 2) resulting in a significant decrease in free estradiol index in the diet and diet-exercise groups but not in the exercise and control groups.
Simple correlation analysis showed no significant correlation between the changes in testosterone or any of the hormones and the changes in PPT either at 6 and 1 year of intervention in either the entire group or limited to the active intervention groups alone (diet, exercise, and diet-exercise groups).
DISCUSSION
Our study showed that despite diet-induced weight loss of ~10% over a one year period, the increase in testosterone level was minimal in this population of frail, obese older men and not enough to raise it to the normal mid-eugonadal range and may even remain below the lower limit of normal (300 ng/dl) set by the Endocrine Society (21). In fact, after adjusting for changes in SHBG, there was no significant change in free testosterone index. However, diet-induced weight loss resulted in a significant decrease in estradiol and free estradiol index. The above findings suggest that weight loss by itself is not an effective method for producing clinically significant improvement in the androgen hormone profile of frail, obese older men.
The syndrome of obesity-associated hypogonadism has been recognized for sometime yet, the treatment of this condition remains controversial. Because hypogonadism in these patients is based on the concept that the high estrogen levels resulting from increase aromatase activity in the large adipose tissue volume results in suppression of gonadotropin production and consequently testosterone by the testis (8;11), reduction in adipose tissue volume appears to be the most appropriate approach. Nevertheless, testosterone replacement remains the standard of therapy for hypogonadism irrespective of body weight (21).
One of the very first studies investigating the effect of weight loss on the hormonal profiles of obese men was that of Stanik et.al. In a group of obese men prescribed a very low caloric diet (320 cal/day) for 8 weeks or until ideal body weight (12), these investigators were able to demonstrate a significant increase in both total and free T accompanied the significant decrease in total and free estradiol after an average weight loss of 19.5 kg with a reduction in obesity from 54±4.2% to 27±3.8% above ideal body weight (12). However, the degree of caloric restriction in this study would be impossible to incorporate in long-term lifestyle changes. Another study among 11 otherwise “healthy” markedly obese men (100 to 305% above IBW), caloric restriction using individualized dietary protocol resulted in massive weight loss (53±36 kg, range: 26-130 kg) after a mean follow-up period of 17 months (11). There was a significant increase in FSH, increase in total and free T but the values at the end of the study were still lower than the normal control subjects. There was no change in LH and no change in total and free estradiol. The authors commented that despite the massive weight loss, these patients were still obese at the end of the study accounting for the failure to significantly reduce estradiol and completely reverse hypogonadotropic hypogonadism.
More studies followed (10;13;14;22-25) which documented a consistent but variable increase in T production associated with weight loss. The meta-analysis of 24 studies by Corona et.al (10) showed that both low-caloric diet and bariatric surgery were associated with significant increases in total and unbound testosterone but bariatric surgery was more effective than low-calorie diet. They also found that the increase in testosterone was greater among those who lost more weight, and in younger, and non-diabetic subjects who had greater degree of obesity at baseline. The degree of weight loss was also found to be associated with the reduction in estradiol and was overall the best determinant of testosterone increase from the multiple regression analysis (10). Thus, although lifestyle intervention is helpful in young non-diabetic obese patients, it may not be as effective in older subjects or in severely obese individuals who may still remain obese at the end of the intervention despite weight loss. In addition, the positive effect of weight loss on testosterone could be short-lived depending on how long the patients can adhere to the prescribed diet and exercise and keep their weight loss. In a previous study, weight loss by a very low-calorie diet was followed by weight regain in the maintenance phase (14). In this study, 28/58 had low T at baseline, which was reduced to 5/57 at the end of the weight loss phase. However, 12/58 had low T once again in the maintenance phase suggesting recurrence of hypogonadism with weight regain.
In our population of frail, obese elderly, weight loss was able to produce little or modest increase in testosterone levels and little or modest reduction in estradiol levels. Hence a 10% weight loss may not be enough to improve the hormonal profile of our obese men, yet a weight loss of over 10% may also be detrimental to this age group (26). Although weight loss is generally regarded as effective and safe in younger obese subjects, it remains a controversial issue in older subjects because it may exacerbate the age-related loss of bone and muscle mass (27). As both aging and increasing adiposity are associated with a decline in testosterone levels (28-31), the problem of obesity-associated hypogonadism could even be more common in men who are both elderly and obese. Furthermore, as both aging and obesity are also associated with loss of lean/muscle mass (3;32;33) it is possible that testosterone lack may aggravate this loss of muscle mass making the elderly obese hypogonadal men at the greatest risk for worse frailty. In our previous publication, we reported that there was improvement in the physical function in patients assigned to diet alone, exercise alone and diet-exercise (15). Similar findings were obtained in this study where analysis was confined to men alone. In the absence of clinically meaningful changes in testosterone levels in this study, we assume that the positive effects of lifestyle intervention on physical function in our study is likely independent of changes in testosterone levels.
Our study has limitations. Given the limited sample size, and the type of subjects enrolled in this study (frail, obese older men), our results may not apply to the population of obese men in general. However, results from this study clarified the role of supervised weight loss from lifestyle intervention in improving testosterone levels in frail, obese older men. Furthermore, if testosterone therapy improves frailty as has been shown in a few studies (34), our results showed that regardless of the changes in testosterone in the select population of frail, obese older men improvement in physical function can be achieved by diet, exercise and diet-exercise. However, whether the combination of lifestyle intervention with exogenous testosterone (to raise levels into the mid-eugonadal range) will result in better improvement in in physical function, remains uncertain and could be the direction of future studies.
In conclusion, because our results are based on a supervised lifestyle intervention with good compliance, we can infer that weight loss is not an effective method for raising testosterone levels in frail, obese older men. Although improvement in physical function was observed with lifestyle intervention in our subjects, this occurred in the absence of a clinically important increase in testosterone level. It is possible that restoration of the physiologic anabolic milieu by raising testosterone levels to the mid-eugonadal range could further improve physical function when added to lifestyle therapy. Although a multifactorial approach (e.g. diet-exercise in combination with testosterone replacement) seems to be an attractive strategy to maximize physical function in the obese elderly with low testosterone, the efficacy of this method remains uncertain. Moreover, the benefits of raising testosterone levels has to be balanced with the risks in older men. Given the emerging concerns on the safety of testosterone replacement raised by recent publications (35;36), and the theoretical possibility of increased conversion of exogenous testosterone to estrogen in the large adipose tissue volume in obese subjects, other treatment modalities may also need some consideration either alone or in combination with weight loss.
ACKNOWLEDGEMENTS
We thank the participants and the staff of the Institute of Clinical and Translational Sciences. This study was supported by grants RO1-AG025501, RO1 AG31176, UL1-RR024992, and DK56341, DK20579. This work was supported with resources at the New Mexico VA Health Care System and Michael E DeBakey VA Medical Center.
Footnotes
The authors declare no conflicts of interest.
REFERENCE LIST
- 1.Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes. 2010;3:285–95. doi: 10.2147/DMSOTT.S7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ard J. Obesity in the US: what is the best role for primary care? BMJ. 2015;350:g7846. doi: 10.1136/bmj.g7846. [DOI] [PubMed] [Google Scholar]
- 3.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes Res. 2004;12:913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 4.Corona G, Mannucci E, Fisher AD, et al. Low levels of androgens in men with erectile dysfunction and obesity. J Sex Med. 2008;5:2454–63. doi: 10.1111/j.1743-6109.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 5.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79:997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- 6.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–8. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 7.Kley HK, Deselaers T, Peerenboom H, Kruskemper HL. Enhanced conversion of androstenedione to estrogens in obese males. J Clin Endocrinol Metab. 1980;51:1128–32. doi: 10.1210/jcem-51-5-1128. [DOI] [PubMed] [Google Scholar]
- 8.Strain GW, Zumoff B, Kream J, et al. Mild Hypogonadotropic hypogonadism in obese men. Metabolism. 1982;31:871–5. doi: 10.1016/0026-0495(82)90175-5. [DOI] [PubMed] [Google Scholar]
- 9.Amatruda JM, Hochstein M, Hsu TH, Lockwood DH. Hypothalamic and pituitary dysfunction in obese males. Int J Obes. 1982;6:183–9. [PubMed] [Google Scholar]
- 10.Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–43. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 11.Strain GW, Zumoff B, Miller LK, et al. Effect of massive weight loss on hypothalamic-pituitary-gonadal function in obese men. J Clin Endocrinol Metab. 1988;66:1019–23. doi: 10.1210/jcem-66-5-1019. [DOI] [PubMed] [Google Scholar]
- 12.Stanik S, Dornfeld LP, Maxwell MH, Viosca SP, Korenman SG. The effect of weight loss on reproductive hormones in obese men. J Clin Endocrinol Metab. 1981;53:828–32. doi: 10.1210/jcem-53-4-828. [DOI] [PubMed] [Google Scholar]
- 13.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–94. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 14.Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab. 2004;6:208–15. doi: 10.1111/j.1462-8902.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- 15.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–9. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napoli N, Shah K, Waters DL, Sinacore DR, Qualls C, Villareal DT. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am J Clin Nutr. 2014;100:189–98. doi: 10.3945/ajcn.113.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napoli N, Villareal DT, Mumm S, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res. 2005;20:232–9. doi: 10.1359/JBMR.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sowers MR, Randolph J, Jr., Jannausch M, Lasley B, Jackson E, McConnell D. Levels of sex steroid and cardiovascular disease measures in premenopausal and hormone-treated women at midlife: implications for the “timing hypothesis”. Arch Intern Med. 2008;168:2146–53. doi: 10.1001/archinte.168.19.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 22.Reis LO, Favaro WJ, Barreiro GC, et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int J Androl. 2010;33:736–44. doi: 10.1111/j.1365-2605.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 23.Hammoud A, Gibson M, Hunt SC, et al. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J Clin Endocrinol Metab. 2009;94:1329–32. doi: 10.1210/jc.2008-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo J, Piantadosi C, Worthley S, Wittert GA. Effects of a low-energy diet on sexual function and lower urinary tract symptoms in obese men. Int J Obes (Lond) 2010;34:1396–403. doi: 10.1038/ijo.2010.76. [DOI] [PubMed] [Google Scholar]
- 25.Alagna S, Cossu ML, Gallo P, et al. Biliopancreatic diversion: long-term effects on gonadal function in severely obese men. Surg Obes Relat Dis. 2006;2:82–6. doi: 10.1016/j.soard.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–34. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 27.Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65 years and older: A review of the controversy. Exp Gerontol. 2013;48:1054–61. doi: 10.1016/j.exger.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araujo AB, O'Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89:5920–6. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 29.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan SA, Lee JY, O'Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male. 2013;16:169–72. doi: 10.3109/13685538.2013.844786. [DOI] [PubMed] [Google Scholar]
- 31.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–92. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frontera WR, Reid KF, Phillips EM, et al. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol (1985) 2008;105:637–42. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–8. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 34.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vigen R, O'Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–36. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 36.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]