Abstract
Recent studies suggest that the Shaker exercise induces fatigue in the upper esophageal sphincter (UES) opening muscles and sternocleidomastoid (SCM), with the SCMs fatiguing earliest. The aim of this study was to measure fatigue induced by the isometric portion of the Shaker exercise by measuring the rate of change in the median frequency (MF rate) of the power spectral density (PSD) function, which is interpreted as proportional to the rate of fatigue, from surface electromyography (EMG) of suprahyoid (SHM), infrahyoid (IHM), and SCM. EMG data compared fatigue-related changes from 20-, 40-, and 60-s isometric hold durations of the Shaker exercise. We found that fatigue-related changes were manifested during the 20-s hold. The findings confirm that the SCM fatigues initially and as fast as or faster than the SHM and IHM. In addition, upon completion of the exercise protocol, the SCM had a decreased MF rate, implying improved fatigue resistance, while the SHM and IHM showed increased MF rates, implying that these muscles increased their fatiguing effort. We conclude that the Shaker exercise initially leads to increased fatigue resistance of the SCM, after which the exercise loads the less fatigue-resistant SHM and IHM, potentiating the therapeutic effect of the Shaker exercise regimen with continued exercise performance.
Keywords: Dysphagia, Electromyography, Exercise, Muscle fatigue, Power spectral density, Surface EMG, Therapeutic exercise, Shaker exercise, UES opening, Deglutition, Deglutition disorders
Swallowing is a complicated process that involves many neck muscles, including the suprahyoid muscle group. During swallowing, this group of muscles, which includes the mylohyoid, geniohyoid, and digastric muscles, contracts and acts as a traction force that contributes to the anteroposterior deglutitive opening of the upper esophageal sphincter (UES), allowing food to pass into the esophagus. The UES is normally in a state of tonic contraction that prevents air passage from the pharynx into the esophagus. Sphincteric relaxation and distensibility also contribute to UES opening.
The Shaker exercise is a simple isotonic/isometric head-raising exercise that has been shown to increase the anteroposterior deglutitive opening diameter and cross-sectional area of the UES [1, 2]. This exercise consists of three isometric repetitions of 1-min sustained head raisings with the patient in the supine position, alternating with 1-min rest periods. Participants are instructed to raise their head high and forward enough to be able to observe their toes without raising their shoulders off the ground. The three sustained head raisings are followed by a second portion of the exercise that includes 30 consecutive, nonsustained, repetitive head raisings in the same supine position.
In prior work, surface EMG (sEMG) studies have been used to evaluate specific muscle activation patterns and the relationship of the timing of hyolaryngeal excursion, pharyngeal constriction, UES opening, and activation of suprahyoid muscles during swallowing [3–9]. In a study by Ferdjallah et al. [6], the shifts in the spectra from recorded sEMGs were used to identify SHM, IHM, and SCM fatigue after completion of the isometric portion of the Shaker exercise. The conclusion of this study showed that the spectral shift for the SHM, IHM, and SCM groups all showed evidence of fatigue; however, this evidence suggested that the SCM group fatigued faster than either the SHM or the IHM.
The original design of the Shaker head-lift exercise was based on patient tolerance and reported fatigue. The overall goal of this project was to begin to refine the exercise by use of objective indices of fatigue. Surface EMG is used as a clinical tool in the study of skeletal muscle fatigue [10–12]. During fatigue, the median (and mean) frequencies of the power spectral density (PSD) function of the sEMG signals shift in a predictable manner [13–15]. The aim of this study was to use EMG spectral analysis to quantify and evaluate the progression of fatigue in the SHM, IHM, and SCM muscle groups in healthy adult subjects during the Shaker exercise.
Methods
This study was approved by the Medical College of Wisconsin institutional review board (IRB).
Study Subjects
Four male and two female healthy elderly volunteers ranging in age from 70 to 78 years (mean = 73 years) were enrolled at an independent senior living community. All subjects reported a negative history of head injury, neurologic dysfunction, neuropathy, myopathy, generalized weakness, neck irradiation, surgery, or swallowing difficulties. All subjects completed the informed consent process prior to being enrolled in the study.
Exercise Protocol
The subjects were given oral, written, and videotaped instructions on the accurate performance of the Shaker exercise. After demonstrating accurate exercise performance, the participants were given a daily log in which to record their independent performance of the Shaker exercise three times per day for 6 weeks. Before and after completing 6 weeks of the exercise, the subjects were instructed to perform the isometric portion of the Shaker exercise while surface EMG electrodes were placed as described above. Direction was given to each participant to perform the isometric or sustained portion of the two-part exercise [1, 2].
For the Shaker exercise trials, the subjects were placed flat on an examining table in a supine position and asked to raise only their head off the table, flexing the neck to look at their feet. The intent of the exercise was to have the subject look at their feet while keeping their shoulders flat on the table with the thoracic muscles relaxed. Only the isometric portion of the Shaker exercise was considered in this study. Thus, the adopted exercise protocol consisted of three durations of sustained head lifts. In the first phase of the preregimen EMG protocol, data from three sEMG channels were recorded simultaneously for 60 s, one channel for each muscle group. The subjects rested for 5 min between trials so that fatigue recovery could take place. The second and third phases were repetitions, with only the durations changed to 40 then 20 s, respectively. After completing 6 weeks of the Shaker exercise regimen, the subjects again repeated the three head-lift trials, as described above, with the sEMG data recorded as described below.
Instrumental Technique
This study used a portable Biometric Datalog system for transportability to the older adult participants who resided in an independent senior-living community setting. A programmable data acquisition unit, the Biometrics Datalog II (NexGen Ergonomics) was used to record sEMG data. Four channels were used: one for the ground and three for the EMG sensors. The high-pass filter was set at 20 Hz and the low-pass filter was set at 450 Hz.
The metric used was the rate of change of the median frequency (MF rate) of the power spectral density (PSD) function, that is, computation from the time-series data captured from the sEMG data of the muscle groups being monitored. The measurements used for sEMG recordings were 20-, 40-, and 60-second head lifts, that is, the isometric portion of the Shaker exercise protocol.
Surface EMG signal digitized time-series data were recorded from the SHM, IHM, and SCM muscle groups during the Shaker exercise durations. The EMG data were recorded and stored with the Biometrics Datalog II programmable data acquisition unit. This portable unit allowed us to collect sampled digitized data from the reusable EMG sensors. The sampling rate was 1000 samples/s.
EMG recordings were done using three electrode pairs that were placed unilaterally over the left SHM, IHM, and SCM muscle groups. Figure 1 depicts the electrode montage, similar to the previous study that examined fatigue related to the Shaker exercise completed by Ferdjallah et al. [6]. Each electrode pair incorporated E1, an active electrode, and E2, a reference electrode, into a single device. The SHM electrode was placed two-thirds of the distance from the mental protuberance to the hyoid bone, proximally, and 1 cm lateral to the midline. The IHM electrode was placed two-thirds of the distance between the jugular notch of the manubrium sterni. The SCM electrode was placed in the middle of the SCM. The ground with the strap was placed around the wrist with the ground resting on a bony surface.
Fig. 1.
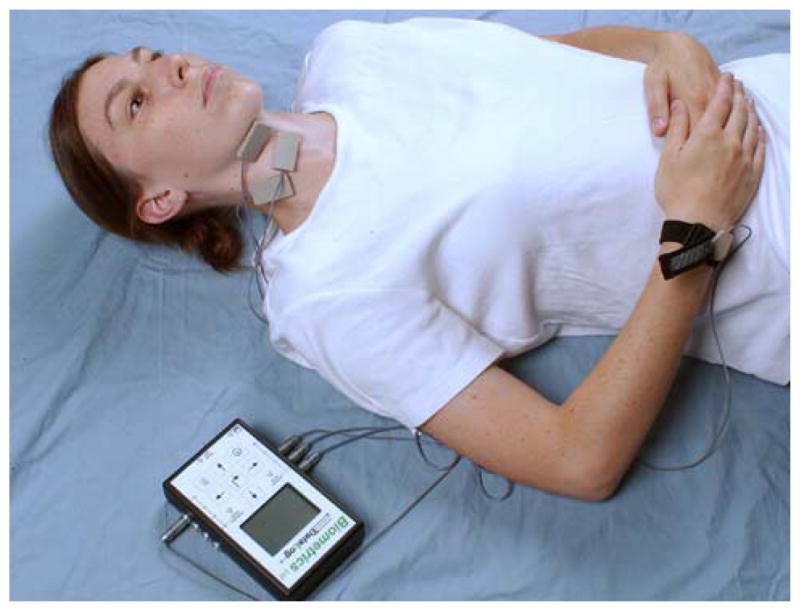
Biometrics datalog montage
Preanalysis Screening of Data Records
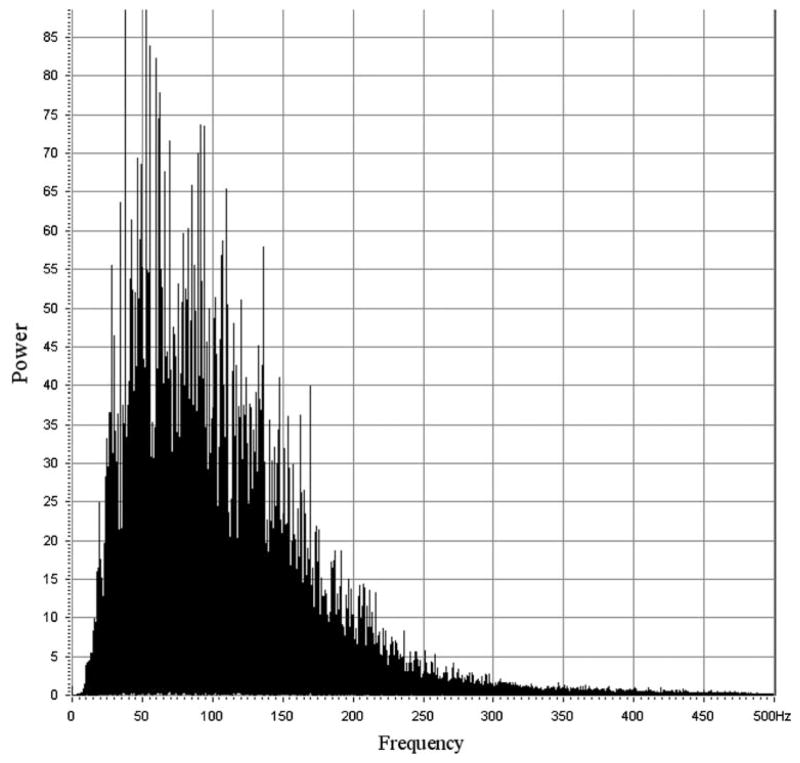
Raw data files were generated by the Biometrics Data Logger hardware, wherein each file contained three channels of digitized time-series data for the SHM, IHM, and SCM for a single exercise trial. The files were later transferred via flash memory to a standard computer for analysis. The file names were coded to identify the subject, trial duration, and whether the data were taken at the beginning or the end of the study. The original equipment manager of the data logger supplied a host application that provided basic analysis tools, including fast Fourier transform (FFT), display, and reformatting functions. The Biometrics Data Logger does not allow the investigator to view or otherwise assess the data as it is being collected, so the investigators could not assess if a sEMG signal was being sensed while in the field. Because of this technical limitation, all files were screened just prior to the analysis stage for the presence of a signal, and files that lacked a signal or appeared corrupt were discarded from the study. The screening process involved visual inspection of both the time-series data and the power spectra and was based on the presence or absence of inclusion and exclusion criteria. The time-series plot of a successfully recorded signal started with several seconds of very-low-amplitude noise (corresponding to the subject’s rest state), followed by rapid onset (<1 s) of a fairly constant-amplitude interference pattern 30–100 times the resting noise amplitude (corresponding to muscle contraction). This interference pattern persisted fairly uniformly for the duration of the trial (20, 40, or 60 s), then returned to the low-voltage baseline that was present at the beginning of the trial. The morphology of a sEMG spectrum had the appearance of a skewed, bell-shaped curve with numerous superimposed and uniformly distributed “spikes.” The amplitudes of these spikes were grossly proportional to the local values of the underlying curve. Most spectral content was found within 250 Hz (Fig. 2). The presence of such morphology was the criterion for inclusion, its absence was the criterion for exclusion. Furthermore, even if only one muscle from a given trial failed inclusion/exclusion criteria, then all records for that trial were excluded. Several excluded spectra were very low amplitude, with bandwidths of less than 60 Hz, which we judged to be caused by excessive distance between the electrodes and target muscle. A few included records contained normal-appearing spectra with superimposed artifacts such as a 60-Hz power-line spike or other low-frequency periodic signals with a few harmonics. The study’s authors judged that these superimposed artifacts would not materially affect the results as long as only median-frequency data were analyzed. An option to analyze mean-frequency data was considered and rejected out of concern that it would be more susceptible to influences from superimposed artifact [10].
Fig. 2.
A representative spectrum of surface EMGs. Spectra from the SCM muscle, IHM, and SHM, whether pre- or postregimen, are grossly indistinguishable
Signal Processing and Analysis
The result of signal processing is the metric rate of change of the median frequency (MF rate) of the power spectral density (PSD) function. Times corresponding to the onset and cessation of effort were established by inspection of the time-series plot to identify when the interference pattern started and stopped. Then PSD calculations were performed, starting with data at the onset time and continuing until the cessation-of-effort time [16]. The PSD software uses an FFT, which necessitated the selection of a computational record length (CRL) that is an integer power of 2, i.e., the number of samples or “batch size” that is to be used in calculating the PSD [16]. For this study a CRL or batch-size of 1024 samples was used. This yielded a resolution of about 1 Hz and processed 1.024 s of time-series data per PSD computation. Then the median frequency of the PSD was computed and logged, and the PSD itself was then discarded. This process resulted in a median-frequency (MF) time series, with about as many MF data points as there were seconds in the trial (about 20, 40, or 60). Finally, a regression analysis was performed on the median-frequency time series. The plotted result shows a line that is the best fit of a scatter of data (Fig. 2). The following equation describes the result:
where MF(t) is median-frequency function, t is time, MF rate is the calculated rate of change of the median frequency, and MF rest is the muscle’s calculated median frequency at rest (not fatigued). For this study, MF rate is the parameter of interest (with one and only one MF rate for each trial for each muscle).
Results
Screening
The data were screened to confirm the presence of a signal. Some records failed the inclusion/exclusion criteria and were excluded from analysis. Of the six subjects, one male’s EMG data had such a low amplitude (about 3–10× noise amplitude) and low bandwidth (<60 Hz) that it suggested that the electrodes were either placed too far from the target sites or that the subject had excessively thick subcutaneous tissue overlying the target muscles, which subjected the signals to overwhelming tissue and spatial filtering effects; records from this subject were excluded from analysis. Of the remaining five subjects, three had partial records from individual trials excluded, but still had pre- and postregimen records that were included in the analysis. Complete records from two subjects met inclusion/exclusion criteria.
Data Analysis
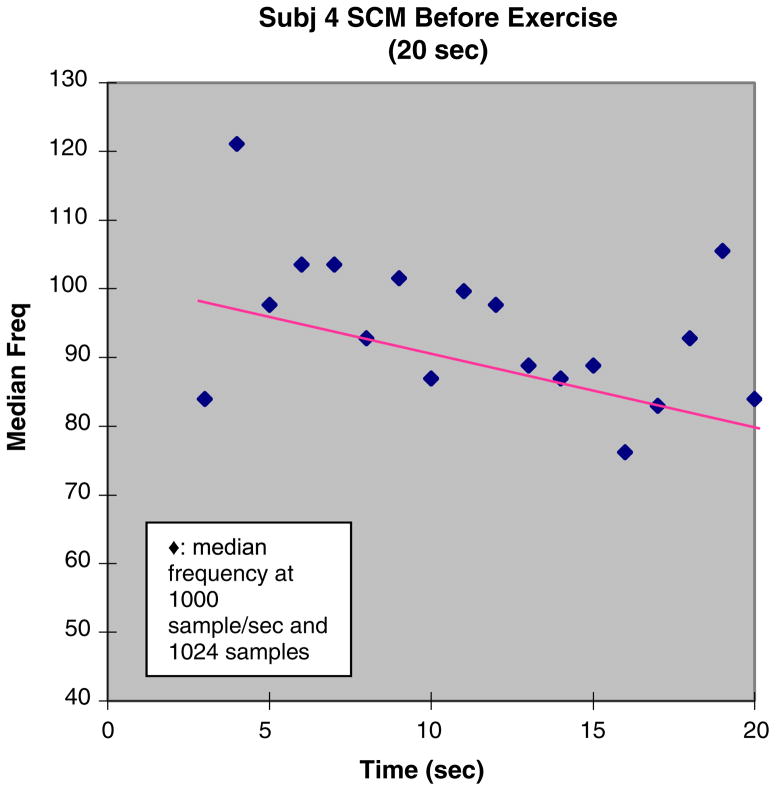
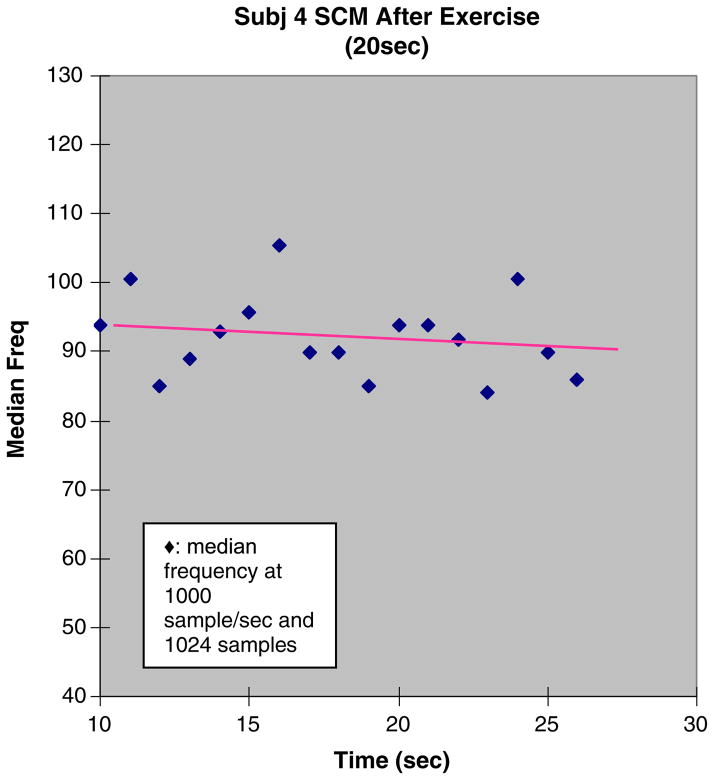
The signal processing procedure to compute MF rate was performed for each of the screened records. Examples of results are seen in Figures 3 and 4, which show scatterplots of median frequency versus time, with superimposed linear regressions. The slope of the regression is the metric MF rate. Given that the trials of the Shaker exercise were isometric, we infer that the rate of fatigue is constant and that MF rate, which is also a constant, reflects this rate.
Fig. 3.
Preregimen Shaker exercise trial. EMG of the sternocleidomas-toid (SCM) for subject 4. This figure shows an example of the median frequency data with linear regression. The slope of the regression, MF rate, is the metric, as it is interpreted to reflect the rate of fatigue
Fig. 4.
Postregimen Shaker exercise trial. EMG of the sternocleido-mastoid (SCM) for subject 4. This figure shows an example of median frequency data with linear regression. The slope in the postexercise testing is less negative than the in pre-exercise testing of the SCM which implies more fatigue resistance
One subject had large, positive MR rates in her pre-regimen trials. Positive median-frequency shifts have not been described in the literature, and we could not reconcile this result with the assumptions that the subject had been exerting a fatiguing level of effort and that her muscles had been correctly instrumented. Therefore, we withdrew her records from the analysis, leaving four subjects.
The MF rate for the four subjects is tabulated in Table 1. The preregimen and postregimen MF rates for each muscle are summarized in a time-weighted average that is weighted proportionally to the trial durations. These results are summarized in Table 2. The preregimen trial results for the SCM showed a more negative averaged MF rate than the IHM and a similar MF rate in the SHM. The postregimen trial results showed a 57% reduction in the averaged SCM MF rate, while the SHM and IHM MF rates showed 12 and 50% increases, respectively. Fatigue-related changes were observed even in the 20-s trials, as suggested by the negative-averaged MF rates.
Table 1.
Rate of change of the median frequency (MF rate)
| Subject ID | Gender | Preregimen | 60-s trial | 40-s trial | 20-s trial | Postregimen | 60-s trial | 40-s trial | 20-s trial |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | SHM | −0.37 | SHM | −0.14 | ||||
| IHM | −0.33 | IHM | −0.3 | ||||||
| SCM | −0.47 | SCM | −0.01 | ||||||
| 2 | M | SHM | −0.44 | SHM | −0.49 | ||||
| IHM | −0.2 | IHM | −0.43 | ||||||
| SCM | −0.16 | SCM | −0.45 | ||||||
| 3 | F | SHM | −0.3 | −0.65 | −1.22 | SHM | −0.49 | −0.9 | −1.04 |
| IHM | −0.27 | −0.51 | −0.45 | IHM | −0.32 | −0.61 | −0.61 | ||
| SCM | −0.31 | −0.67 | −1.17 | SCM | −0.21 | −0.16 | −0.58 | ||
| 4 | F | SHM | −0.5 | −0.34 | −0.34 | SHM | −0.55 | −0.76 | −1.3 |
| IHM | −0.12 | −0.11 | −0.41 | IHM | −0.42 | −0.69 | 0.12 | ||
| SCM | −0.61 | −0.12 | −0.89 | SCM | −0.1 | −0.13 | −0.26 |
Units in Hz/s
No entry indicates that data failed inclusion/exclusion criteria
Table 2.
Summary of EMG
| Preregimen | Postregimen | |
|---|---|---|
| SHM | −0.56 | −0.63 |
| IHM | −0.27 | −0.40 |
| SCM | −0.46 | −0.20 |
Average rates of change of the median frequency (MF rate) for the suprahyoid (SHM), infrahyoid (IHM), and sternocleidomastoid (SCM) muscles before and after the 6-week Shaker Exercise regimen. Units are in Hz/s
Discussion
The Shaker exercise is a simple isotonic/isometric head-raising exercise that has been shown to increase the anteroposterior deglutitive opening diameter and cross-sectional area of the UES [1, 2]. The original design of the Shaker exercise was based on patient tolerance and reported fatigue and consisted of three isometric repetitions of 1-min sustained head raisings with 1-min rest periods.
The purpose of this study was to begin to refine the exercise by using an objective physiologic measure of fatigue, specifically, the rate of spectral shift seen on surface EMG. When we observed a muscle under a fatiguing load via surface EMG, then periodically calculated the median frequency of the power spectral density function, we anticipated that a process of progressive downward shifting of the MF rate would occur, based on prior work by other researchers. Indeed, this is what we observed. This frequency shift has long been thought to be related to underlying physiologic changes resulting from muscle fatigue; these changes include a decrease in muscle-fiber conduction velocity along with other factors [13].
Our previous work [6–9] was done in a hospital EMG laboratory setting with large, dedicated EMG equipment. With the advances in instrumentation we were able to do this study in the field with a portable data acquisition system. Another refinement was the reconsideration of fatigue not as an endpoint but as a dynamic process. The “process of fatigue” is defined as the reversible physiologic change that progresses as a muscle is exerted with effort sufficient to result in endpoint fatigue as defined above. The spectral shift associated with these changes is assumed to vary continuously from an initial reading of zero to the previously described shift noted at the endpoint fatigue.
Skeletal muscle fatigue has been defined as a failure to maintain the required or expected force during a muscle contraction [9], although this definition of fatigue was not used for this study for practical reasons. Functions of the central nervous system, the muscle cell membrane, muscle end plate, muscle T-tubular system, and the energy supply to the muscle may all contribute to muscle fatigue. Surface EMG is often used as a clinical tool in the study of skeletal muscle fatigue [3–5]. During fatigue, the median (and mean) frequencies of the PSD function of the sEMG signals shift in a predictable manner. Several studies have demonstrated that sustained isometric contractions produce a shift of the mean power frequency to lower values. An increase in power at lower frequencies and a decrease in power at higher frequencies have also been observed [10–15]. Because of the fatigue-induced shifts in the spectra of sEMG, this technique allows the evaluation of which muscles are most affected by an exercise.
In this study we redefine fatigue as a dynamic process rather than an endpoint. We have several reasons for this: Since the Shaker exercise incidentally fatigues the SCM, the target suprahyoid muscles may never reach the fatigue endpoint, and in some situations, such as in elderly subjects, it may not be possible to mount adequate effort (i.e., central fatigue). The rate of change in the median frequency (MF rate) allows us to consider fatigue as a process taking place well in advance of exhausting a muscle. This measure also gives us the ability to assess relative fatigue rates in different muscles. We can assess fatigue onset, progression, and response to exercise therapy in targeted muscles without the subject ever having to exert himself to a fatigue endpoint.
Surface EMG signals were detected from the SCM, SHM, and IHM muscle groups, indicating their involvement in the Shaker exercise. This study confirms prior findings that have shown that the onset of muscular fatigue begins immediately after the start of the exercise [6–9]. Initial findings suggested that the early fatiguing of the SCM might limit the therapeutic efficacy of this exercise because it does not have a role in the swallowing process. The findings from this study are consistent with earlier studies showing early fatiguing of the SCM [6–9]. However, our current findings suggest that the Shaker exercise also promotes fatigue resistance in the SCM. The findings go on to suggest that the SCM is strengthened, while the contractility of the SHM and IHM is increased (Table 2). We can infer that if the subjects were to continue to practice the Shaker exercise, the SHM and IHM muscles would increase their fatigue resistance, as the increased fatigue resistance of the SCM has enabled increased loading and effort in the targeted muscles.
Conclusion
In summary, our findings agree with our previous work [6–9] that showed that during the isometric portion of the Shaker exercise, the SCM muscle group fatigued as soon as or sooner than the SHM and the IHM groups. We also found that the SHM, IHM, and SCM groups all begin to show EMG evidence of fatigue within 20 s of activation. Remarkable findings in this study show that after completing 6 weeks of the Shaker exercise, the SCM has a decreased (less negative) MF rate, implying improved fatigue resistance, while the SHM and IHM show an increased (more negative) MF rate, implying greater exertion. We interpret this result to show that the Shaker exercise initially leads to increased fatigue resistance of only the SCM, after which the exercise loads the less fatigue-resistant SHM and IHM, potentiating the therapeutic effect of the Shaker exercise with continued practice. This would account for the therapeutic effect of this exercise in spite of the seemingly limiting role of the SCM.
Acknowledgments
Sincere thanks to the residents of Alexian Village Senior Independent Community for their time, dedication, and cooperation. This research was supported in part by grants from the NIH/NIDDK (R01 DK25731) and The Retirement Research Foundation (#2001-188).
Contributor Information
Kevin T. White, Email: kevin.white2@va.gov, Department of Physical Medicine and Rehabilitation (PMR), Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Caryn Easterling, Email: caryn@uwm.edu, Department of Communicational Sciences and Disorders, University of Wisconsin-Milwaukee, Milwaukee, WI 53201, USA.
Niles Roberts, Email: nroberts@mcw.edu, Department of Physical Medicine and Rehabilitation (PMR), Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Jacqueline Wertsch, Email: wertschj@mcw.edu, Department of Physical Medicine and Rehabilitation (PMR), Medical College of Wisconsin, Milwaukee, WI 53226, USA. Clement J Zablocki VA Medical Center, 5000 W National Avenue, Milwaukee, WI 53095, USA.
Reza Shaker, Email: rshaker@mcw.edu, Division of Gastroenterology and Hepatology, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
References
- 1.Shaker R, Kern M, Bardan E, Taylor A, Stewart ET, Hoffman RG, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol. 1997;272:G1518–22. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]
- 2.Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, et al. Rehabilitation of swallowing by exercise in tube fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122:1314–21. doi: 10.1053/gast.2002.32999. [DOI] [PubMed] [Google Scholar]
- 3.Goyal RK. Disorders of the cricopharyngeus muscle. Otolaryngol Clin North Am. 1984;17:115–30. [PubMed] [Google Scholar]
- 4.Perlman AL, Palmer PM, McCulloch TM, VanDaele DJ. Electromyography activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1999;86:1663–9. doi: 10.1152/jappl.1999.86.5.1663. [DOI] [PubMed] [Google Scholar]
- 5.Crary MA, Carnaby (Mann) GD, Groher ME. Biomechanical correlates of surface electromyography signals obtained during swallowing by healthy adults. J Speech Lang Hear Res. 2006;49:186–93. doi: 10.1044/1092-4388(2006/015). [DOI] [PubMed] [Google Scholar]
- 6.Ferdjallah M, Wertsch J, Shaker R. Spectral analysis of surface EMG of upper esophageal sphincter opening muscles during head lift exercise. JRRD. 2000;37(3):335–40. [PubMed] [Google Scholar]
- 7.Alfonso M, Ferdjallah M, Shaker R, Wertsch JJ. Electrophysiologic validation of deglutitive UES opening head lift exercise. Gastroenterology. 1998;114(4):A711. [Google Scholar]
- 8.Jurell KC, Shaker R, Mazur A, Haig AJ, Wertsch JJ. Spectral analysis to evaluate hyoid muscles involvement in neck exercise. Muscle Nerve. 1996;19:1224. [Google Scholar]
- 9.Jurell KC, Shaker R, Mazur A, Haig AJ, Wertsch JJ. Effect of exercise on upper esophageal sphincter opening muscles: a spectral analysis. Gastroenterology. 1997;112(4):A757. [Google Scholar]
- 10.Basmajian JV, De Luca CJ. Muscle alive. 5. Baltimore MD: Williams & Wilkins; 1985. [Google Scholar]
- 11.Kramer CG, Hagg T, Kemp B. Real time measurement of muscle fatigue related changes in surface EMG. Med Biol Eng Comp. 1987;25:627–30. doi: 10.1007/BF02447329. [DOI] [PubMed] [Google Scholar]
- 12.Lyons M, Rouse M, Baxendale R. Fatigue and EMG changes in the masseter and temporalis muscles during sustained contractions. J Oral Rehabil. 1993;20:321–31. doi: 10.1111/j.1365-2842.1993.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 13.Merletti R, Knaflitz M, De Luca CJ. Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J Appl Physiol. 1990;69(5):1810–20. doi: 10.1152/jappl.1990.69.5.1810. [DOI] [PubMed] [Google Scholar]
- 14.Merletti R, Lo Conte LR, Orizio C. Indices of muscle fatigue. J Electromyogr Kinesiol. 1991;1:20–33. doi: 10.1016/1050-6411(91)90023-X. [DOI] [PubMed] [Google Scholar]
- 15.Moritani T, Muro M, Nagata A. Intramuscular and surface electromyogram changes during muscle fatigue. J Appl Physiol. 1986;60:1179–85. doi: 10.1152/jappl.1986.60.4.1179. [DOI] [PubMed] [Google Scholar]
- 16.Brigham EO. The fast Fourier transform and its applications. Englewood Cliffs, NJ: Prentice Hall; 1988. [Google Scholar]