Abstract
Objective
To improve our understanding of patients’ treatment preferences for chronic hepatitis C (HCV).
Methods
Subjects with HCV were recruited from 2 VA medical centers. Preferences were ascertained using conjoint analysis. We used segmentation analysis to examine whether there were groups of respondents with similar preferences that were systematically different from the preferences of others. We then measured the associations between treatment preference with subjects’ characteristics and their gist principles related to living with HCV and the burden of therapy.
Results
A total of 199 subjects participated in this study. The segmentation analysis demonstrated that subjects could be classified into 2 distinct groups. The larger group [group 1, n = 118 (59%)] opted for current treatment and the other [group 2, n = 81 (41%)] preferred to defer. Patients with cirrhosis were less likely to belong to group 2 (prefer to defer) compared with those without cirrhosis (40.5% vs. 21.3%), whereas subjects self-identifying as African American were more likely to belong to group 2 than white subjects (51.3% vs. 30.5%). Members of group 1 had a more positive overall gist principles related to HCV compared with members of group 2 [mean (SD) score = 28.63 (3.06) vs. 26.46 (2.79), P < 0.0001]. These gist principles mediated the relationship between race and treatment preference (Sobel test statistic = −2.68, 2-tailed P = 0.007).
Conclusions
Our findings indicate that there are groups of HCV patients with similar preferences that are distinct from other groups’ preferences. Patients’ gist principles related to the significance of having a chronic viral infection and the burdens of therapy are strongly related to their current treatment decisions. These findings help inform how best to initiate and deliver treatment for patients with HCV.
Keywords: hepatitis C, patient preferences, treatment deferral
Chronic hepatitis C virus (HCV) is a major health care burden and can lead to cirrhosis, liver failure, hepatocellular carcinoma, and death.1 Although definitive data on long-term outcomes are lacking, studies have shown that sustained virological response (SVR) to treatment seems to be associated with a decreased rate of disease progression and improved survival.2–5 The efficacy of antiviral therapy, however, must be considered in the context of the natural history of HCV. Cirrhosis develops in approximately 30% of patients, whereas liver-related mortality may occur in 20% of patients overall.6 Moreover, HCV does not progress at a uniform rate in all patients.6 Understanding patients’ preferences to treat now versus defer is therefore critical to help inform drug development, formulary decision making, and resource allocation (eg, number of clinic slots, physicians and nurses to support drug initiation, and monitoring).
In a previous study, we found that patients’ preferences for HCV treatment with pegylated interferon and ribavirin were sensitive to the degree of toxicity, with 67% and 51% of subjects being willing to accept the risk of mild and severe side effects, respectively.7 Preferences for treatment of HCV were stronger among subjects with a higher perceived risk of developing cirrhosis, more severe underlying liver disease, and worse HCV-related quality of life. More recently, Kauf et al8 found that patients were willing to accept the risk of additional side effects associated with HCV triple therapy with pegylated interferon, ribavirin, and a protease inhibitor for improved SVR rates. However, neither of these studies mirrored the choices that patients face in clinical practice, which includes the option to defer.
In some situations, such as in elective surgery, the severity of symptoms and functional impairment strongly influence when patients are willing to accept the risks inherent to a specific procedure (such as a total joint replacement). In HCV, however, symptoms may be minimal and the reasons underlying variability in preference to treat now or defer are less well understood. In this study, we therefore sought to examine patient preferences for triple therapy or deferral and explore the reasons underlying variability.
We quantified preferences using conjoint analysis. This method predicts preferences for specific options based on how people value trade-offs, such as the amount of benefit patients demand before accepting a specified risk of toxicity.9 Subsequent segmentation of conjoint data allows one to subdivide a large population into meaningful groups whose preferences are similar within groups, but systematically different from other groups. To understand the reasons underlying variability in preferences, we developed a list of gist principles (simple values related to living with HCV and the burden of therapy). Gist principles are general but contextually relevant social or moral values that are mentally represented in a simple meaningful form called “gist” in long-term memory.10,11 We hypothesized that these principles would be a stronger predictor of preference than the traditional patient-level factors.
Although there are now newer therapies available for HCV, the results of this study provide new insights on the methods used to examine patients’ preferences and the major determinants of their treatment choices.
METHODS
Subjects were drawn from patients referred for evaluation of HCV or previously treated for HCV in the VA Connecticut and San Francisco Healthcare Systems. We performed a limited chart review to identify adults aged 18 and above who had chronic HCV genotype 1 infection; were able to speak and read English; and had a telephone and mailing address. Patients with contraindications to treatment (decompensated liver disease; hepatocellular carcinoma; visual or hearing impairments; current alcohol or other substance abuse; uncontrolled psychiatric illness; severe comorbidities including end-stage chronic obstructive pulmonary disease, end-stage renal disease, or end-stage congestive heart failure, or cancer) were excluded.
Letters were sent to those meeting these initial eligibility criteria. The letter notified the potential participants that they would be telephoned by a research assistant and offered them the opportunity to refuse this contact by calling an answering machine and leaving a message. The research assistant telephoned all patients who did not “opt out” to describe the study and schedule interviews. Full written consent was obtained at the beginning of the in-person study interview. All subjects underwent a formal education class about the natural history of HCV and the outcomes associated with both treatment and deferral before participating in the study. The study was approved by the VA Central Institutional Review Board.
Each subject participated in a single face-to-face interview administered by a research assistant. Preferences were ascertained using choice-based conjoint analysis (CBC). CBC is a computerized questionnaire that assesses preferences by asking respondents to choose a preferred option from a set of hypothetical alternatives (see example in Fig. 1). Conjoint analysis assumes that each option is a composite of different characteristics, and that each characteristic represents one of a number of levels. Levels refer to the range of estimates for each characteristic. Respondents do not evaluate treatment alternatives directly; instead preferences are calculated based on how participants value differences between competing options.
FIGURE 1.
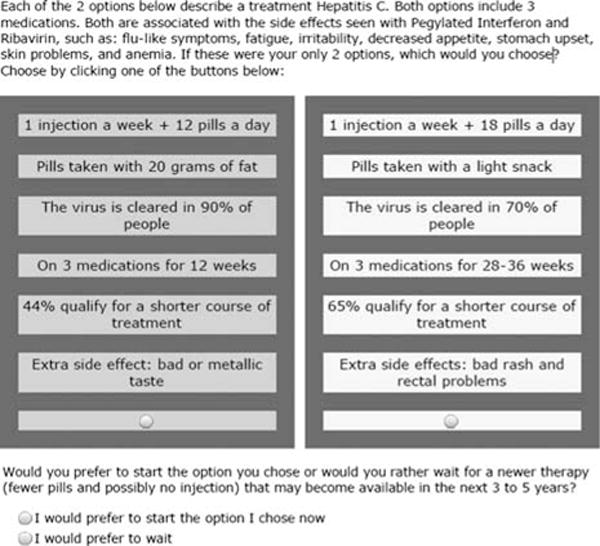
Example of choice-based conjoint analysis task.
The survey was designed using Sawtooth Software, SSI Web 8.0. It was composed of 6 attributes (detailed in Appendix A, Supplemental Digital Content 1, http://links.lww.com/JCG/A186): (1) route of administration (1 injection a week + 12 pills a day vs. 1 injection a week + 18 pills a day), (2) food requirement (pills taken with 20 g of fat vs. pills taken with a light snack), (3) expected SVR (virus is cleared in 30% vs. 70% vs. 90% of people), (4) number of weeks on triple therapy (on 3 medications for 12 wk vs. 28 to 36 wk), (5) percent qualifying for a shorter course of treatment based on initial response (44% vs. 65% qualify for a shorter course of treatment), unique side effects (bad rash and rectal problems vs. metallic taste). The survey included an educational component describing all medication characteristics using lay terminology (Appendix, Supplemental Digital Content 1, http://links.lww.com/JCG/A186).
Subjects performed 10 choice tasks drawn randomly from the experimental design. Each task included 2 hypothetical medications and a subsequent option to defer (see example in Fig. 1). We used the software’s complete enumeration strategy to construct the choice sets. The complete enumeration method ensures that (1) each level is shown as few times as possible in a single task, (2) each level is shown approximately an equal number of times across the choice tasks, and (3) the level of 1 characteristic is chosen independently of the levels of other characteristics. The program was set to generate a design for 300 versions of the survey. The a priori estimates of standard errors for all attribute levels were between 0.03 or 0.04 and the efficiencies reported were all 0.99 or 1.00. In this context, efficiency refers to the ability of the design to estimate each parameter, compared with the hypothetical fully orthogonal design (please see: http://www.sawtoothsoftware.com/support/technical-papers/cbc-related-papers/cbc-technical-paper-2013 for further illustrative examples and further details).
In addition to the 10 choice sets where levels were assigned as per the above described strategy, we included 2 fixed tasks in which the investigators defined the options in the choice set to gauge the accuracy of the utility estimates. The first task held all attributes constant except for SVR, which was set at 30% for the first option and 70% for the second. The second-fixed task described levels representative of telaprevir and boceprevir for all attributes except SVR and the percent qualifying for shorter duration of treatment, which were set at 70% and 65%, respectively. The responses of the fixed tasks were not included in the simulations.
We also collected demographic and clinical characteristics. Ten gist principles, 5 positive and 5 negative, related to HCV treatment were composed based on input from patients, a nurse practitioner who specializes in HCV, and 3 hepatologists. These principles were modeled on others used to account for risk-related decision making in antibiotics expectations, HIV prevention, arthritis medications, and breast cancer genetics.12–14 Participants rated each statement on a 4-point scale (“strongly agree” = 1 and “strongly disagree” = 4). Statement order was determined using a random-numbers generator. Positive items were reverse coded and summed. The gist principles were ascertained before the preference task and are listed in Table 2. The gist principles and conjoint analysis attributes were pilot tested and revised based on feedback obtained from subjects during cognitive interviews with 10 subjects.
TABLE 2.
Subjects’ Mean Belief Scores by Group Membership
| Direction | Gist Principles | Group 1 (n = 118) |
Group 2 (n = 81) |
P |
|---|---|---|---|---|
| Positive | Postponing treatment is risky because it allows liver damage to progress | 3.32 (0.66) | 3.16 (0.54) | 0.07 |
| Positive | Having side effects is worth it if there is ANY possibility of eliminating the virus | 3.18 (0.73) | 2.98 (0.59) | 0.03 |
| Negative | It is important to avoid strong medications unless absolutely necessary | 3.08 (0.70) | 3.13 (0.54) | 0.60 |
| Positive | Coping with side effects is worth it to protect my liver | 3.29 (0.65) | 2.99 (0.67) | 0.002 |
| Negative | Having side effects is worth it only if there is a REALLY STRONG possibility of eliminating the virus | 3.10 (0.75) | 3.09 (0.64) | 0.86 |
| Negative | Negative It is better to wait for treatments that are easier to take, even if they aren’t available for another 5 y | 2.22 (0.71) | 2.70 (0.77) | < 0.0001 |
| Positive | It is unhealthy to live with a virus, even if it isn’t causing any symptoms | 3.10 (0.76) | 2.89 (0.71) | 0.05 |
| Negative | It is not reasonable to undergo treatment if it interferes with your responsibilities (whether at work or home) | 2.16 (0.71) | 2.31 (0.61) | 0.10 |
| Negative | There is no reason to take medications for a virus that is not bothering me | 1.97 (0.70) | 2.29 (0.64) | 0.001 |
| Positive | Undergoing treatment now is important to ensure a healthy future | 3.24 (0.78) | 2.96 (0.63) | 0.006 |
Bold indicates t test statistically significant <0.05.
Statistical Analysis
For each respondent, utilities (values) were calculated for each level of each attribute using Hierarchical Bayes (HB) modeling (Sawtooth CBC/HB system for hierarchical Bayes estimation version 4.0). HB modeling has the advantage that it can better model preference heterogeneity among respondents.15 In HB modeling, the sample averages (prior information) are used to update the individual utilities in a number of iterations until the sample averages stop changing between iterations. After this convergence, the cycle is run several thousand more times and the estimates of each iteration are saved and averaged. Utilities were entered in SAS software, version 8.2 (SAS Institute, Cary, NC) and merged with the respondents’ characteristics. We calculated the percentage of importance that respondents assigned to each attribute by dividing the range of utilities for each attribute by the sum of the ranges and multiplying by 100. We subsequently performed Latent Class analysis (Sawtooth Software, SSI Web 8.0) to examine whether preferences clustered by specific segments. Class solutions were replicated 5 times from random starting seeds. This approach has been used successfully in recent stated preference studies.16–20
We used Sawtooth Software Market Research Tools (SMRT) to estimate preferences separately for HCV treatment with triple therapy (telaprevir or boceprevir) versus deferral. SMRT is a platform that enables investigators to perform simulations to predict preferences for specified products based on the utilities obtained during the conjoint analysis survey. In this study, treatment preferences were generated using the randomized first choice model in which utilities are summed across the levels corresponding to each option and then exponentiated and rescaled so that they sum to 100.21,22 This model is based on the assumption that subjects’ prefer the option with the highest utility. The randomized first choice model accounts for the error in the point estimates of the utilities as well as the variation in each respondent’s total utility for each option. This model has been shown to have better predictive ability than other models.21 Further details can be found at: https://www.sawtoothsoftware.com/help/issues/ssiweb/online_help/hid_smrt_randomizedfirstchoice.
Telaprevir was described as: 1 injection a week + 12 pills a day, taken with 20 g of fat, virus cleared in 76% of people, on 3 medications for 12 weeks, 65% qualify for shorter course of treatment, associated with risk of a bad rash and rectal problems. Boceprevir was described as: 1 injection a week + 18 pills a day, taken with a light snack, virus cleared in 65% of people, on 3 medications for 28 to 36 weeks, 44% qualify for shorter course of treatment, associated with risk of a metallic taste. We subsequently ran sensitivity analyses to determine whether subjects preferring to defer, switch to choosing treatment when triple therapy is described as having the most favorable level for each attribute. Differences in patient characteristics across groups were examined using t tests and χ2 tests for continuous and categorical variables, respectively. Variables significantly associated with preference were subsequently examined using logistic regression.
RESULTS
At VA Connecticut, 190 letters were mailed, 2 patients opted out before being telephoned, 30 were never reached by telephone, 13 were not eligible, 46 declined to participate. At the San Francisco VA, 242 letters were sent, 49 subjects were not eligible, 126 declined to participate. Of the 214 subjects agreeing to participate, 199 arrived for the interview and completed the survey. The study population (n = 199) was predominantly (96%) male. Thirty-nine percent were African American, 9% were Hispanic, 55% had at least some college education, and 24% were currently employed. With respect to their clinical characteristics, 28% reported having a very good or excellent health status, 33% had cirrhosis at the time of the interview, and 71% had previously been treated with pegylated interferon and ribavirin for HCV. The superior option in the first-fixed task was chosen 98% of the time. Forty-four percent of the subjects chose to defer treatment when presented with the second-fixed task.
The segmentation analysis demonstrated that subjects could be classified into 2 distinct groups (probability of membership = 100%) based on their treatment preferences; with the larger group [group 1, n = 118 (59%)] opting for current treatment and the other [group 2, n = 81 (41%)] preferring to defer. The latter are consistent with the findings of the second-fixed task. Treatment preferences among members of group 1 were equally divided between both antiviral regimens with 47.4% (SE = 0.14) of the subjects preferring telaprevir, 48% (SE = 0.15) preferring boceprevir, and 4.6% (SE = 0.06) preferring to defer. Treatment preference was uniform among members of group 2, with 100% (SE = 0.01) preferring to defer. Members of group 2 continued to choose to defer when presented with treatment reflecting the best case scenario (ie, the most valued level of each attribute).
We subsequently sought to determine whether we could identify differences between members of groups 1 and 2 (Table 1). Age, education, and current employment were not associated with treatment preference. Subjects self-identifying as African American were more likely to belong to group 2 (ie, prefer to defer treatment) than white subjects (51.3% vs. 30.5%). Patients with cirrhosis were less likely to defer treatment compared with those without cirrhosis (40.5% vs. 21.3%). Duration since diagnosis, health status, and quality of life, and previous treatment status were not related to treatment preference.
TABLE 1.
Subjects’ Demographic and Clinical Characteristics by Group Membership
| Subjects’ Characteristics | Group 1 (n = 118) |
Group 2 (n = 81) |
P |
|---|---|---|---|
| Demographic | |||
| Age [mean (SD)] | 59.03 (6.18) | 59.65 (7.25) | 0.52 |
| Race [n (%)] | |||
| White | 82 (69.49) | 37 (46.25) | 0.0008† |
| African American | 36 (30.51) | 41 (51.25) | |
| Asian/Pacific Islander | 0 | 1 (1.25) | |
| American Indian/ | 0 | 1 (1.25) | |
| Alaska Native Others | 0 | 1 (1.25) | |
| College education [n (%)] | 65 (55.08) | 45 (55.56) | 0.95 |
| Employed [n (%)] | 30 (25.42) | 18 (22.22) | 0.60 |
| Clinical | |||
| Years since diagnosis [median (IQR)] | 14 (8–20) | 13 (7.5–18) | |
| Cirrhosis [n (%)] | 47 (40.52) | 17 (21.25) | 0.005 |
| Excellent/very good health status [n (%)] | 36 (30.51) | 20 (24.69) | 0.37 |
| Previously treated* [n (%)] | 90 (76.27) | 52 (64.20) | 0.06 |
| Achieved SVR [n (%)] | 69 (76.67) | 41 (78.85) | 0.76 |
Previously received at least some treatment with pegylated interferon and ribavirin.
White versus nonwhite.
Subjects’ gist principles related to HCV by group are illustrated in Table 2. Members of group 1 had a more positive overall score compared with members of group 2 [mean (SD) score = 28.63 (3.06) vs. 26.46 (2.79), P < 0.0001]. White subjects had significantly higher scores (P < 0.05) for gist principles 1, 4, and 10 within group 1 and for gist principles 2, 4, 8, and 9 within group 2. We then tested whether gist principles mediated the relationship observed between race and treatment preference using sequential logistic regression models (Table 3, Fig. 2). The relationship between race and treatment preference after controlling for the presence of cirrhosis [adjusted odds ratio (95% confidence interval) = 2.30 (1.26–4.20)] was no longer significant after adding gist principles to the model [adjusted odds ratio (95% confidence interval) = 1.66 (0.87–3.16)] indicating that they mediated the relationship between race and treatment preference (Sobel test statistic = −2.68, 2-tailed P = 0.007).
TABLE 3.
Regression Models Demonstrating Mediation
| Estimate | SE | Wald χ2 | P | |
|---|---|---|---|---|
| Step 1: demonstrate that the independent variable (race) is associated with the outcome (group membership) | ||||
| Cirrhosis | 0.78 | 0.34 | 5.27 | 0.02 |
| Race (white vs. nonwhite) | 0.83 | 0.31 | 7.31 | 0.007 |
|
| ||||
| Estimate | SE | t value | P | |
|
| ||||
| Step 2: demonstrate that the independent variable (race) is associated with the mediator (gist principles) | ||||
| Cirrhosis | 0.84 | 0.47 | 1.90 | 0.07 |
| Gist principles | 1.79 | 0.45 | 3.99 | < 0.0001 |
|
| ||||
| Estimate | SE | Wald χ2 | P | |
|
| ||||
| Step 3: demonstrate that the mediator affects the relationship between the independent variable (race) and the outcome (group membership) | ||||
| Cirrhosis | 0.65 | 0.36 | 3.33 | 0.07 |
| Race | 0.51 | 0.33 | 2.38 | 0.12 |
| Gist principles | 0.23 | 0.06 | 14.43 | 0.0001 |
FIGURE 2.

Mediation model.
DISCUSSION
Until recently, all available medications used to treat HCV carried a significant risk of toxicity, including fatigue, flu-like symptoms, joint pain, and depression. Therefore, for many years patients with HCV have faced a difficult treatment decision: whether to undergo treatment now or whether to wait for future less burdensome treatment options. In this study, we found that patient preferences for the HCV treatments of pegylated interferon, ribavirin, and either telaprevir or boceprevir cluster into 2 distinct groups: those preferring to begin treatment now and a slightly smaller group preferring to defer. Among those preferring to start treatment, preferences were evenly divided among the currently available treatment options, suggesting that most patients would be willing to accept either regimen. In contrast, patients preferring to defer, were unwilling to consider beginning treatment now, even for a hypothetical regimen having the maximum benefits and minimal risks associated with telaprevir and boceprevir. These findings indicate that there are groups of HCV patients with similar preferences that are distinct from other groups’ preferences and illustrate the value of using segmentation analysis to understand preference heterogeneity.
Few patient characteristics predicted treatment preference. Patients with cirrhosis were less likely to defer. This is expected given that patients with cirrhosis are frequently encouraged to undergo treatment sooner rather than later given their poor prognosis. We also found that African American patients were more likely to defer treatment. This finding is consistent with other studies demonstrating a greater likelihood of medical and surgical treatment refusal among African Americans compared with white patients of similar disease severity.23–30 Our results suggest that the explanation for this finding is due to a difference in gist principles. African American patients were more likely to endorse negative gist principles and less likely to endorse positive principles compared with their white counterparts. The strong association between gist principles and treatment preferences found in this study is consistent with literature across many disciplines demonstrating that gist principles, attitudes, and values are strong predictors of both preferences and choice behavior.10,31,32 Moreover, gist principles and values tend to have a greater impact on preferences than objective knowledge.33,34
Treatment for HCV currently involves selecting among several available multidrug, all-oral, interferon-free regimens. Treatment duration is between 8 and 24 weeks, rather than the 24 and 48 weeks considered by patients in this study, interferon is generally not used, and cure rates frequently exceed 90%.35–41 Treatment does, however, require in-person visits at the medical center every 2 weeks to pick up medications and for close follow-up. It is clear to treating providers that more patients now opt to be treated than was the case with earlier generations of therapy.42
There are several important strengths of this study. We used conjoint analysis, a robust preference measurement tool, to quantify preferences. An important advantage of using this approach is that preferences are quantified based on trade-offs between specific risks and benefits and therefore are not biased by physicians’ preferences. The responses to the first-fixed task demonstrated that subjects could identify dominant choices. The similar proportions of subjects deferring under the fixed task and latent class analyses support the 2 clusters of preferences found. In addition, while many studies have demonstrated disparities between African American and white patients’ treatment preferences, the mediation of this effect by gist principles, improves our understanding of why these disparities exist.
There are also significant limitations to the present study. Although veterans are at higher risk for HCV and important stakeholders in HCV-related treatment decisions, our sample may not be representative of other patient populations as participants were from 2 VA medical centers. Further studies are needed to examine preference patterns and the relationship between values and preferences among women, and to examine the influence of gist principles on treatment preferences for newer options.
In summary, deferral is the preferred option for a significant percentage of patients. Preference to defer is stronger among African American than white veterans, and this difference is explained by differences in gist principles. Outside of cirrhosis, patient characteristics do not predict preference. This study illustrates how understanding preference heterogeneity can enable physicians to better communicate with their patients. Future studies are needed to examine patients’ preferences regarding treatment in context of new all-oral, interferon-free regimens for chronic HCV infection.
Supplementary Material
Acknowledgments
The authors would like to thank Dorthe Welch and Lanette Errante for recruiting and interviewing the subjects for this study. The authors are, as always, indebted to the Veterans.
Supported in full by the VA Health Services and Research Department (IIR 10-131). Analyses were performed by Liana Fraenkel. All authors had a substantial role in the design of the study and writing the manuscript.
Footnotes
J.L. has served as a consultant for Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, Janssen, Merck, and has received research funding from Abbott, Achillion, Bristol-Myers Squibb, Gilead, and Janssen (paid to the institution). V.R. has served on including committees of the National Academy of Sciences, Psychonomic Society, and other nonprofits. She has been a paid consultant for Xerox. G.G.-T. has served as a consultant for Abbvie and Fibrogen, is an associate editor for Hepatology and serves on committees of the American Association for the Study of Liver Diseases. The remaining authors declare that they have nothing to disclose.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.jcge.com.
References
- 1.Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429–2438. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 2.Shiratori Y, Imazeki F, Moriyama M, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 3.Shindo M, Hamada K, Oda Y, et al. Long-term follow-up study of sustained biochemical responders with interferon therapy. Hepatology. 2001;33:1299–1302. doi: 10.1053/jhep.2001.24100. [DOI] [PubMed] [Google Scholar]
- 4.Kasahara A, Hayashi N, Mochizuki K, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Hepatology. 1998;27:1394–1402. doi: 10.1002/hep.510270529. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology. 2002;123:483–491. doi: 10.1053/gast.2002.34785. [DOI] [PubMed] [Google Scholar]
- 6.Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 7.Fraenkel L, Chodkowski D, Lim J, et al. Patients’ preferences for treatment of hepatitis C. Med Decis Making. 2010;30:45–57. doi: 10.1177/0272989X09341588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kauf TL, Mohamed AF, Hauber AB, et al. Patients’ willingness to accept the risks and benefits of new treatments for chronic hepatitis C virus infection. Patient. 2012;5:265–278. doi: 10.1007/BF03262498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauber BA, Fairchild AO, Johnson FR. Quantifying benefit-risk preferences for medical interventions: An overview of a growing empirical literature. Appl Health Economics Health Policy. 2013;11:319–329. doi: 10.1007/s40258-013-0028-y. [DOI] [PubMed] [Google Scholar]
- 10.Reyna VF. A theory of medical decision making and health: Fuzzy trace theory. Med Decis Making. 2008;28:850–865. doi: 10.1177/0272989X08327066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyna VF, Estrada SM, DeMarinis JA, et al. Neurobiological and memory models of risky decision making in adolescents versus young adults. J Exp Psychol Learn Mem Cogn. 2011;37:1125–1142. doi: 10.1037/a0023943. [DOI] [PubMed] [Google Scholar]
- 12.Broniatowski DA, Klein EY, Reyna VF. Germs are germs, and why not take a risk? Patients’ expectations for prescribing antibiotics in an inner-city emergency department. Med Decis Making. 2015;35:60–67. doi: 10.1177/0272989X14553472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraenkel L, Peters E, Charpentier P, et al. Decision tool to improve the quality of care in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:977–985. doi: 10.1002/acr.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe CR, Reyna VF, Widmer CL, et al. Efficacy of a web-based intelligent tutoring system for communicating genetic risk of breast cancer: a fuzzy-trace theory approach. Med Decis Making. 2015;35:46–59. doi: 10.1177/0272989X14535983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. (Sawtooth Software Technical Paper Series).HB-Reg v4 For Hierarchical Bayes Regression. Available at: http://www.sawtoothsoftware.com/support/technical-papers/sawtooth-software-products/hb-reg-technical-paper-2013. Accessed June 22, 2015.
- 16.Carroll FE, Al-Janabi H, Flynn T, et al. Women and their partners’ preferences for Down’s syndrome screening tests: a discrete choice experiment. Prenat Diagn. 2013;33:449–456. doi: 10.1002/pd.4086. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham CE, Walker JR, Eastwood JD, et al. Modeling mental health information preferences during the early adult years: a discrete choice conjoint experiment. J Health Commun. 2014;19:413–440. doi: 10.1080/10810730.2013.811324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feudtner C, Walter JK, Faerber JA, et al. Good-parent beliefs of parents of seriously ill children. JAMA Pediatr. 2015;169:39–47. doi: 10.1001/jamapediatrics.2014.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goossens LM, Utens CM, Smeenk FW, et al. Should I stay or should I go home? A latent class analysis of a discrete choice experiment on hospital-at-home. Value Health. 2014;17:588–596. doi: 10.1016/j.jval.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Woodward AT. A latent class analysis of age differences in choosing service providers to treat mental and substance use disorders. Psychiatr Serv. 2013;64:1087–1094. doi: 10.1176/appi.ps.201200401. [DOI] [PubMed] [Google Scholar]
- 21.Huber J, Orme B, Miller R. The value of choice simulators. In: Gustafsson A, Herrmann AF, editors. Conjoint Measurement. 4th. New York, NY: Springer; 2007. pp. 347–362. [Google Scholar]
- 22.Orme B. Market Simulators for Conjoint Analysis Getting Started With Conjoint Analysis: Strategies for Product Design and Pricing Research. 2nd. Madison, WI: Research Publishers LLC; 2010. [Google Scholar]
- 23.Constantinescu F, Goucher S, Weinstein A, et al. Racial disparities in treatment preferences for rheumatoid arthritis. Med Care. 2009;47:350–355. doi: 10.1097/MLR.0b013e31818af829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constantinescu F, Goucher S, Weinstein A, et al. Understanding why rheumatoid arthritis patient treatment preferences differ by race. Arthritis Rheum. 2009;61:413–418. doi: 10.1002/art.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braverman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 26.Byrne MM, Souchek J, Richardson M, et al. Racial/ethnic differences in preferences for total knee replacement surgery. J Clin Epidemiol. 2006;59:1078–1086. doi: 10.1016/j.jclinepi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Med Care. 2003;41:479–489. doi: 10.1097/01.MLR.0000053228.58042.E4. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim SA, Siminoff LA, Burant CJ, et al. Differences in expectations of outcome mediate African American/white patient differences in “willingness” to consider joint replacement. Arthritis Rheum. 2002;46:2429–2435. doi: 10.1002/art.10494. [DOI] [PubMed] [Google Scholar]
- 29.Long JA, Chang VW, Ibrahim SA, et al. Update on the health disparities literature. Ann Intern Med. 2004;141:805–812. doi: 10.7326/0003-4819-141-10-200411160-00013. [DOI] [PubMed] [Google Scholar]
- 30.Suarez-Almazor ME, Souchek J, Kelly PA, et al. Ethnic variation in knee replacement: patient preferences or uninformed disparity? Arch Intern Med. 2005;165:1117–1124. doi: 10.1001/archinte.165.10.1117. [DOI] [PubMed] [Google Scholar]
- 31.Redelmeier DA, Rozin P, Kahneman D. Understanding patients’ decisions: cognitive and emotional perspectives. JAMA. 1993;270:72–76. [PubMed] [Google Scholar]
- 32.Keeney RL. Value-focused Thinking: A Path to Creative Decisionmaking. Cambridge, MA: Harvard UP; 1992. [Google Scholar]
- 33.Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459–464. doi: 10.1016/j.vaccine.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Meszaros JR, Asch DA, Baron J, et al. Cognitive processes and the decisions of some parents to forego pertussis vaccination for their children. J Clin Epidemiol. 1996;49:697–703. doi: 10.1016/0895-4356(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 35.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 36.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV Genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 37.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with Abt-450/r–ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 38.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 39.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 40.Poordad F, Hezode C, Trinh R, et al. Abt-450/r–ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 41.Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with abt-450/r–ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 42.Kohli A, Shaffer A, Sherman A, et al. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


