Abstract
Background
A number of proteins secreted from adipose tissue, known as adipokines, are involved in the inflammatory process. The expression and secretion of adipokines are altered with obesity, leading to a pro-inflammatory state, with an enhanced vascular immune response. Although weight loss reduces inflammation, the time course for these changes during massive weight loss after bariatric surgery is not well described. We examined the changes in the biomarkers of inflammation after laparoscopic Roux-en-Y gastric bypass (RYGB) in morbidly obese individuals in a university hospital.
Methods
The fasting levels of plasma inflammatory adipokines, including leptin, adiponectin, C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and soluble receptor 1 for TNF-α were measured before surgery (baseline) and 3 weeks, 3 months, and 6 months after surgery in 15 morbidly obese patients who underwent Roux-en-Y gastric bypass without a major complication.
Results
The mean weight loss at 6 months was 25.7% ± 4.5% of the total body weight. The body mass index decreased from a mean of 55.1 ± 6.6 kg/m2 to 40.5 ± 5.5 kg/m2. The concentrations of leptin, CRP, and soluble receptor 1 for TNF-α decreased, and the adiponectin levels had increased from the baseline measures by 6 months postoperatively. The baseline and 6-month TNF-α and CRP levels correlated with each other. No other significant associations among the biomarkers were seen.
Conclusion
RYGB reduced the pro-inflammatory biomarkers and increased the anti-inflammatory mediators of obesity, independent of the magnitude of weight loss. The lack of correlations between the changes in biomarkers and weight loss suggests that the driving force behind the changes in the inflammatory markers is multifactorial and needs further investigation to clarify the health changes that occur after RYGB.
Keywords: Adipokines, Anti-inflammatory, Pro-inflammatory, Weight loss
Historically, adipose tissue's function was thought to be exclusively a storage depot for body energy (fat). Current knowledge has shown that this tissue consists of several cell types, including mature adipocytes, preadipocytes, fibroblasts, endothelial cells, and macrophages. The tissue behaves as an endocrine organ because it produces and secretes signaling proteins known as adipokines [1]. Obesity, specifically abdominal or visceral obesity, leads to high levels of most pro-inflammatory adipokines, such as leptin, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and the soluble receptor 1 for TNF-α (TNFSR1). In contrast, adiponectin, an anti-inflammatory adipokine, is decreased in obesity. Functionally, adipokines modulate a number of processes, including the immune response, insulin sensitivity, and energy balance, to name a few. The chronic inflammatory state in obesity is purported to contribute to the disease processes linked with obesity, such as type 2 diabetes and cardiovascular disease, owing to their involvement in vascular reactivity, thrombosis, angiogenesis, direct effects on insulin sensitivity, sympathetic nervous activity, and inflammation [1–3]. Furthermore, the expression and release of adipokines from fat tissue are affected by the specific depot sites, because the visceral site has greater macrophage infiltration and, subsequently, greater expression of pro-inflammatory and lower expression of anti-inflammatory adipokines than does subcutaneous fat [4,5].
The success of bariatric weight loss procedures for treating obesity and its related co-morbidities was recently documented in a meta-analysis [6]. However, the biologic mechanisms by which bariatric surgery resolves these conditions are not fully understood. One probable mechanism is the inflammatory hypothesis, which suggests that the health risks of obesity are caused by the presence of a low-grade inflammatory state in obesity [7,8]. Although several studies have examined the effect of inflammatory markers after surgery, only a few have examined both pro- and anti-inflammatory markers in the same study. Additionally, studies of the response at several points, when weight loss is greatest (during the first 6 mo), are limited. Earlier work has demonstrated inconsistencies in the response of these inflammatory biomarkers after surgery, possibly resulting from differences in the type of weight loss procedure performed and the follow-up duration [9–11]. The present analysis has added to these data by analyzing the serial changes in leptin, C-reactive protein (CRP), IL-6, TNF-α, TNFSR1, and adiponectin before (baseline) and 3 weeks, 3 months, and 6 months after Roux-en-Y gastric bypass (RYGB). Furthermore, we studied the associations among the various inflammatory biomarkers, body mass index (BMI), and weight loss at baseline and 6 months postoperatively.
Methods
Study population
Both men and women were recruited from the general surgery clinic scheduled for laparoscopic RYGB at Wake Forest University Baptist Medical Center. To be eligible for RYGB, the patients must have had a minimum BMI of 40.0 kg/m2 or a BMI of ≥35.0 kg/m2 with an obesity-related co-morbidity, such as diabetes, hypertension, or dyslipidemia, had to be willing to report to the medical center to undergo testing procedures at the scheduled times, and had to have plans to remain in the area for the duration of the study. Pregnant women or women planning to become pregnant during the follow-up period were excluded. All participants had met with the surgeon and clinic staff and were scheduled for surgery before learning about and being enrolled in the present study. All clinic patients who met the eligibility criteria were given the option to participate in the present study. No selection biases were apparent in the study recruitment.
The institutional review board of Wake Forest University Health Sciences approved the study. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The staff from the general surgery clinic obtained consent for study participation and acquired the demographic information and health history from the subjects.
Outcome variables
Data were collected at baseline (before surgery) and 3 weeks, 3 months, and 6 months after surgery. At each of these points, the participants reported to the General Clinical Research Center of Wake Forest University Baptist Medical Center after a 12-hour overnight fast and the following measures were collected.
Body weight and height
Standard techniques were used to obtain the patients’ body weight and height. In brief, the body weight and height were determined with the shoes, jackets, and outer garments removed. The measurement instruments were calibrated weekly.
Inflammatory biomarkers
Whole blood samples were collected in ethylenediaminetetraacetic acid-treated vacutainers using venipuncture in the early morning (7–9 am) after a 12-hour fast. Before blood sampling, the participants were queried about their medication use and health status. Any participant who reported currently taking any antibiotic medication or having an overt infection (e.g., urinary tract, respiratory) or fever (>99.0°F) were rescheduled. The samples were put immediately on ice and separated by centrifugation for 20 minutes at 4°C within 30 minutes of collection. After separation, the specimens were stored in 1-mL aliquots at −80°C until analysis.
Fasting plasma concentrations of leptin, adiponectin, IL-6, TNF-α, and TNFSR1 were determined using enzyme-linked immunosorbent assay kits (high sensitivity for IL-6 and TNF-α) from R&D Systems (Minneapolis, MN). CRP was measured using an automated immunoanalyzer (Immulite, Diagnostics Products, Los Angeles, CA). All samples were measured in duplicate, and the average of the 2 values was used for data analyses. Samples with values greater than the maximal detection limit were diluted (1:2) and reanalyzed. Duplicate samples that did not provide a coefficient of variation of <15% were reanalyzed. The intra-assay and interassay coefficient of variation for IL-6, TNF-α, and the soluble receptor assay was 7% and 16%, 8% and 23%, and <15%, respectively. The inter-assay and intra-assay coefficient of variation for the CRP assay (ALPCO, Windham, NH) was 8% and 7%, respectively.
Statistical analysis
The inflammatory biomarker data were not normally distributed; therefore, the data were transformed by obtaining the natural log of the raw values. However, for clarity, the nontransformed values are shown in Figures 1 and 2, and these values have been referenced in the “Results” and “Discussion” sections. Repeated measures analysis of covariance was used to assess the changes in the natural log concentrations of the inflammatory markers, BMI, and body weight during follow-up. The estimated marginal means are presented from the analysis. The covariates in the model were age, initial BMI, and number of co-morbidities. Spearman correlations were determined for BMI, weight loss, and inflammatory biomarkers. Statistical significance was set at P ≤.05. All analyses were performed using PASW, version 17.0 (SPSS, Chicago, IL).
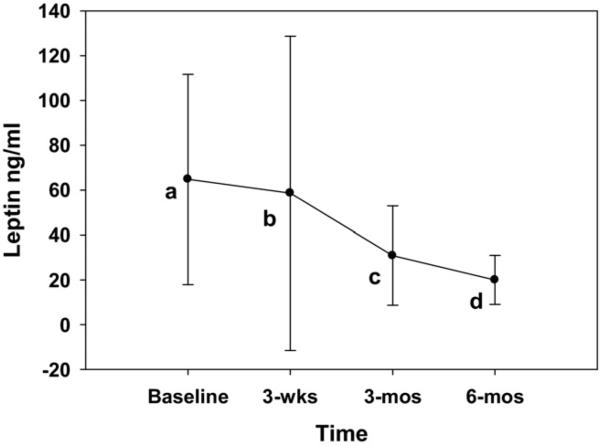
Fig. 1.
Plasma levels of leptin during 6-month follow-up period. Values are estimated marginal mean with standard deviation. Value at each point significantly different from others.
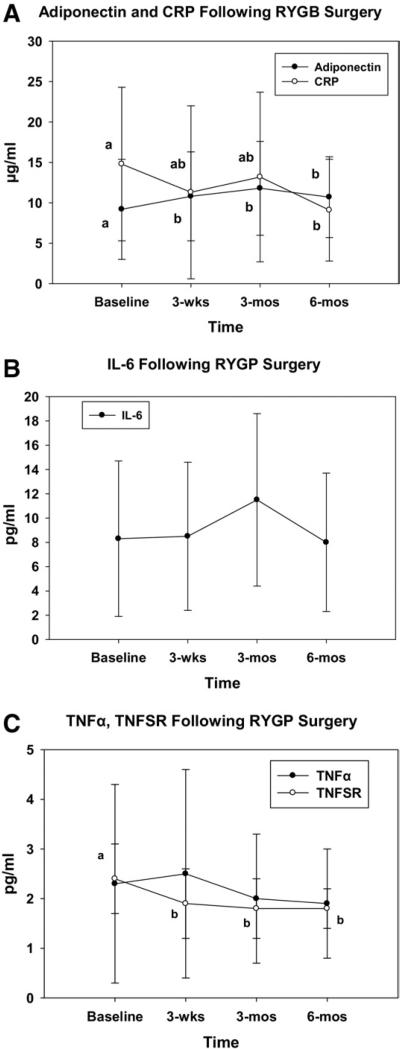
Fig. 2.
Plasma levels of (A) adiponectin and CRP, (B) IL-6, and (C) TNF-α and TNFSR during 6-month follow-up period. Values are estimated marginal mean with standard deviation. Points with different letters are significantly different from each other. For example, in Fig. A, CRP at baseline was significantly different from that at 6 months (a versus b), but 3-week and 3-month values were not different from each other or different from baseline or 6-month value (ab designation for 3 wk and 3 mo).
Results
A total of 27 patients agreed to participate in the present study and underwent laparoscopic RYGB. During the study period, 6 serious adverse events in 3 different participants were noted; none of the events were the result of the study's testing procedures. A total of 6 participants withdrew from the study (including 2 of the 3 with serious adverse events) and another 6 patients were not included in the study results because they were lost to at least some part of follow-up testing. The reasons for being withdrawn from the study were surgery complications, no longer being followed up by the surgeon, and personal (lack of time and no child care). Subsequently, only those with a blood sample at all 4 points were included in the data analysis (n = 15). No significant differences were found in demographics or baseline body weight and inflammatory marker levels between those who completed and did not complete the study. Age, gender, and prevalence of co-morbidities are listed in Table 1. The cohort was 45.9 ± 8.9 years old, and most were women (n = 14) and white (n = 13). Of the 15 patients with complete data, 13 (87%) reported ≥4 obesity-related co-morbidities. The BMI and body weight at baseline was 55.1 ± 6.6 kg/m2 and 152.9 ± 23.1 kg, respectively (Table 2). Nearly 10% of the total body weight was lost at 3 weeks and ~25% at 6 months postoperatively.
Table 1.
Demographics and obesity co-morbidities
| Variable | Value |
|---|---|
| Mean age (yr) | 45.9 ± 8.9 |
| Women (n) | 14 (93) |
| Ethnicity (n) | |
| White | 13 (87) |
| Black | 2 (13) |
| Co-morbidity (n) | |
| Degenerative joint disease | 14 (93) |
| Gastroesophageal reflux disease | 11 (73) |
| Hypertension | 11 (73) |
| Sleep apnea | 8 (53) |
| Dyslipidemia | 6 (40) |
| Type 2 diabetes mellitus | 5 (33) |
Data in parentheses are percentages.
Table 2.
Body mass index, weight, and natural log values for inflammatory markers at baseline and follow-up (n = 15)
| Variable | Baseline | Follow-up point |
||
|---|---|---|---|---|
| 3wk | 3mo | 6mo | ||
| BMI (kg/m2) | 55.1 ± 6.6* | 50.5 ± 6.2* | 45.2 ± 5.7* | 40.5 ± 5.5§ |
| Weight (kg) | 152.9 ± 23.1* | 140.0 ± 20.4* | 125.8 ± 17.6* | 111.3 ± 15.7§ |
| Weight loss (% from baseline) | –8.4 ± 1.5* | –17.5 ± 3.0* | –25.7 ± 4.5‡ | |
| Natural log leptin (ng/mL) | 4.02 ± .54* | 3.68 ± .83† | 3.15 ± .85‡ | 2.73 ± 0.93§ |
| Natural log adiponectin (μg/mL) | 2.00 ± .70* | 2.23 ± .59† | 2.34 ± .55† | 2.26 ± .53† |
| Natural log CRP (μg/mL) | 2.46 ± .78a | 2.08 ± .84*† | 2.18 ± 1.06*† | 1.92 ± .87† |
| Natural log IL-6 (pg/mL) | 1.85 ± .73 | 1.81 ± .96 | 2.14 ± .99 | 1.79 ± .85 |
| Natural log TNF-α (pg/mL) | .62 ± .64 | .62 ± .82 | .53 ± .64 | .48 ± .55 |
| Natural log TNFSR1 (pg/mL) | .82 ± .32* | .60 ± .33† | .53 ± .34† | .58 ± .21† |
BMI = body mass index; CRP = C-reactive protein; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-α; TNFSR1 = soluble receptor 1 for TNF-α.
Data with different superscript symbols significantly different from each other (e.g., adiponectin was significantly lower at baseline than at other points, with no differences in values at 3 weeks, 3 months, and 6 months.
The natural log values were used in all statistical analyses of the inflammatory markers (Table 2). The leptin concentration was nearly 65 ng/mL at baseline and had decreased 70% to 20 ng/mL by 6 months (Fig. 1). Adiponectin was higher at each postoperative follow-up point compared with at baseline, although no significant differences were found in the adiponectin concentrations at the 3-week, 3-month, or 6-month points (Fig. 2A). The acute phase reactant protein, CRP, was significantly lower at 6 months than at baseline (14.77 μg/mL versus 9.10 μg/mL, respectively; Fig. 2A). However, no differences were found in the CRP concentrations between the baseline, 3-week, and 3-month points. The IL-6 and TNF-α levels were not different among the 4 sampling points, with IL-6 ranging from 7.97 to 11.46 pg/mL and TNF-α from 1.86 to 2.55 pg/mL (Fig. 2B,C). The TNFSR1 was lower at each postoperative point compared with at baseline, although no significant differences were found in the TNFSR1 concentrations at 3 weeks, 3 months, or 6 months postoperatively (Fig. 2C).
The Spearman correlations between the biomarkers and BMI at baseline and 6 months postoperatively are listed in Table 3. At baseline, leptin was the only biomarker that showed a correlation with BMI (r = .78). This relationship was still apparent at 6 months (r = .79). TNF-α and CRP correlated with each other at baseline (r = .51) and at 6 months (r = .53). At 6 months, TNF-α also correlated with TNFSR1. Adiponectin showed a trend for an association with TNFSR1 at baseline (P = .06); leptin also showed a trend for an association with TNFSR1 at 6 months (P = .09). The analysis was also performed between changes (absolute and relative) in the inflammatory biomarkers and changes in the BMI. However, because no statistically significant correlations were found, the data are not shown.
Table 3.
Spearman correlations between body mass index and inflammatory biomarkers at baseline and 6 months (n = 15)
| Variable | CRP | IL-6 | TNF-α | TNFSR1 | Adiponectin | Leptin |
|---|---|---|---|---|---|---|
| BMI | ||||||
| Baseline | .27 (.33) | .08 (.78) | –.08 (.78) | .32 (.24) | < .01 (.99) | .78 (< .01)* |
| 6 mo | .07 (.81) | –.03 (.93) | .17 (.57) | .38 (.18) | –.14 (.64) | .79 (< .01)* |
| Leptin | ||||||
| Baseline | .43 (.11) | .04 (.90) | .12 (.67) | .23 (.42) | –.14 (.62) | — |
| 6 mo | .21 (.45) | .01 (.97) | .20 (.48) | .44 (.09)† | –.36 (.18) | |
| Adiponectin | ||||||
| Baseline | –.34 (.22) | –.44 (.12) | –.40 (.14) | .49 (.06)† | — | |
| 6 mo | –.28 (.31) | –.20 (.50) | –.03 (.93) | –.09 (.75) | ||
| TNFSR1 | ||||||
| Baseline | –.33 (.23) | –.35 (.22) | < .01 (.99) | — | ||
| 6 mo | .20 (.47) | –.24 (.42) | .54 (.04)* | |||
| TNF-α | ||||||
| Baseline | .51 (.05)* | .15 (.62) | — | |||
| 6 mo | .53 (.04)* | –.45 (.11) | ||||
| IL-6 | ||||||
| Baseline | .04 (.90) | — | ||||
| 6 mo | –.10 (.73) |
BMI = body mass index; CRP = C-reactive protein; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-; TNFSR1 = soluble receptor 1 for TNF-α.
Data presented as r, with P values in parentheses.
Significant at P <.05.
Trend for significance with P = .05 to .10.
Discussion
Our aim was to examine the serial responses of both pro- and anti-inflammatory markers to extreme weight loss by studying the changes in adipokines and acute phase reactant proteins after laparoscopic RYGB surgery. Furthermore, the correlations of the inflammatory markers with BMI, weight loss, and each other were investigated. Reductions in the pro-inflammatory biomarkers have been put forth as a physiologic mechanism for health improvement with weight loss [8]; however, there is still much to learn and understand regarding their response after bariatric surgery. The present study adds to the published data by measuring the inflammatory markers at a number of follow-up points ≤6 months postoperatively, during the period of intense weight loss.
The pro-inflammatory state of obesity has been proposed as a causative factor for a number of metabolic health conditions of obesity, including diabetes, cardiovascular disease, and cancer [1]. In a large longitudinal observational study, IL-6 was found to be related to insulin resistance, which was strengthened with increasing BMI [12]. Additional support for the relationship between obesity co-morbidities and inflammation has been shown by Swarbrick et al. [13], because they found that adipokines might be involved in the long-term treatment of diabetes after RYGB.
A number of other studies had follow-up points ≤6, 12, and 24 months but did not assess before 6 months [10,11,14], except for van Dielen et al. [9], who performed measurements at 3, 6, 12, and 24 months after surgery. Consistent with earlier work [9,11,14–16], we found significant decreases in several inflammatory markers, including leptin, CRP, and TNFSR1, from baseline to 6 months postoperatively. In addition, adiponectin significantly increased during this period. Iannelli et al. [11] showed reductions in CRP by 6 months and this response was greater by 12 months. Larger decreases in CRP were also apparent at 12 months in patients undergoing RYGB compared with laparoscopic sleeve gastrectomy [11]; however, the level of initial obesity did not affect the response [10]. CRP is the most often measured inflammatory marker, and it has consistently shown reductions no earlier than 6 months [9,11,14]. Iannelli et al. [11] observed additional decreases by 12 months. The lack of change in CRP before 6 months was surprising considering that others have found a 26% decrease in CRP after a 12-week dietary intervention [17]. This could be attributed to recovering from the surgical procedure versus the amount of body fat or weight lost [9].
The changes observed after RYGB in adiponectin and TNFSR1 were apparent at 3 weeks, with no further changes seen with greater weight loss at 6 months. In contrast to what we reported with CRP, this supports the theory that the impetus for the changes in adiponectin and TNFSR1 might be an acute negative energy balance and not necessarily modifications in body fat. The lack of an observed change in TNF-α can be explained in that it has a shorter half-life than CRP. Consistent with this hypothesis is the significant decrease in TNFSR1 observed in the present study, because TNFSR1 is more stable in the circulation and has a longer half-life than TNF-α [18,19]. The TNFSR1 response supports earlier work for this inflammatory marker after gastric bypass [20,21]. We anticipated that IL-6 would decrease after RYGB, because 25% of IL-6 is released from adipose tissue [22] and because of the significant decrease in CRP, whose production is stimulated in the liver by IL-6. However, we are uncertain why no decrease occurred in the plasma concentrations of IL-6 after RYGB.
A recent study examined both plasma and adipose tissue expression of inflammatory markers during weight loss caused by consuming a very low-energy diet, followed by weight maintenance during an isocaloric period [23]. They observed reductions in selected plasma inflammatory markers (e.g., CRP, serum amyloid A, and IL-6) during weight loss. However, during weight maintenance, the markers tended to increase toward baseline. Additionally, during the isocaloric period, increases occurred in subcutaneous adipose expression for several markers (adiponectin, IL-6, and TNF-α). Albeit this constitutes a different model than surgery-induced weight loss, it appears that the low-energy intake drives the reduction in pro-inflammatory markers and not the loss in body fat, because these markers increased to preweight loss levels during weight maintenance.
In another study, in a 2-year follow-up after restrictive weight loss surgery, patients showed variable changes in inflammatory markers over time [9]. In general, most inflammatory markers reached the plasma levels of controls at 2 years after surgery. However, during the early period, the biomarkers had either increased or showed no changes compared with baseline. This was evident across a broad range of bariatric procedures, including RYGB, biliopancreatic diversion, laparoscopic adjustable gastric band, and vertical banded gastroplasty and LapBand. This suggests that the surgery might initiate an inflammatory state, with the reductions in biomarkers from baseline not seen until ≥3 months after surgery for some markers and longer for others. The complications associated with the surgery could, at least in part, underlie some of these responses in the inflammatory markers. The present study was limited in that we were unable to attain specific information on certain items, such as surgery duration and complications during surgery. We did eliminate patients with postoperative complications from the analysis. Another explanation for the potential delay in the reduction of the inflammatory biomarkers is that the severe energy restriction after this type of surgery leads to persistent stress that can elevate certain markers [9]. This could explain the equivocal data in this area during the first several months after surgery.
Leptin was the only adipokine that showed additional reductions at each measurement point throughout the 6-month follow-up period, suggesting it is directly linked to the loss in body fat. Although leptin correlated with the BMI at baseline and 6 months, the change in weight loss did not correlate with the change in leptin. Other factors in addition to the fat mass are also involved in leptin's metabolism and levels, such as nutrient intake, eicosanoids, energy metabolizing hormones, and disease states [24,25]. The high initial levels of leptin likely resulted from our subjects’ high initial BMI (55.1 kg/m2); also 14 of the 15 individuals were women [15,16,26].
The increase in the anti-inflammatory adipokine adiponectin is consistent with the findings of others for various weight loss treatments [26,27]. Adiponectin increased from before surgery to 3 weeks postoperatively, but no additional increase was apparent at 6 months, even with continued reductions in body weight. This lack of relationship between adiponectin and BMI was surprising, although it could, at least in part, be explained by the accumulating evidence demonstrating that skeletal muscle cells synthesize and secrete adiponectin as a part of the myokine milieu [28,29]. We did not have body composition data available for the present analysis.
It has been proposed by others [13,30] that the adipokine response from weight loss might be affected by the presence of diabetes or insulin resistance. Whitson et al. [30] found in their preliminary study that CRP showed greater reductions with weight loss after bariatric surgery in patients with diabetic than in those without. Although we attempted to study the differences between those with and without diabetes in our analysis, we did not find differences in the changes in any of our inflammatory markers between those with and without diabetes. This might have been because of our limited sample size.
The results from the present study were drawn from a small homogenous sample size (white middle-age women), which could have biased the results. Additional limitations included a lack of information on the menopausal status of sample, surgical details (i.e., operative length, complications), measures of total body composition (i.e., fat mass and fat-free mass), and assessment of disease conditions and treatment regimens at the follow-up points.
Conclusion
A number of inflammatory biomarkers changed during the follow-up period after bariatric RYGB. Although participants had lost >25% of their body weight by 6 months, the level of inflammation remained high. Several of the inflammatory biomarkers demonstrated different patterns of changes, with TNFSR1, leptin, and adiponectin having significant changes in as few as 3 weeks, and CRP not decreasing until 6 months. Additional understanding of the mechanisms underlying they changes in these markers, as well as the time course of the changes, is warranted.
Acknowledgments
Supported by the Wake Forest University, Claude D. Pepper Older American Independence Center (National Institutes of Health grant P30 AG21332), the Wake Forest University General Clinical Research Center (National Institutes of Health grant M01-RR07122), and the Wake Forest University Cross-Campus Collaboration Grant.
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- 1.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediat Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissebah AH, Freedman DS, Peiris AN. Health risks of obesity. Med Clin North Am. 1989;73:111–38. doi: 10.1016/s0025-7125(16)30695-2. [DOI] [PubMed] [Google Scholar]
- 3.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–66. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 4.Zoico E, Di Francesco V, Mazzali G, et al. Adipocytokines, fat distribution, and insulin resistance in elderly men and women. J Gerontol A Biol Sci Med Sci. 2004;59:M935–9. doi: 10.1093/gerona/59.9.m935. [DOI] [PubMed] [Google Scholar]
- 5.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 2010;18:879–83. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 7.Cottam DR, Mattar SG, Barinas-Mitchell E, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004;14:589–600. doi: 10.1381/096089204323093345. [DOI] [PubMed] [Google Scholar]
- 8.Esposito K, Giugliano G, Scuderi N, Giugliano D. Role of adipokines in the obesity-inflammation relationship: the effect of fat removal. Plast Reconstr Surg. 2006;118:1048–57. doi: 10.1097/01.prs.0000232281.49432.ce. [DOI] [PubMed] [Google Scholar]
- 9.van Dielen FM, Buurman WA, Hadfoune M, Nijhuis J, Greve JW. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89:4062–8. doi: 10.1210/jc.2003-032125. [DOI] [PubMed] [Google Scholar]
- 10.Iannelli A, Anty R, Piche T, et al. Impact of laparoscopic Roux-en-Y gastric bypass on metabolic syndrome, inflammation, and insulin resistance in super versus morbidly obese women. Obes Surg. 2009;19:577–82. doi: 10.1007/s11695-008-9764-8. [DOI] [PubMed] [Google Scholar]
- 11.Iannelli A, Anty R, Schneck AS, Tran A, Gugenheim J. Inflammation, insulin resistance, lipid disturbances, anthropometrics, and metabolic syndrome in morbidly obese patients: a case-control study comparing laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy. Surgery. 2010;149:364–70. doi: 10.1016/j.surg.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Herbert A, Karamohamed S, Liu J, Manning A, Fox CS, Meigs JB, et al. BMI modifies associations of IL-6 genotypes with insulin resistance: the Framingham Study. Obesity. 2006;14:1454–61. doi: 10.1038/oby.2006.165. [DOI] [PubMed] [Google Scholar]
- 13.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, et al. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–11. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anty R, Dahman M, Iannelli A, et al. Bariatric surgery can correct iron depletion in morbidly obese women: a link with chronic inflammation. Obes Surg. 2008;18:709–14. doi: 10.1007/s11695-007-9276-y. [DOI] [PubMed] [Google Scholar]
- 15.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 16.García de la Torre N, Rubio MA, Bordiú E, et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008;93:4276–81. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 17.Heilbronn LK, Noakes M, Clifton PM. Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Arterioscler Thromb Vasc Biol. 2001;21:968–70. doi: 10.1161/01.atv.21.6.968. [DOI] [PubMed] [Google Scholar]
- 18.Peters M, Jacobs S, Ehlers M, et al. The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med. 1996;183:1399–406. doi: 10.1084/jem.183.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–9. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilarrasa N, Vendrell J, Sanchez-Santos R, et al. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-alpha receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol (Oxford) 2007;67:679–86. doi: 10.1111/j.1365-2265.2007.02945.x. [DOI] [PubMed] [Google Scholar]
- 21.Molina A, Vendrell J, Gutierrez C, et al. Insulin resistance, leptin and TNF-alpha system in morbidly obese women after gastric bypass. Obes Surg. 2003;13:615–21. doi: 10.1381/096089203322190844. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 23.Salas-Salvado J, Bullo M, Garcia-Lorda P, et al. Subcutaneous adi-pose tissue cytokine production is not responsible for the restoration of systemic inflammation markers during weight loss. Int J Obes (Lond) 2006;30:1714–20. doi: 10.1038/sj.ijo.0803348. [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Aliaga MJ, Lorente-Cebrian S, Martinez JA. Regulation of adipokine secretion by n-3 fatty acids. Proc Nutr Soc. 2010;69:324–32. doi: 10.1017/S0029665110001801. [DOI] [PubMed] [Google Scholar]
- 25.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–56. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 26.Trakhtenbroit MA, Leichman JG, Algahim MF, et al. Body weight, insulin resistance, and serum adipokine levels 2 years after 2 types of bariatric surgery. Am J Med. 2009;122:435–42. doi: 10.1016/j.amjmed.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manco M, Fernandez-Real JM, Equitani F, et al. Effect of massive weight loss on inflammatory adipocytokines and the innate immune system in morbidly obese women. J Clin Endocrinol Metab. 2006;92:483–90. doi: 10.1210/jc.2006-0960. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Chewchuk S, Lavigne C, et al. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab. 2009;297:E657–64. doi: 10.1152/ajpendo.00186.2009. [DOI] [PubMed] [Google Scholar]
- 29.Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology. 2004;145:5589–97. doi: 10.1210/en.2004-0503. [DOI] [PubMed] [Google Scholar]
- 30.Whitson BA, Leslie DB, Kellogg TA, et al. Adipokine response in diabetics and nondiabetics following the Roux-en-Y gastric bypass: a preliminary study. J Surg Res. 2007;142:295–300. doi: 10.1016/j.jss.2007.03.036. [DOI] [PubMed] [Google Scholar]