Abstract
We previously observed that the human bitter taste receptor T2R38 is an important component of upper respiratory innate defense because it detects acyl homoserine lactone (AHL) quorum sensing molecules secreted by gram-negative bacteria. T2R38 activation in human sinonasal epithelial cells stimulates calcium and nitric oxide signals that increase mucociliary clearance, the major physical respiratory defense against inhaled pathogens. While mice do not have a clear T2R38 orthologue, they do have bitter taste receptors capable of responding to T2R38 agonists, suggesting that T2R-mediated innate immune mechanisms may be conserved in mice. We examined whether AHLs activate calcium and nitric oxide signaling in mouse nasal epithelial cells and utilized pharmacology as well as cells from knockout mice lacking important components of canonical taste signal transduction pathways to determine if AHL-stimulated responses require taste signaling molecules. We found that AHLs stimulate calcium-dependent NO production that increases mucociliary clearance and thus likely serves an innate immune role against gram-negative bacteria. These responses require PLCβ2 and TRPM5 taste signaling components, but not α-gustducin. These data suggest the mouse may be a useful model for further studies of T2R-mediated innate immunity.
Keywords: Acyl-homoserine lactone, chronic rhinosinusitis, innate immunity, mucociliary clearance, nitric oxide, T2R bitter taste receptor
Introduction
Mucociliary clearance is the primary physical innate defense of both the upper and lower respiratory tract.1–3 Inhaled pathogens, toxins, and irritants are trapped by the mucus that overlays the airway epithelium. Coordinated ciliary beating by airway epithelial cells drives the transport of this debris-laden mucus toward the oropharynx, where it is removed by expectoration or swallowing.4–6 In chronic rhinosinusitis (CRS), impairment of mucociliary clearance in the upper respiratory tract can result in recurrent bacterial infections that require prolonged medical therapy having a detrimental impact on patient quality of life 4, 5 and creating an annual aggregated healthcare costs of $6 billion. Because CRS is responsible for approximately 1 in 5 antibiotic prescriptions in adults, it has been hypothesized that antibiotic resistance may become a significant problem in CRS,7–9 and therefore new treatment modalities are needed. An attractive therapeutic strategy for CRS patients would be to target endogenous host defense pathways to stimulate mucociliary clearance and/or innate immune mechanisms such as antimicrobial peptide secretion.
An emerging potential target pathway is that mediated by T2R bitter taste receptors.10–12 There are approximately 25 functional isoforms of human T2Rs, and many of these are also expressed in the ciliated epithelial cells of the human respiratory tract,13 including the sinonasal cavity.14 T2Rs in type II taste cells of the tongue protect against the ingestion of harmful compounds, including toxic bacterial and plant products, and at least one T2R in the human airway, described below, detects bacterial products and activates innate immune responses.14 Ciliated epithelial cells have long been known to serve an important sentinel role in adaptive and innate sinonasal immunity,15, 16 particularly through expression of Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), lipoteichoic acid (LTA), and flagellin.15, 16 We previously demonstrated that the human bitter taste receptor T2R38 similarly contributes to the sentinel function of ciliated epithelial cells. T2R38 is expressed in the cilia of human upper respiratory epithelial cells and detects physiological concentrations of acyl-homoserine lactone (AHL) quorum sensing molecules secreted by gram-negative bacteria such as Pseudomonas aeruginosa.14 Once activated, T2R38-mediated calcium signals stimulate nitric oxide synthase (NOS) and production of intracellular nitric oxide (NO), which serves two innate defense roles. The first is stimulation of ciliary beating through protein kinase G (PKG) activation to increase ciliary beat frequency and mucociliary clearance. The second role is direct bacterial killing upon diffusion of the NO into the airway surface liquid. NO and its reactive derivatives, including S-nitrosothiols and peroxynitrites, can damage bacterial DNA, inactivate enzymes containing reactive thiol groups or metal cofactors, and react with membrane lipids causing bacterial permeabilization.17, 18
The AHL-induced antibacterial response of human sinonasal epithelial cells is correlated with genetic polymorphisms known to regulate T2R38 functionality. T2R38 is encoded by the TAS2R38 gene, which has 2 common polymorphisms in Caucasian populations. One polymorphism encodes a functional receptor variant containing a proline (P), alanine (A), and valine (V) at amino acid positions 49, 262, and 296, respectively, while the other polymorphism encodes a receptor variant with alanine (A), valine (V), and isoleucine (I) at these positions, respectively.19–22 Homozygous PAV/PAV individuals (~20% frequency in Caucasian populations19) are termed “supertasters” for certainT2R38-specific agonists, such as phenylthiocarbamide (PTC) or 6-propyl-2-thiouracil (PROP), while AVI/AVI individuals (~30% frequency in Caucasian populations) are “non-tasters” for these bitter compounds. AVI/PAV heterozygotes have varying intermediate levels of taste for these compounds. Sinonasal epithelial cells derived from PAV/PAV “supertaster” individuals exhibited enhanced NO production, mucociliary clearance, and bacterial killing compared with AVI/PAV and AVI/AVI cells. Furthermore, preliminary clinical data suggest that patients with enhanced T2R38 function (PAV/PAV “supertasters”) may be less susceptible to gram-negative sinonasal infection than patients with low or intermediate levels of T2R38 function (AVI/AVI or AVI/PAV, respectively).14 Additionally, T2R38 supertasters are may be less likely to require surgical intervention for CRS.23 The T2R38 pathway is thus a potential target to promote bacterial clearance and innate immunity in patients with upper respiratory infections, but because there is a large subset of patients that are sub-optimally responsive to T2R38 agonists, it is critical to further define the T2R38-mediated signaling pathway in airway epithelial cells.
The availability of biochemical and molecular biological tools in mice, including knockout animals, makes the mouse an attractive model organism for dissecting out airway T2R signaling. While mice express T2R bitter taste receptors that can respond to the T2R38 agonists PTC and PROP,24–27 they do not have a clear T2R38 orthologue and the mechanisms reported to underlie PTC and/or PROP avoidance in mice are complex and may not be solely based on taste.28–33 However, we sought to examine whether mice exhibit a sinonasal epithelial innate immune response to PTC and P. aeruginosa AHLs and determine whether this response is dependent on taste signaling pathway. Our data suggest that important components of the sinonasal response to AHLs are conserved between mice and humans, revealing important mechanistic insights into this response. Also, this study suggests that the mouse is a useful model for future investigation of airway innate immune responses mediated by taste signaling molecules.
Methods
Reagents and solutions
All reagents and solutions used were previously described.14 Fluo-4, DAF-FM diacetate, thapsigargin, ionomycin, Texas Red dextran (10,000 MW), BAPTA-AM, and fluorescent microspheres were from Invitrogen (Grand Island, NY). N-butyryl-L-homoserine lactone (C4HSL), N-3-oxo-dodecanoyl-homoserine lactone (C12HSL), S-Nitroso-N-Acetyl-D,L-Penicillamine (SNAP), and carboxy-PTIO (cPTIO) were from Cayman Chemical (Ann Arbor, MI). U73122 and U73343 were from Tocris Biosciences/R&D Systems, Inc. (Minneapolis, MN USA). Unless indicated, all other reagents were from Sigma-Aldrich (St. Louis, MO USA). Physiological experiments were performed with Dulbecco’s PBS (DPBS; containing 1.8 mM calcium) on the apical side of the cultures. The basolateral side was bathed in modified HEPES-buffered Hank’s Balanced Salt Solution (HBSS) containing 1× MEM amino acids (Invitrogen) to provide a source of arginine (0.6 mM) for NO production. DPBS contained (in mM) 138 NaCl, 2.7 KCl, 1.5 KH2PO4, 8 Na2HPO4, 1.8 CaCl2, and 1.5 MgCl2, with pH adjusted to approximately 7.2. HBSS contained (in mM) 137 NaCl, 5 KCl, 0.4 KH2PO4, 0.3 Na2HPO4, 5.5 glucose, 1.8 CaCl2, 1.5 MgCl2, 10 HEPES, pH 7.4. Experiments performed in the absence of extracellular calcium utilized the above solutions with no added calcium and containing 1 mM EGTA.
Mouse septal sinonasal air-liquid interface (ALI) cultures
All mouse work was done with full approval of the University of Pennsylvania and Philadelphia VA Medical Center Institutional Animal Care and Use Committees (IACUCs). TRPM534 and α-gustducin35 knockout mice used were on an otherwise wildtype C57BL/6 background. ALI cultures were set-up as previously described36, 37 from excised nasal septae. Epithelial cells were isolated by collagenase and pronase digestion before culture on Costar 6.5 mm transwell permeable filter supports (Corning Inc. Life Sciences, Lowell, MA USA) with apical side submerged. After 7 days, cells reached confluence and the medium was removed from the apical surface with feeding from the basolateral side. Differentiation and ciliogenesis were observed within 10–14 days after exposure to air, and cultures were used within 4–6 weeks.
Bacterial culture
Bacterial culture was performed as previously described.14 Pseudomonas aeruginosa strains PAO1 (Wt), PAO-JP2 (ΔlasI, ΔrhlI; Tcr, HgCl2r),38 and Sad36 ([flgK]::Tn5B30[TCr])39 were grown for 3 days in Luria Broth (LB) media (PAO1) or LB plus tetracycline (15 μg/ml; PAO-JP2 and Sad36). Resultant medium was centrifuged at 2000 g for 15 min at room temperature and filtered through a 0.2 μm filter. The resultant conditioned medium (CM) was adjusted to an OD655 = 0.35 (blanked against LB) to normalize to pyocyanin concentrations.40 LB was used as a control for all experiments because no differences were observed between responses induced by LB and LB supplemented with tetracycline.
Calcium and nitric oxide imaging
Calcium and nitric oxide imaging were performed as previously described14, 36 using the calcium sensitive fluorophore Fluo-4 and reactive nitrogen species indicator DAF-FM, respectively. The validation of DAF-FM use as a method for measuring NO produced by sinonasal ALI cultures was previously described.14, 36 After loading of mouse sinonasal cultures with Fluo-4 AM (10 μM applied to apical side only for 2 hrs) or DAF-FM diacetate (10 μM applied to apical side only for 45 min), cultures were copiously washed and incubated in the dark at room temperature for 20 min to allow cells to de-esterify loaded dye and recover. Imaging was performed using the 488 nM laser line of an Olympus Fluoview confocal system with an Olympus IX-81 microscope and 10x (0.3 NA UPlanFLN objective; Olympus). Images were analyzed in Olympus Fluoview software and/or ImageJ as previously described.14, 36 No offset or gamma alterations were used. Fluo-4 fluorescence changes were normalized after subtraction of the background fluorescence, which was estimated for each experiment by measuring unloaded ALIs at identical settings. Baseline Fluo-4 fluorescence (Fo) was determined by averaging the first 10 frames of each experiment. The magnitudes of DAF-FM fluorescence changes were used to approximate NO production, and thus care was taken to follow the loading protocol strictly to normalize DAF-FM fluorescence loading.
Imaging of mucociliary transport
Mucociliary transport velocity was measured as previously described14 using 2 μm polystyrene fluorescent microspheres (0.0025% by weight in 30 μl) that were added to the apical surface of the cultures after copious washing with PBS to remove mucus clumps. Beads were imaged using an inverted Nikon TE2000E epifluorescence microscope (20x, 0.5 NA PlanFluor objective) equipped with a 12-bit QImaging camera and computer running ImageJ (NIH) and μManager.41 To qualify for inclusion in the statistical analysis, a streak had to have a visible beginning and ending within the field of view.
Data analysis and statistics
All statistical analyses (Student’s t test or ANOVA, as indicated) were performed in Excel and/or GraphPad Prism as indicated; P < 0.05 was considered statistically significant. For multiple comparisons, ANOVA with indicated post-test was performed. For all figures, one asterisk (*) indicates P <0.05, two asterisks (**) indicates P <0.01, and “n.s.” indicates no statistical significance.
Results
To examine sinonasal epithelial cell responses, we utilized air-liquid interface (ALI) cultures derived from mouse nasal septum.36, 37 ALI cultures are the state-of-the-art respiratory cell culture model, as they mimic the polarized respiratory epithelium with differentiated ciliated and goblet cells.42, 43 We have found that the expression of the bitter taste receptor T2R38 is identically localized to respiratory cilia in human sinonasal ALI cultures and human sinonasal tissue explants, suggesting the receptor-mediated responses are physiologically relevant. Because taste receptors are linked to downstream calcium signaling, we loaded mouse septal ALI cultures with the calcium-sensitive fluorophore Fluo-4 to measure relative changes in cytoplasmic free calcium concentration as previously reported.36
When mouse epithelial cells were stimulated by application of the bitter tastant PTC to the apical side of the ALI cultures, they exhibited sustained calcium responses with lower magnitude and slower kinetics relative to the purinergic receptor-induced responses stimulated by ATP (Fig. 1A). PTC-induced responses were blocked by U73122 (Fig. 1B), an inhibitor of PLCβ2, an important component of taste signaling10–12, 44–46 at a concentration that did not inhibit the ATP-induced response (Fig. 1C). These results appear to be very similar to the PTC-induced T2R38-mediated calcium signaling previously observed in human sinonasal ALI cultures and dissociated human sinonasal ciliated cells.14 We thus hypothesized that mouse ALI cultures express bitter taste receptors that respond to PTC and potentially also to bacterial AHL molecules.
Fig. 1.
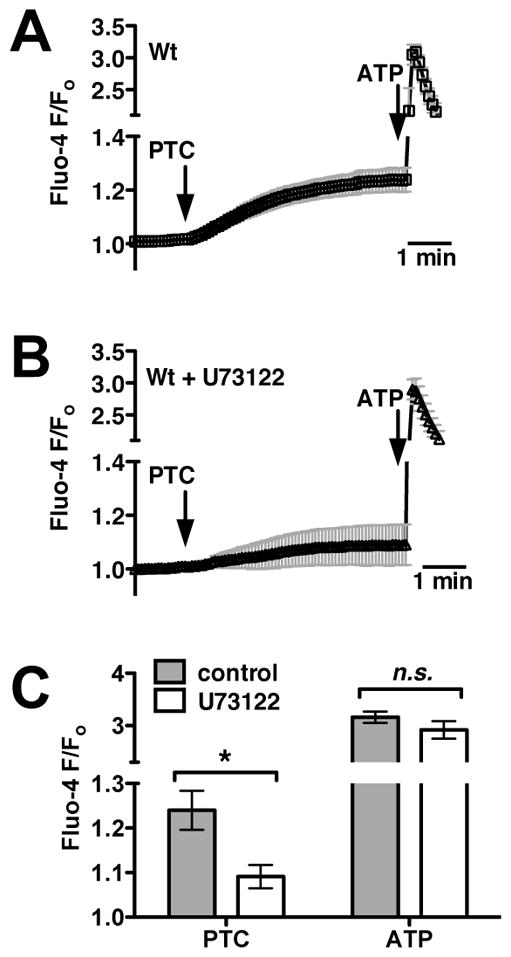
Mouse nasal septal epithelial cells respond to the bitter tastant PTC with an increase in intracellular calcium that is dependent upon the taste signaling component PLCβ2. (A) Average Fluo-4 trace (mean ± SEM) from mouse nasal septal ALIs (n = 5 cultures) during apical stimulation with 1 mM PTC and subsequent 100 μM ATP. Note the break in left y-axis because of the larger magnitude of the ATP response. (B) Average trace showing responses in the presence of the PLCβ2 inhibitor U73122 (5 μM; 10 min pre-incubation; apical side only; n = 5). (C) Bar graph of peak calcium responses (mean ± SEM from A–B) after 5 min stimulation with PTC (F/Fo = 1.24 ± 0.04 and 1.09 ± 0.03 in the absence and presence of U713122, respectively) and during ATP stimulation (F/Fo = 3.16 ± 0.1 and 2.92 ± 0.2, respectively). Symbols denote significance of indicated paired comparisons via 1-way ANOVA with Bonferroni post-test; *P <0.05, n.s. not significant.
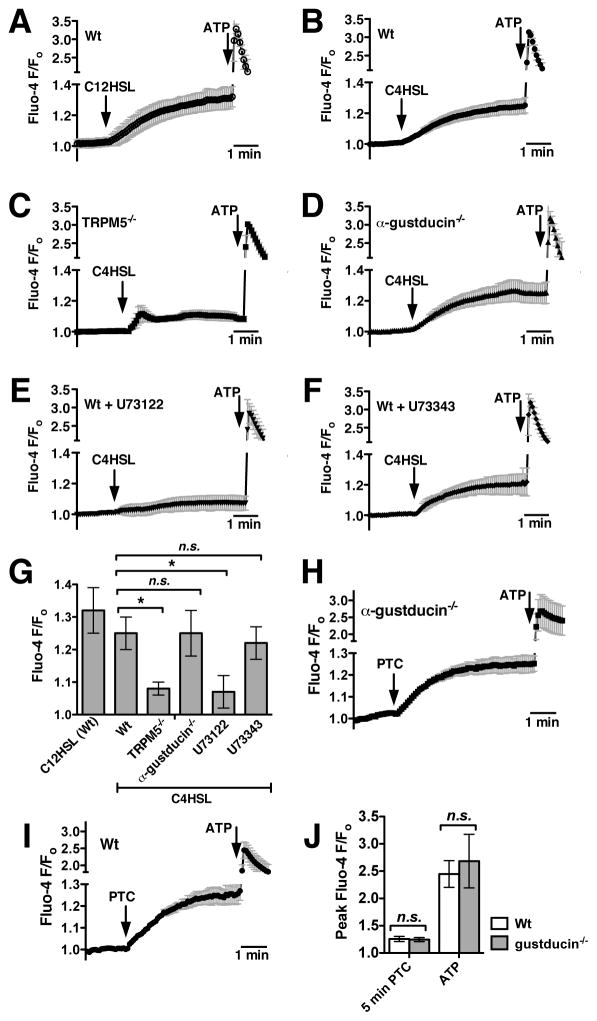
We next examined if mouse sinonasal epithelial cells used components of the classic taste signal transduction pathway to respond to the two most common AHL molecules used by airway pathogen Pseudomonas aeruginosa, C4HSL and C12HSL. AHL-mediated quorum sensing is a mechanism for bacteria to regulate gene expression in response to the physical constraints of their environment and external signals, including those coming from other individual bacteria. The quorum sensing in gram-negative bacteria can regulate many processes, including virulence, motility and swarming, and biofilm formation.38, 47–49 Mouse ALI cultures stimulated with either C12HSL or C4HSL (100 μM each) exhibited calcium responses that appeared very similar to those elicited by PTC (Fig. 2A–B). Interestingly, the magnitude and kinetics of this calcium response were altered significantly in TRPM5 knockout mouse cultures (Fig. 2C). In taste cells, TRPM5 is not itself calcium permeable but is activated by initial receptor-dependent intracellular calcium release and serves to allow cation influx and support sustained signaling by depolarizing the cell membrane potential and activating voltage-gated channels.34, 46, 50–52 From these data, TRPM5 appears to be required for maintaining sustained calcium signaling in response to C4HSL in mouse sinonasal epithelial cells. Interestingly, C4HSL-induced calcium signaling was completely intact in ALI cultures derived from mice lacking the taste G protein subunit α-gustducin (Fig. 2D).53–56 However, C4HSL-induced calcium signaling was inhibited by the PLCβ2 inhibitor U73122 (Fig. 2E) but not by its inactive analogue U73343 (Fig. 2F). These results are summarized in Fig. 2G, and suggest that AHLs induce calcium signals in mouse sinonasal epithelial cells that go through two important proteins also used in taste signaling, PLCβ2 and TRPM5. The involvement of PLCβ2 and TRPM5 but not α-gustducin was initially surprising, so this was confirmed by testing the responses to PTC in Wt and α-gustducin knockout cultures, which were not significantly different (Fig. 2H–J). Wt and α-gustducin knock-out status of cultures was confirmed by PCR genotyping of animals before isolating and culturing cells as well as afterwards by genotyping the same cultures used for the experiments shown in Fig. 2.
Fig. 2.
Pseudomonas aeruginosa AHLs stimulate mouse nasal calcium signaling that also requires components of taste signaling. (A–B) Average traces showing calcium responses in Wt cultures stimulated with 100 μM C12HSL (A; n = 3 cultures) or C4HSL (B; n = 5 cultures). (C–D) Traces showing calcium responses to C4HSL in TRPM5 knockout (TRPM5−/−; C, n = 7) and α-gustducin knockout (α-gustducin−/−; D, n = 3) ALIs. (D–E) Traces showing C4HSL-induced calcium responses in Wt cultures treated with the PLCβ2 inhibitor U73122 (D, n = 4) and the inactive U73343 (E, n = 4). (F) Bar graph showing calcium response after 5 min stimulation with C4HSL. Fluo-4 F/Fo values (mean ± SEM) after 5 min stimulation were 1.32 ± 0.07 (C12HSL; Wt), 1.25 ± 0.05 (C4HSL; Wt), 1.08 ± 0.02 (C4HSL; TRPM5−/−), 1.25 ± 0.07 (C4HSL; gustducin−/−), 1.07 ± 0.05 (C4HSL + U73122; Wt), and 1.22 ± 0.05 (C4HSL ± U73343). (H–I) To confirm the lack of role for α-gustducin, cultures from Wt (n = 5) and α-gustducin−/− (n = 5) mice were also stimulated with 1 mM PTC. (J) Bar graph showing calcium response after 5 min stimulation with PTC and peak calcium response after stimulation with ATP. Fluo-4 F/Fo values (mean ± SEM) after PTC were 1.26 ± 0.05 (Wt) and 1.24 ± 0.04 (α-gustducin−/−). Fluo-4 F/Fo values (mean ± SEM) after ATP were 2.45 ± 0.25 (Wt) and 2.68 ± 0.49 (α-gustducin−/−). Symbols denote significance determined by 1-way ANOVA with Dunnett’s (G) or Bonferroni (J) post-tests; *P <0.05, n.s. not significant.
ALI cultures are made up of numerous cell types, including ciliated epithelial cells, goblet cells, and dendritic cells.37, 57–59 We previously showed T2R38 expressed in human sinonasal ciliated epithelial cells detects AHLs and activates calcium and NO responses directly in the ciliated cells14. However, other studies in mice have shown that AHLs and bitter tastants stimulate calcium responses from dissociated nonciliated epithelial cells in the nose termed solitary chemosensory cells (SCCs) that do require α-gustducin in addition to PLCβ2 and TRPM5.27, 60, 61 We tested whether the responses observed in our cultures originated from ciliated epithelial cells by taking dissociated ciliated epithelial cells from mouse airway (visualized under simultaneous differential interference contrast and confocal fluorescence microscopy), loading them with Fluo-4, and stimulating them with PTC (Fig. 3A) or C4HSL (Fig. 3B). Ciliated epithelial cells did indeed respond to both agonists with similar calcium signals, suggesting that the responses we observe in ALI cultures indeed originate from the ciliated epithelial cells themselves.
Fig. 3.
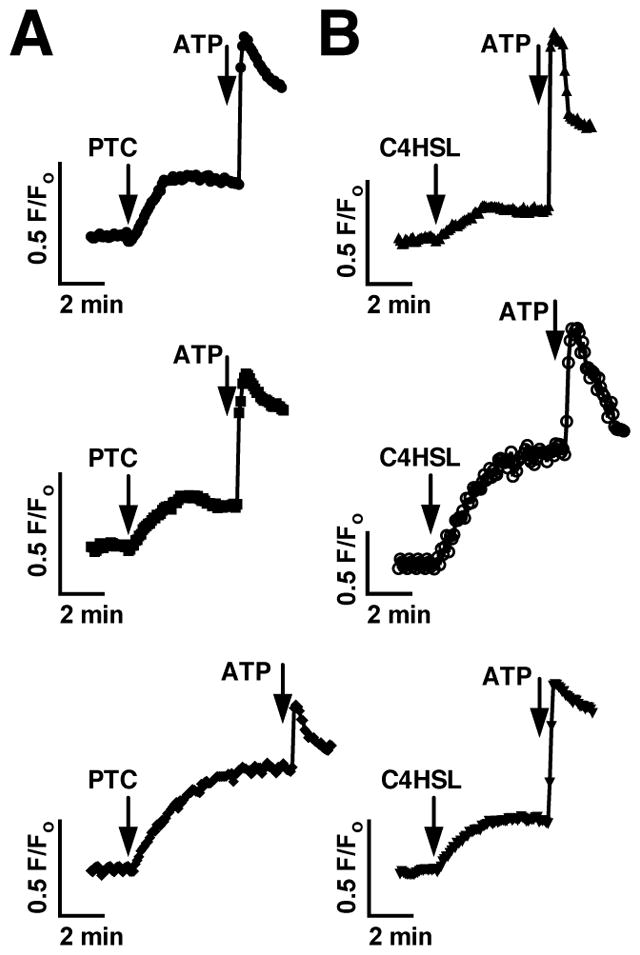
Dissociated mouse septal ciliated epithelial cells exhibit calcium responses to PTC and C4HSL. (A–B) Mouse septal ciliated epithelial cells were dissociated as previously described and stimulated with 1 mM PTC (A) or 150 μM C4HSL (B). Three responses from each condition are shown, representative of 5–7 experiments each.
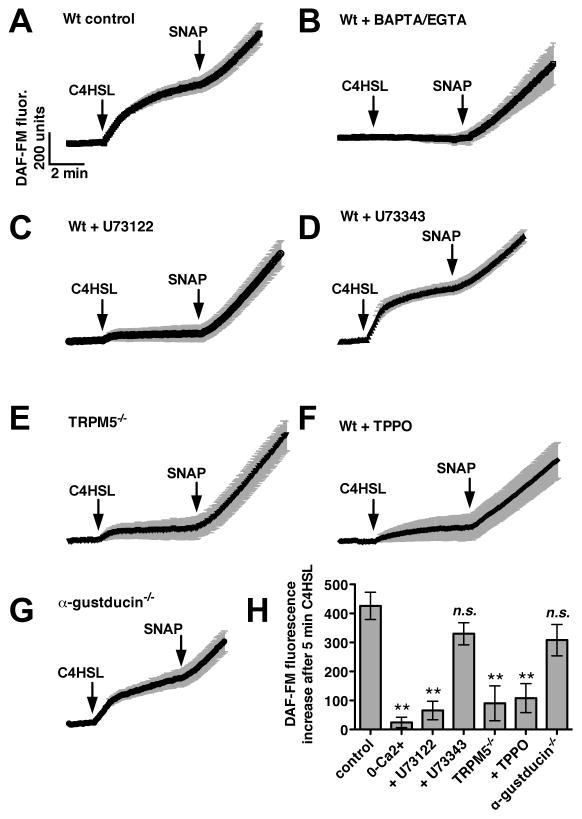
We next examined whether AHL-induced mouse sinonasal calcium signaling results in production of nitric oxide, an important signaling component that can regulate two important icomponents of airway innate immunity by increasing ciliary beating and mucociliary clearance 14, 62 as well as by directly killing bacteria.14, 17, 18 Nitric oxide synthase (NOS) isoforms can be activated in response to low-level calcium signaling and calmodulin activation in airway epithelial cells.14, 63 Mouse sinonasal ALI cultures were loaded with the fluorophore DAF-FM, which exhibits an increase in fluorescence when it reacts with NO and derived nitrogen radicals.14 A DAF-FM fluorescence increase in mouse nasal septal ALIs was previously shown to reflect NO production.36 C4HSL (100 μM) stimulated a robust increase in DAF-FM fluorescence (Fig. 4A). The non-specific NO donor SNAP was added at the end of all experiments as a control for dye loading. C4HSL-induced fluorescence increase was inhibited when calcium signals were eliminated by loading cultures with the calcium chelator BAPTA-AM (10 μM for 15 min) and using extracellular media that contained the calcium chelator EGTA (0.5 mM; Fig. 4B), despite the fact that SNAP was still able to induce robust fluorescence increases. This result suggests that DAF-FM fluorescence increases in response to C4HSL reflect calcium-stimulated NOS activity, likely activation of eNOS.63 Like the calcium signals observed in Fig. 2, the C4HSL-induced DAF-FM fluorescence increases were inhibited by the PLCβ2 inhibitor U73122 (Fig. 4C) but not its inactive analogue U73343 (Fig. 4D). The DAF-FM fluorescence increases were also inhibited in cultures derived from TRPM5 knockout mice (Fig. 4E) as well as Wt cultures in the presence of triphenylphosphine oxide (TPPO; 80 μM Fig. 4F), a TRPM5 inhibitor.64 However, the DAF-FM fluorescence increases did not depend on α-gustducin, as they were completely intact in cultures derived from α-gustducin knockout mice (Fig. 4G). These data are summarized in Fig. 4H, and suggest that C4HSL activates NO production in mouse sinonasal epithelial cells and that this response also requires two important components of taste signaling, PLCβ2 and TRPM5.
Fig. 4.
C4HSL stimulates mouse septal epithelial cell NO production that requires taste signaling components. (A) Average trace (n = 7 cultures) showing DAF-FM fluorescence (increase reflects reactive nitrogen species production) in Wt cultures during stimulation with 100 μM C4HSL as well as the non-specific NO donor SNAP (10 μM). (B–D) Average traces of C4HSL-stimulated fluorescence increases in Wt cultures under 0-calcium conditions (BAPTA/EGTA; n = 7, B), in the presence of U73122 (n = 5, C) and U73343 (n = 6, D). (E–F) Average traces of C4HSL-induced fluorescence increases in TRPM5−/− cultures Wt cultures in the presence of the TRPM5 inhibitor TPPO (n = 5 each). (G) Traces of DAF-FM fluorescence increase in α-gustducin−/− cultures (n = 7). (H) Bar graph of fluorescence changes after stimulation with C4HSL as shown in A-F. DAF-FM fluorescence increases were 426 ± 47 (Wt; control), 24 ± 18 (Wt with 0-Ca2+), 65 ± 32 (Wt + U73122), 330 ± 38 (Wt + U73343), 90 ± 60 (TRPM5−/−), 108 ± 50 (Wt + TPPO), 308 ± 54 (α-gustducin−/−). Symbols denote significance compared with control determined via 1-way ANOVA with Dunnett’s post-test; **P <0.01, n.s. not significant.
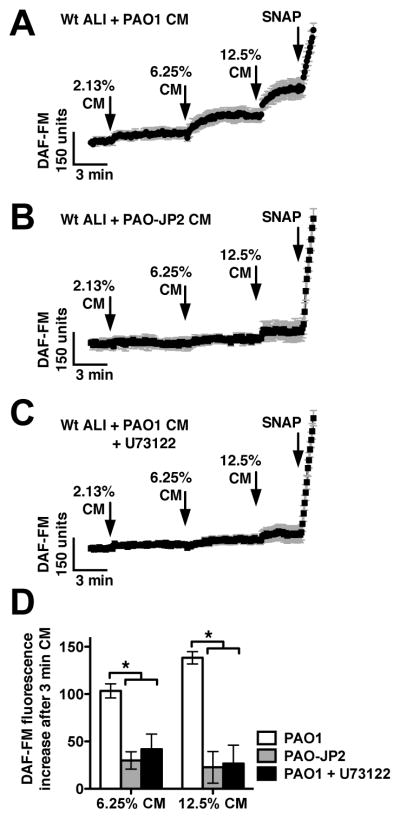
To examine whether AHLs secreted by intact P. aeruginosa bacteria can likewise activate mouse sinonasal epithelial cell NO, we took DAF-FM loaded ALIs and stimulated them with dilute concentrations of P. aeruginosa-conditioned medium (CM) from a 3-day planktonic growth culture as previously described.14 CM from a Wt P. aeruginosa (strain PAO1) induced robust DAF-FM fluorescence increases (Fig. 5A), while medium from a strain that cannot produce AHLs (PAO-JP2) did not (Fig. 5B). DAF-FM fluorescence increases in response to PAO1 CM were also blocked by U73122 (Fig. 5C), suggesting they require the PLCβ2 component of taste signaling. These data are summarized in Fig. 5D.
Fig. 5.
Conditioned medium from Wt but not AHL-deficient Pseudomonas aeruginosa stimulates taste-dependent NO production. (A–C) Traces of DAF-FM fluorescence from Wt mouse nasal septal ALI cultures stimulated with dilute conditioned medium (CM) from Wt P. aeruginosa (PAO1; A; n = 6 cultures), AHL-deficient P. aeruginosa (PAO-JP2; B; n = 7 cultures), and Wt P. aeruginosa in the presence of U73122 (C; n = 6 cultures). (D) Bar graph showing DAF-FM fluorescence increases (mean ± SEM) after 3 min stimulation with 6.25% CM and 12.5% CM. DAF-FM fluorescence increases were [6.25% CM] 103 ± 7 (PAO1), 33 ± 10 (PAO-JP2), 42 ± 16 (PAO1 + U73122) and [12.5% CM] 138 ± 7 (PAO1), 27 ± 20 (PAO-JP2), and 26 ± 20 (PAO1 + U73122). Asterisks indicate significance determined by 1-way ANOVA with Bonferroni post-test; *P <0.05.
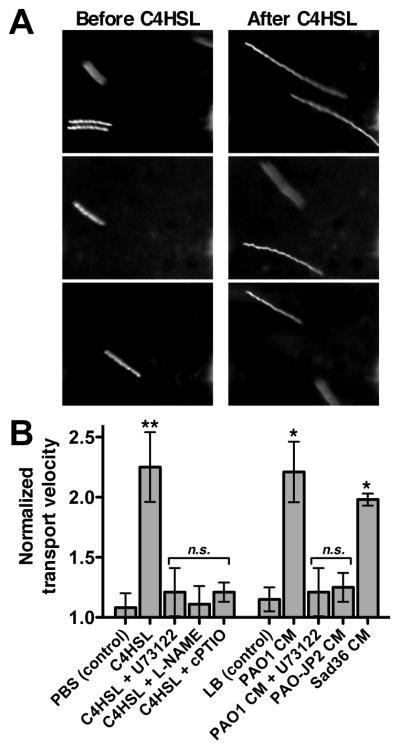
We next examined if AHL-activated taste signaling and NO production stimulates mucociliary clearance to confirm that this reflects an innate immune response. A particle-tracking assay was used to measure relative changes in mucociliary transport, as previously described.14, 65 Fluorescence microspheres were overlayed on top of the ALI culture and images during 2 second camera exposures, resulting in images of “streaks” denoting the distance traveled during the 2 second period. Relative streak length approximates the relative increase in mucociliary transport rate. Stimulation of Wt cultures with 10 μM C4HSL induced a ~2-fold increase in mucociliary transport rate (Fig. 6A) that was blocked by U73122 or the NOS inhibitor L-NAME (20 μM for 15 min preincubation; Fig. 6B) or the NO scavenger carboxy-PTIO (10 μM; Fig. 6B). This suggests that C4HSL increases mucociliary clearance that requires both PLCβ2/taste signaling as well as NOS activity and NO production. Likewise, we found that dilute (12.5%) PAO1 CM induced an increase in mucociliary clearance that required PLCβ2/taste signaling, while 12.5% LB alone had no effect (Fig. 6B). CM from the AHL-deficient strain PAO-JP2 had no effect (Fig. 6B), while CM from a flagellin-deficient flgK mutant strain (Sad36) activated responses similar to PAO1 CM (Fig. 6B), supporting the specific role for secreted AHLs in initiating this response.
Fig. 6.
C4HSL and Wt Pseudomonas aeruginosa -conditioned medium stimulate an increase in mucociliary transport. (A) Representative images of one field of view showing streaks representing particle transport by a Wt mouse septal ALI culture before stimulation (left) and after 3 min stimulation with 100 μM C4HSL (right). (B) Graph showing mean ± SEM (n = ALI 3–5 cultures each) of normalized changes in particle transport after addition of PBS alone (reflecting the mechanical force of pipetting alone, 1.08 ± 0.12), C4HSL (2.25 ± 0.29), C4HSL + U73122 (1.21 ± 0.2), C4HSL + L-NAME (1.11 ± 0.15), C4HSL + cPTIO (1.21 ± 0.08), 12.5 % LB alone (1.15 ± 0.10), 12.5% PAO1 CM (2.21 ± 0.25), PAO1 CM + U73122 (1.21 ± 0.20), PAO-JP2 CM (1.25 ± 0.12), and Sad36 CM (1.98 ± 0.05). Symbols denote significance vs. appropriate control condition (PBS or LB alone) determined via 1-way ANOVA with Dunnett’s post-test; *P <0.05, **P <0.01.
Discussion
T2R bitter receptors are emerging as important players in the innate immunity of the upper14, 23 and lower13 airways, with preclinical14 and clinical14, 23 data suggesting the human receptor T2R38 plays a key sentinel role in upper airway gram-negative bacteria infection and CRS by detecting AHL quorum-sensing molecules secreted by gram-negative bacteria. The role of taste receptors in innate immunity is particularly intriguing, because taste receptors have a uniquely high density of naturally occurring genetic variants;66 variation in individual T2R function may partly underlie differences in susceptibility to CRS and partly explain previous studies that have suggested CRS has one or more unknown genetic components.67–69 Because of the potential importance of this signaling pathway to human innate immunity, we examined if molecules involved in taste signaling are also required for detection of AHLs in mouse nasal septal epithelial cells, both to define mechanisms of this pathway as well as to determine if the mouse is a useful model in which to study it.
The data shown above demonstrate that the bitter tastant PTC and Pseudomonas quorum-sensing AHLs stimulate calcium signals with similar magnitude and kinetics. The AHL-dependent calcium response drives production of NO that stimulates an increase in mucociliary clearance, the “first-line” of defense in sinonasal innate immunity. This response requires two important components that are also involved in taste signaling, namely PLCβ2 and TRPM5, identically as previously observed in human sinonasal epithelial cells.14 Notably, neither the PTC-induced nor C4HSL-induced calcium and NO responses observed here require the G protein subunit α-gustducin, a central component of the canonical signaling pathway in taste receptor cells of the tongue.44, 46 Gustducin-independent taste-receptor signaling has been previously observed,35, 54, 55, 70–73 but to our knowledge, the finding that a T2R-receptor-linked signaling pathway is completely intact in the absence of α-gustducin would be highly novel. While our results are strongly suggestive of a T2R receptor-mediated response, they do not demonstrate a requirement for T2R receptors because specific knockout mice do not yet exist. Further work must be directed at determining which T2R receptors may be required for this response in order to confirm whether or not this pathway is truly an α-gustducin-independent T2R-dependent chemosensory pathway. The Gα-protein(s) involved in the sinonasal mucosal response to AHLs also remain to be determined. It is also notable that, because no specific pharmacological gustducin inhibitor exists, this elucidation of this molecular insight into AHL airway signaling required use of the mouse model and α-gustducin knockout.
The responses observed here originate from ciliated epithelial cells. As mentioned above, other researchers have observed AHL-dependent calcium responses originating from discreet nonciliated cells termed solitary chemosensory cells (SCCs) that synapse with trigeminal neurons and regulate breath holding in response to bitter chemicals.27, 60, 61 While the responses reported here were independent of α-gustducin, the responses of SCCs to AHLs have been reported to require α-gustducin.27, 60, 61 This likely reflects a difference in the signaling pathways of these two very different cell types. While the role of reported SCC responses in the mouse airway appears to be to prevent further inhalation of toxic substances,27, 60, 61 our data here suggest that the ciliated epithelial cells themselves can also detect AHLs and mount rapid local innate immune responses mediated by calcium-dependent NO production. While it is yet to be determined whether the SCC responses27, 60, 61 are mimicked in the human nasal epithelium, the data here suggest that the ciliated epithelial cell NO response is partially conserved between species.
In addition to its role in controlling airway innate immunity by increasing mucociliary clearance rates, NO is thought to be an important direct mediator of innate immunity. NO is thought to be able to rapidly diffuse into bacteria, and NO and its reactive derivatives (eg, S-nitrosothiols and peroxynitrites) can damage bacterial DNA, react with proteins containing sulfhydryl or thiol groups, inactive enzymes with metal cofactors, and damage membrane lipids.17,18 It has been suggested that the majority of airway NO originates in the nose74–76 with high levels of NOS expression in the cilia and microvilli of the paranasal sinus epithelium.77, 78 Polymorphisms in the NOS1 gene have been correlated with severity of CRS,79 but levels of exhaled NO have been both positively and negatively correlated with CRS.80–84 The evidence is not yet consistent enough nor are there reliable enough measurement techniques to make exhaled NO a useful measure for or predictor of sinonasal infection.85 Nonetheless, high concentrations of NO can be toxic to the common nasal pathogens P. aeruginosa14 and Staphylococcus aureus,86 making NO an attractive therapeutic molecule for treatment of sinonasal disease. There are thus significant potential clinical implications for the ability to harness the T2R-mediated NO production pathway for treatment of respiratory infections. The exact mouse T2R receptor(s) that may be involved in AHL-induced NO production are yet unknown due to the lack of a clear T2R38 orthologue. However, the data outlined in this study demonstrate that important components of this pathway are conserved between human and mouse, revealing important molecular insights into this pathway and suggesting that the mouse is a very useful and important model with which to study the mechanisms and downstream effects of sinonasal AHL-detection and potentially T2R signaling in vitro and potentially in vivo.
Acknowledgments
We thank B.H. Igelwski and J.M. Schwingel for Pseudomonas aeruginosa strain PAO-JP2 and G. O’Toole for strain Sad36. This work was supported by a grant from the Flight Attendants Medical Research Institute (082478) and a charitable donation from the RLG Foundation, Inc., both to N.A.C., as well as a National Institutes of Health grant (DC03055) to R.F.M.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article.
References
- 1.Satir P, Sleigh MA. The physiology of cilia and mucociliary interactions. Annu Rev Physiol. 1990;52:137–55. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- 2.Sleigh MA, Blake JR, Liron N. The propulsion of mucus by cilia. American Rev Respir Dis. 1988;137:726–41. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- 3.Eliezer N, Sade J, Silberberg A, Nevo AC. The role of mucus in transport by cilia. Am Rev Respir Dis. 1970;102:48–52. doi: 10.1164/arrd.1970.102.1.48. [DOI] [PubMed] [Google Scholar]
- 4.Antunes MB, Gudis DA, Cohen NA. Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29:631–43. doi: 10.1016/j.iac.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Cohen NA. Sinonasal mucociliary clearance in health and disease. Ann Otol Rhinol Laryngol Suppl. 2006;196:20–6. doi: 10.1177/00034894061150s904. [DOI] [PubMed] [Google Scholar]
- 6.Gudis D, Zhao KQ, Cohen NA. Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:1–6. doi: 10.2500/ajra.2012.26.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kingdom TT, Swain RE., Jr The microbiology and antimicrobial resistance patterns in chronic rhinosinusitis. Am J Otolaryngol. 2004;25:323–8. doi: 10.1016/j.amjoto.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Genoway KA, Philpott CM, Javer AR. Pathogen yield and antimicrobial resistance patterns of chronic rhinosinusitis patients presenting to a tertiary rhinology centre. J Otolaryngol Head Neck Surg. 2011;40:232–7. [PubMed] [Google Scholar]
- 9.Bhattacharyya N, Kepnes LJ. Assessment of trends in antimicrobial resistance in chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2008;117:448–52. doi: 10.1177/000348940811700608. [DOI] [PubMed] [Google Scholar]
- 10.Behrens M, Meyerhof W. Bitter taste receptor research comes of age: From characterization to modulation of TAS2Rs. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Behrens M, Meyerhof W. Oral and extraoral bitter taste receptors. Results Probl Cell Differ. 2010;52:87–99. doi: 10.1007/978-3-642-14426-4_8. [DOI] [PubMed] [Google Scholar]
- 12.Meyerhof W, Batram C, Kuhn C, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–70. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 13.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–4. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–59. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi EH, Wormald PJ, Tan LW. Innate immunity in the paranasal sinuses: a review of nasal host defenses. Am J Rhinol. 2008;22:13–9. doi: 10.2500/ajr.2008.22.3127. [DOI] [PubMed] [Google Scholar]
- 16.Ramanathan M, Jr, Lane AP. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2007;136:348–56. doi: 10.1016/j.otohns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–25. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcinkiewicz J. Nitric oxide and antimicrobial activity of reactive oxygen intermediates. Immunopharmacology. 1997;37:35–41. doi: 10.1016/s0162-3109(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 19.Bufe B, Breslin PA, Kuhn C, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–7. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan J, Abrol R, Trzaskowski B, Goddard WA., 3rd 3D Structure Prediction of TAS2R38 Bitter Receptors Bound to Agonists Phenylthiocarbamide (PTC) and 6-n-Propylthiouracil (PROP) J Chem Inf Model. 2012 doi: 10.1021/ci300133a. [DOI] [PubMed] [Google Scholar]
- 21.Biarnes X, Marchiori A, Giorgetti A, et al. Insights into the binding of Phenyltiocarbamide (PTC) agonist to its target human TAS2R38 bitter receptor. PloS one. 2010;5:e12394. doi: 10.1371/journal.pone.0012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floriano WB, Hall S, Vaidehi N, Kim U, Drayna D, Goddard WA., 3rd Modeling the human PTC bitter-taste receptor interactions with bitter tastants. J Mol Model. 2006;12:931–41. doi: 10.1007/s00894-006-0102-6. [DOI] [PubMed] [Google Scholar]
- 23.Adappa ND, Howland TJ, Palmer JN, et al. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol. 2013;3:184–187. doi: 10.1002/alr.21140. [DOI] [PubMed] [Google Scholar]
- 24.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA. 2002;99:2392–7. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen MC, Wu SV, Reeve JR, Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006;291:C726–39. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- 26.Jeon TI, Seo YK, Osborne TF. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J. 2011;438:33–7. doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol. 2008;99:2929–37. doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics. 2005;22:139–49. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 29.Nelson TM, Munger SD, Boughter JD., Jr Taste sensitivities to PROP and PTC vary independently in mice. Chem Senses. 2003;28:695–704. doi: 10.1093/chemse/bjg062. [DOI] [PubMed] [Google Scholar]
- 30.Boughter JD, Jr, Raghow S, Nelson TM, Munger SD. Inbred mouse strains C57BL/6J and DBA/2J vary in sensitivity to a subset of bitter stimuli. BMC Genet. 2005;6:36. doi: 10.1186/1471-2156-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitney G, Harder DB. Phenylthiocarbamide (PTC) preference among laboratory mice: understanding of a previously “unreplicated” report. Behav Genet. 1986;16:605–10. doi: 10.1007/BF01066287. [DOI] [PubMed] [Google Scholar]
- 32.Whitney G, Harder DB. Genetics of bitter perception in mice. Physiol Behav. 1994;56:1141–7. doi: 10.1016/0031-9384(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 33.St John SJ, Pour L, Boughter JD., Jr Phenylthiocarbamide produces conditioned taste aversions in mice. Chem Senses. 2005;30:377–82. doi: 10.1093/chemse/bji032. [DOI] [PubMed] [Google Scholar]
- 34.Damak S, Rong M, Yasumatsu K, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–64. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 35.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 36.Zhao KQ, Cowan AT, Lee RJ, et al. Molecular modulation of airway epithelial ciliary response to sneezing. FASEB J. 2012;26:3178–87. doi: 10.1096/fj.11-202184. [DOI] [PubMed] [Google Scholar]
- 37.Antunes MB, Woodworth BA, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43:195–6. 8. doi: 10.2144/000112531. 200 passim. [DOI] [PubMed] [Google Scholar]
- 38.Pearson JP, Pesci EC, Iglewski BH. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–67. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 40.Reszka KJ, O’Malley Y, McCormick ML, Denning GM, Britigan BE. Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide. Free Radic Biol Med. 2004;36:1448–59. doi: 10.1016/j.freeradbiomed.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer control of microscopes using microManager. Curr Protoc Mol Biol. 2010;Chapter 14(Unit 14):20. doi: 10.1002/0471142727.mb1420s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai Y, Chen B, Shi J, Palmer JN, Kennedy DW, Cohen NA. Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2011;128:1207–15. e1. doi: 10.1016/j.jaci.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Ramanathan M, Jr, Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. American journal of rhinology. 2007;21:373–7. doi: 10.2500/ajr.2007.21.3034. [DOI] [PubMed] [Google Scholar]
- 44.Gilbertson TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol. 2000;10:519–27. doi: 10.1016/s0959-4388(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 45.Kinnamon SC, Margolskee RF. Mechanisms of taste transduction. Curr Opion Neurobiol. 1996;6:506–13. doi: 10.1016/s0959-4388(96)80057-2. [DOI] [PubMed] [Google Scholar]
- 46.Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- 47.Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–4. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearson JP, Van Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181:1203–10. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flickinger ST, Copeland MF, Downes EM, et al. Quorum sensing between Pseudomonas aeruginosa biofilms accelerates cell growth. J Am Chem Soc. 2011;133:5966–75. doi: 10.1021/ja111131f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao N, Lu M, Echeverri F, et al. Voltage-gated sodium channels in taste bud cells. BMC Neurosci. 2009;10:20. doi: 10.1186/1471-2202-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27:5777–86. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogura T, Margolskee RF, Kinnamon SC. Taste receptor cell responses to the bitter stimulus denatonium involve Ca2+ influx via store-operated channels. J Neurophysiol. 2002;87:3152–5. doi: 10.1152/jn.2002.87.6.3152. [DOI] [PubMed] [Google Scholar]
- 53.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–9. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 54.Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci. 2003;23:9947–52. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozeck M, Brust P, Xu H, Servant G. Receptors for bitter, sweet and umami taste couple to inhibitory G protein signaling pathways. Eur J Pharmacol. 2004;489:139–49. doi: 10.1016/j.ejphar.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Clapp TR, Trubey KR, Vandenbeuch A, et al. Tonic activity of Galpha-gustducin regulates taste cell responsivity. FEBS letters. 2008;582:3783–7. doi: 10.1016/j.febslet.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol. 2007;21:533–7. doi: 10.2500/ajr.2007.21.3068. [DOI] [PubMed] [Google Scholar]
- 58.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–21. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 59.Dimova S, Brewster ME, Noppe M, Jorissen M, Augustijns P. The use of human nasal in vitro cell systems during drug discovery and development. Toxicol In Vitro. 2005;19:107–22. doi: 10.1016/j.tiv.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Tizzano M, Gulbransen BD, Vandenbeuch A, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107:3210–5. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–22. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 63.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58:175–82. doi: 10.1136/thorax.58.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer RK, Atwal K, Bakaj I, et al. Triphenylphosphine oxide is a potent and selective inhibitor of the transient receptor potential melastatin-5 ion channel. Assay Drug Dev Technol. 2010;8:703–13. doi: 10.1089/adt.2010.0334. [DOI] [PubMed] [Google Scholar]
- 65.Worthington EN, Tarran R. Methods for ASL measurements and mucus transport rates in cell cultures. Methods Mol Biol. 2011;742:77–92. doi: 10.1007/978-1-61779-120-8_5. [DOI] [PubMed] [Google Scholar]
- 66.Kim U, Wooding S, Ricci D, Jorde LB, Drayna D. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat. 2005;26:199–204. doi: 10.1002/humu.20203. [DOI] [PubMed] [Google Scholar]
- 67.Greisner WA, 3rd, Settipane GA. Hereditary factor for nasal polyps. Allergy Asthma Proc. 1996;17:283–6. doi: 10.2500/108854196778662192. [DOI] [PubMed] [Google Scholar]
- 68.Cohen NA, Widelitz JS, Chiu AG, Palmer JN, Kennedy DW. Familial aggregation of sinonasal polyps correlates with severity of disease. Otolaryngol Head Neck Surg. 2006;134:601–4. doi: 10.1016/j.otohns.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 69.Hamilos DL. Chronic rhinosinusitis patterns of illness. Clin Allergy Immunol. 2007;20:1–13. [PubMed] [Google Scholar]
- 70.He W, Danilova V, Zou S, et al. Partial rescue of taste responses of alpha-gustducin null mice by transgenic expression of alpha-transducin. Chem Senses. 2002;27:719–27. doi: 10.1093/chemse/27.8.719. [DOI] [PubMed] [Google Scholar]
- 71.He W, Yasumatsu K, Varadarajan V, et al. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci. 2004;24:7674–80. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glendinning JI, Bloom LD, Onishi M, et al. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- 73.Ruiz-Avila L, Wong GT, Damak S, Margolskee RF. Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in alpha -gustducin. Proc Natl Acad Sci USA. 2001;98:8868–73. doi: 10.1073/pnas.151235798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haight JS, Djupesland PG, Qjan W, et al. Does nasal nitric oxide come from the sinuses? J Otolaryngol. 1999;28:197–204. [PubMed] [Google Scholar]
- 75.Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Comm. 1991;181:852–7. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 76.Maniscalco M, Sofia M, Pelaia G. Nitric oxide in upper airways inflammatory diseases. Inflamm Res. 2007;56:58–69. doi: 10.1007/s00011-006-6111-1. [DOI] [PubMed] [Google Scholar]
- 77.Deja M, Busch T, Bachmann S, et al. Reduced nitric oxide in sinus epithelium of patients with radiologic maxillary sinusitis and sepsis. Am J Resp Critical Care Med. 2003;168:281–6. doi: 10.1164/rccm.200207-640OC. [DOI] [PubMed] [Google Scholar]
- 78.Degano B, Valmary S, Serrano E, Brousset P, Arnal JF. Expression of nitric oxide synthases in primary ciliary dyskinesia. Human pathology. 2011;42:1855–61. doi: 10.1016/j.humpath.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Endam LM, Filali-Mouhim A, Bosse Y, Castano R, Desrosiers M. Polymorphisms in the nitric oxide synthase 1 gene are associated with severe chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:e49–54. doi: 10.2500/ajra.2011.25.3588. [DOI] [PubMed] [Google Scholar]
- 80.Bommarito L, Guida G, Heffler E, et al. Nasal nitric oxide concentration in suspected chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2008;101:358–62. doi: 10.1016/S1081-1206(10)60310-9. [DOI] [PubMed] [Google Scholar]
- 81.Noda N, Takeno S, Fukuiri T, Hirakawa K. Monitoring of oral and nasal exhaled nitric oxide in eosinophilic chronic rhinosinusitis: a prospective study. Am J Rhinol Allergy. 2012;26:255–9. doi: 10.2500/ajra.2012.26.3772. [DOI] [PubMed] [Google Scholar]
- 82.Weschta M, Deutschle T, Riechelmann H. Nasal fractional exhaled nitric oxide analysis with a novel hand-held device. Rhinology. 2008;46:23–7. [PubMed] [Google Scholar]
- 83.Deroee AF, Naraghi M, Sontou AF, Ebrahimkhani MR, Dehpour AR. Nitric oxide metabolites as biomarkers for follow-up after chronic rhinosinusitis surgery. Am J Rhinol Allergy. 2009;23:159–61. doi: 10.2500/ajra.2009.23.3289. [DOI] [PubMed] [Google Scholar]
- 84.Naraghi M, Deroee AF, Ebrahimkhani M, Kiani S, Dehpour A. Nitric oxide: a new concept in chronic sinusitis pathogenesis. Am J Otolaryngol. 2007;28:334–7. doi: 10.1016/j.amjoto.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Phillips PS, Sacks R, Marcells GN, Cohen NA, Harvey RJ. Nasal nitric oxide and sinonasal disease: a systematic review of published evidence. Otolaryngl Head Neck Surg. 2011;144:159–69. doi: 10.1177/0194599810392667. [DOI] [PubMed] [Google Scholar]
- 86.Jardeleza C, Foreman A, Baker L, et al. The effects of nitric oxide on Staphylococcus aureus biofilm growth and its implications in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1:438–44. doi: 10.1002/alr.20083. [DOI] [PubMed] [Google Scholar]