Abstract
Objective:
To describe the phenotype of a patient with classical features of X-linked L1 syndrome associated with novel brain malformations.
Methods:
Diagnostic analysis included physical and dysmorphology examinations, MRI of the brain, and exome sequencing of the family trio.
Results:
We report a 2.5-year-old boy with developmental delay, dysmorphic facies, and adducted thumbs. MRI of the brain showed a truncated corpus callosum and periventricular heterotopias associated with polymicrogyria (PMG). Variant segregation analysis with exome sequencing discovered a novel maternally derived hemizygous variant in exon 14 of the L1CAM gene (c.1759 G>C; p.G587R).
Conclusions:
This novel L1CAM mutation was located in the protein's sixth immunoglobin domain and involved glycine-587, a key residue in the structure of L1CAM because of its interactions with lysine-606, which indicates that any mutation at this site would likely affect the secondary structure and function of the protein. The replacement of the small nonpolar glycine residue with a large basic arginine would have an even more dramatic result. The presentation of periventricular nodular heterotopias with overlying PMG is very uncommon, and its association with L1CAM may provide insight into other similar cases. Furthermore, this presentation indicates the important role that L1CAM plays in neuronal migration and brain development and extends the phenotype associated with L1CAM-associated disorders.
The L1 family of adhesion molecules is involved in several processes during the development of the brain. These events occur through L1CAM's interactions with various proteins and involve myelination, neuronal outgrowth, axon fasciculation, and neuron migration.1 Mutations in L1CAM have previously been associated with 4 X-linked neurologic conditions, collectively known as L1 syndrome: X-linked hydrocephalus, MASA syndrome (mental retardation, aphasia, shuffling gait, and adducted thumbs), spastic paraplegia type 1 (SPG-1), and X-linked agenesis of the corpus callosum.2,3
L1CAM consists of a conserved cytoplasmic, 5 fibronectin type III, and 6 extracellular immunoglobulin (Ig1–6) domains.1 The Ig1–6 and the second fibronectin domain are responsible for homophilic binding that triggers neurite outgrowth, while heterophilic binding involves the neurocan protein or the integrins interacting with other L1CAM domains.4,5 Of note, the sixth Ig domain promotes neurite outgrowth through both homophilic binding and heterophilic binding to integrins.5
More than 200 L1CAM mutations have been reported, with a range of phenotypes.1 Patients were classified as having a “severe” phenotype if they had macrocephaly that required shunting or died before 2 years of age; all others were classified as having a “mild” phenotype.3 Patients with cytoplasmic domain mutations often presented with a mild phenotype, while extracellular domain mutations were more severe. Further analysis of missense mutations noted that variants involving the surface of the extracellular domain were milder than those affecting its core.3
We report a 2.5-year-old boy with a novel L1CAM mutation. Classical features of L1 syndrome were present, including callosal hypoplasia, intellectual disability, adducted thumbs, and SPG.1,2 Unique to this patient was the presence of periventricular nodular heterotopias (PNHs) associated with overlying polymicrogyria (PMG).
METHODS
Standard protocol approvals, registrations, and patient consents.
Research protocols were approved through the institutional review board, and the family gave informed consent (CSMC IRB protocol Pro00037131).
Patient.
The proband was a 2.5-year-old boy who presented with dysmorphic features, motor disabilities, and cognitive delay. He was the second child of a nonconsanguineous couple of Thai descent. He had an unaffected sister and no family history of neurologic disease.
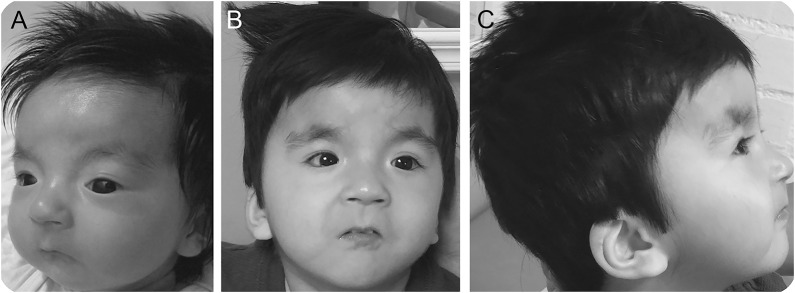
At birth, the child was described as having several dysmorphic features, including a wide nasal bridge, hypertelorism, sparse eyebrows/eyelashes, microstomia, retrognathia, and posteriorly rotated ears (figure 1A). He had a high palate and a bifid uvula. He had arachnodactyly, overlapping digits, and adducted thumbs. His first and second toes were widely spaced bilaterally, and there was partial syndactyly between the second and third toes on the left foot. His birth weight was 3,319 g, length was 48 cm, and head circumference was 33 cm, all within normal limits. He exhibited poor feeding and required gastrostomy tube placement.
Figure 1. Photographs of the patient.
Patient (A) as an infant and (B) at 2.5 years of age. Note the microcephaly, sparse lashes, broad nasal bridge, and small-appearing mouth with downturned corners. (C) Profile view at 2.5 years of age with posteriorly rotated ears and retrognathia.
On examination, the child was awake and alert. He did not speak or follow commands. He had limited receptive language. Head circumference and length were below the 5th percentile. He had developed bushy eyebrows, low posterior hairline, and thick hair with frontal upsweep and disorganized growth pattern (figure 1B). He had a mild apparent hypertelorism, wide nasal bridge, a small-appearing mouth with downturned corners and retrognathia, high palate, and bifid uvula (figure 1B). He had low-set posteriorly rotated ears with an abnormal helix (figure 1C). Visual tracking was decreased. He had adducted thumbs and SPG. Strength was mildly and diffusely decreased. His reflexes were brisk. His ankles were dorsiflexed with talipes calcaneovalgus deformities.
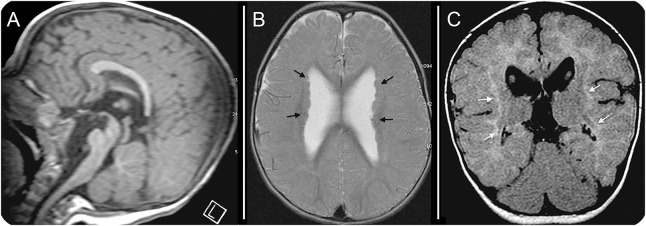
MRI of the brain at 1 year of age revealed a truncated corpus callosum with incomplete formation of the genu and rostrum anteriorly and the splenium posteriorly (figure 2A). There was deformation of the lateral ventricles with larger anterior horns. Bilateral PMG was present and associated with PNHs (figure 2B). White matter signal abnormalities were present, with reduced volume consistent with dysmyelination (figure 2C). Both the pons and medulla were small and not fully developed (figure 2A).
Figure 2. Brain MRI at 1 year of age.
(A) Sagittal T1 imaging revealed truncation of the rostrum, genu, and splenium of the corpus callosum. (B) Axial T1 imaging showed periventricular nodular heterotopias (black arrows), polymicrogyria, and enlarged lateral ventricles that are widely separated and abnormal in shape. (C) Coronal axial fluid-attenuated inversion recovery imaging showed hyperintense white matter (white arrows) with reduced volume, consistent with dysmyelination.
Genetic evaluation.
Genomic DNA was extracted from blood, and exome sequencing was performed as per previous protocols (GeneDx, Gaithersburg, MD).6
RESULTS
Exome sequencing identified a novel maternally derived missense mutation (c.1759 G>C; p.Gly587Arg) in exon 14 of the L1CAM gene (NM_000425.3). This variant was not observed in approximately 6,500 individuals in the NHLBI Exome Sequencing Project.
DISCUSSION
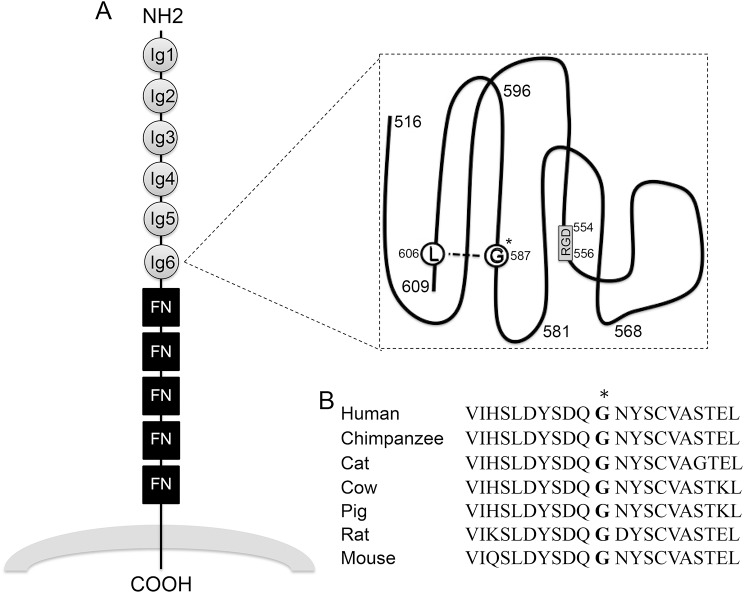
Our proband was hemizygous for a missense mutation in the sixth Ig domain of the L1CAM gene. This mutation involves the substitution of a nonpolar glycine residue with a larger basic arginine residue. Previously, glycine-587 was identified as a key residue in the structure of L1CAM because of its interactions with lysine-606, which indicates that any mutation at this site would likely affect the secondary structure and function of the protein (figure 3A).4 Furthermore, glycine-587 residue is conserved across mammalian species (figure 3B), and neither this variant nor any other variant has been previously reported to affect this residue. Consistent with these findings, the child had several features associated with L1CAM disorders, in addition to some unique migrational anomalies of his brain. Other nearby mutations (p.Y589H, p.A593P, p.D598N) that were previously associated with L1 syndrome (corpus callosum hypoplasia, retardation, adducted thumbs, and spasticity) provide additional evidence that this substitution is responsible for his phenotype.7 Furthermore, investigations in C57BL/6 mice with deletion of L1CAM's sixth Ig domain (L1-6D mice) resulted in a typical L1 phenotype consisting of hydrocephalus and embryonic lethality.8 This phenotype was likely the result of loss of L1–L1 homophilic binding that is important for axon fasciculation, extension, and guidance, as well as loss of heterophilic integrin binding that triggers signaling pathways involved in cell migration.1,5
Figure 3. Diagram of L1CAM extracellular domains and glycine-587 conservation.
(A) Schematic of the L1CAM protein extracellular domains with glycine-587 (G*) and lysine-606 (L) residues highlighted.1,4 Fibronectin type III domains = FN in square; immunoglobulin domain = Ig in circle; RGD motif = RGD in box. (B) Glycine-587 (*) is conserved across several mammalian species.
PNHs are defined as heterotopic nodules of disorganized neurons along lateral ventricles of the brain. It has been hypothesized that these abnormalities are caused by impaired departure of neurons from the ventricular zone (VZ). In murine neocortex, shRNA knockdown of L1CAM showed impaired migration of neurons from the VZ to the intermediate zone (IZ) and the cortical plate.9 Altered distribution of the leading processes of neurons led to aberrant locomotion.9 A similar defect was observed in slice culture experiments of neuronal L1CAM knockdown (L1-KD) mice, in which L1-KD neurons had decreased locomotion in the IZ and longer processes when translocating through the primitive cortical zone.10 These defects were likely caused by impaired homophilic binding and inadequate activation of MAPK, resulting in slowed migration. In addition, it has been reported that integrins such as α3β1 and α5β1 modulate the interaction between the leading processes of the neurons and substrate as they migrate through the cortical zone.10 It is likely that our proband's mutation and its proximity to a nearby RGD motif in Ig6 disrupted both homophilic and integrin binding, resulting in impaired cell signaling and causing migrational abnormalities.
Our patient's presentation with PNHs associated with PMG appears to be novel in L1CAM-associated disorders. Several patients with an X-linked inheritance pattern of familial hydrocephalus thought to be due to L1CAM mutations were also noted to have PMG.11 Unfortunately, a genetic diagnosis was never confirmed.10 More interesting is that PNHs with overlying frontal-perisylvian PMG have been previously described in only a handful of patients,12,13 and nearly all of the individuals with this subtype were males with limb abnormalities.12 Unfortunately, the genetic factors causing this combination also have yet to be identified. Further investigations of those cases with an emphasis on L1CAM may be appropriate.
We have described a case of an L1CAM-associated disorder caused by a hemizygous missense mutation in its sixth Ig domain. This mutation likely disrupted the secondary structure of L1CAM, resulting in abnormal homophilic and integrin binding and leading to the common symptoms of L1 syndrome along with a novel presentation of specific migrational abnormalities.
ACKNOWLEDGMENT
The authors are grateful to the patient and the parents for their cooperation, and they thank Kelli DeJohn and Shantel Brown for excellent administrative assistance and Barrington Burnett for critical analysis. T.M.P. was supported by Cedars-Sinai Medical Center institutional funding and the Diana and Steve Marienhoff Fashion Industries Guild Endowed Fellowship in Pediatric Neuromuscular Diseases.
GLOSSARY
- Ig
immunoglobulin
- IZ
intermediate zone
- PMG
polymicrogyria
- PNH
periventricular nodular heterotopia
- SPG-1
spastic paraplegia type 1
- VZ
ventricular zone
AUTHOR CONTRIBUTIONS
Christine Shieh: study concept and design and writing of manuscript. Franklin Moser: acquisition of data, analysis, and interpretation. John Graham: acquisition of data, analysis, and interpretation. Valerie Watiker: acquisition of data; critical revision of the manuscript for important intellectual content. Tyler Pierson: study concept and design, writing of manuscript; study supervision; critical revision of the manuscript for important intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
Christine Shieh and Franklin Moser report no disclosures. John Graham has served on the editorial boards of the European Journal of Medical Genetics, Clinical Pediatrics, the American Journal of Medical Genetics, Congenital Anomalies, Global Pediatric Health, and the International Advisory Board for Indian Academy of Medical Genetics; has received royalties for Smith's Recognizable Patterns of Human Deformation; and has had involvement in the following legal proceedings: Zoloft Product Liability Litigation (Pfizer, Inc.), Depakote Product Liability (Abbott), Murphy v. NXP Semiconductors (Paternally derived NKX2.2 disruption) Organic Solvent Exposure, Bustamante v. Children's Hospital of Los Angeles, Bartlett, Wolf v du Pont Co. (Perfluorooctanoic acid in water supply), Numkena v Motorola (Cerebral Palsy) (Organic Solvents), and Kirk and Goode v Varco (Fetal Akinesia: Autism and Asthma) (Organic Solvents). Valerie Watiker and Tyler Pierson report no disclosures. Go to Neurology.org/ng for full disclosure forms.
REFERENCES
- 1.Hortsch M. The L1 family of neural cell adhesion molecules: old proteins performing new tricks. Neuron 1996;17:587–593. [DOI] [PubMed] [Google Scholar]
- 2.Stumpel C, Vos YJ. L1 syndrome. In: GeneReviews [online]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK1484/. Accessed May 22, 2015. [Google Scholar]
- 3.Fransen E, Van Camp G, D'Hooge R, Vits L, Willems PJ. Genotype-phenotype correlation in L1 associated diseases. J Med Genet 1998;25:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman A, Jouet M, MacFarlane J, Du JS, Kenwrick S, Chothia C. Outline structure of the human L1 cell adhesion molecule and the sites where mutations cause neurological disorders. EMBO J 1996;15:6050–6059. [PMC free article] [PubMed] [Google Scholar]
- 5.De Angelis E, MacFarlane J, Du JS, et al. Pathological missense mutations of neural cell adhesion molecule L1 affect hemophilic and heterophilic binding activities. EMBO J 1999;18:4744–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka AJ, Cho MT, Millan F, et al. Mutations in SPATA5 are associated with microcephaly, intellectual disability, seizures, and hearing loss. Am J Hum Genet 2015;97:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenson PD, Mort M, Ball EV, et al. The Human Gene Mutation Database: providing a comprehensive central mutation database for molecular diagnostics and personalized genomics. Hum Genomics 2009;4:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh K, Cheng L, Kamei Y, et al. Brain development in mice lacking L1-L1 homophilic adhesion. J Cell Biol 2004;165:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishimoto T, Itoh K, Umekage M, et al. Downregulation of L1 perturbs neuronal migration and alters the expression of transcription factors in murine neocortex. J Neurosci Res 2013;91:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonosaki M, Itoh K, Umekage M, et al. L1CAM is crucial for cell locomotion and terminal translocation of the soma in radial migration during murine corticogensis. PLoS One 2014;9:e86186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graf WED, Born DE, Sarnat HB. The pachygyria-polymicrogyria spectrum of cortical dysplasia in X-linked hydrocephalus. Eur J Pediatr Surg 1998;8:10–14. [DOI] [PubMed] [Google Scholar]
- 12.Wieck G, Leventer RJ, Squier WM, et al. Periventricular nodule heterotopia with overlying polymicrogyria. Brain 2005;128:2811–2821. [DOI] [PubMed] [Google Scholar]
- 13.Abe Y, Kikuchi A, Kobayashi S, et al. Xq26.1-26.2 gain identified on array comparative genomic hybridization in bilateral periventricular nodular heterotopia with overlying polymicrogyria. Dev Med Child Neurol 2014;56:1221–1224. [DOI] [PubMed] [Google Scholar]