Abstract
Malignant phyllodes tumor is a rare breast malignancy with sarcomatous overgrowth and with limited effective treatment options for recurrent and metastatic cases. Recent clinical trials indicated a potential for anti-angiogenic, anti-EGFR and immunotherapeutic approaches for patients with sarcomas, which led us to investigate these and other targetable pathways in malignant phyllodes tumor of the breast. Thirty-six malignant phyllodes tumors (including 8 metastatic tumors with two cases having matched primary and metastatic tumors) were profiled using gene sequencing, gene copy number analysis, whole genome expression, and protein expression. Whole genome expression analysis demonstrated consistent over-expression of genes involved in angiogenesis including VEGFA, Angiopoietin-2, VCAM1, PDGFRA, and PTTG1. EGFR protein overexpression was observed in 26/27 (96%) of cases with amplification of the EGFR gene in 8/24 (33%) cases. Two EGFR mutations were identified including EGFRvIII and a presumed pathogenic V774M mutation, respectively. The most common pathogenic mutations included TP53 (50%) and PIK3CA (15%). Cases with matched primary and metastatic tumors harbored identical mutations in both sites (PIK3CA/KRAS and RB1 gene mutations, respectively). Tumor expression of PD-L1 immunoregulatory protein was observed in 3/22 (14%) of cases. Overexpression of molecular biomarkers of increased angiogenesis, EGFR and immune checkpoints provides novel targeted therapy options in malignant phyllodes tumors of the breast.
Keywords: breast, fibroepithelial tumors, malignant phyllodes tumor, biomarkers, molecular profiling
INTRODUCTION
Phyllodes tumors (PT) of the breast are rare, biphasic (fibro-epithelial) neoplasms, constituting ≤ 1% of all breast cancers [1], and are histologically classified as benign, borderline or malignant. The malignant variant is characterized by an overgrowth of the malignant stromal (sarcomatous) component and constitutes ∼20% of all phyllodes tumors. There is a significant potential (∼22%) for both local recurrence and distant metastasis [1]. These tumors may also pose a potential therapeutic challenge as no effective targeted therapy has been reported yet [1, 2].
Previous studies of the molecular genetics of PT showed the importance of the TP53 gene and p53 protein in progression from benign to malignant phyllodes tumors [3–6]. A gene expression study conducted by Vidal et al. [7] revealed activation of gene clusters related to hypoxia and angiogenesis in malignant phyllodes cases. Expression of various angiogenic factors including CD34, CD105, vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-alpha has also been reported in malignant phyllodes tumors [8–11]. Tse et al. [12] also found the tumor angiogenesis (measured by microvessel density) as an independent predictor of malignancy in phyllodes tumors. Recent gene sequencing studies revealed the presence of variety of mutations in malignant phyllodes tumors of which MED12 [Mediator Complex Subunit 12] mutation appears to be the most consistent and shared by both malignant and benign fibroepithelial tumors [13–15]. However, truly actionable (targetable by specific drugs) genetic alterations in PT appear to be rare. Majority of the previous studies showed EGFR protein overexpression and EGFR gene amplification in PT [13, 16–18]; However, Tse et al. [19] reported a low EGFR amplification rate (8%) in EGFR protein positive phyllodes tumors. No targetable EGFR activating mutations have been reported thus far.
Recently, cancer immunoediting involving immune check point proteins programmed cell death-1 (PD-1/CD279) expressed on tumor infiltrating T-lymphocytes and its ligand (PD-L1/CD274) expressed on tumor cells have come into the clinical focus due the remarkable therapeutic benefits caused by their specific inhibitors in patients with advanced melanoma, renal cell carcinoma and non-small cell lung carcinoma [20–21]. PD-1/PD-L1 expression was also reported in soft tissue sarcomas, which indicated a potential for targeting this pathway in a variety of cancers [20, 22–26].
In the present study, we comprehensively profiled a series of malignant phyllodes tumors of the breast in an attempt to identify potentially targetable pathways/biomarkers.
RESULTS
Patients
The study included 36 malignant (high grade) phyllodes tumors of which 24 were primary, 8 metastatic and 4 recurrent malignant phyllodes tumors of the breast (Table 1). Two metastatic phyllodes cases (lung metastasis) had their primary site samples available for molecular testing. All patients were females with the mean age of 50.8 years (range, 17–76 years).
Table 1. Results of multiplatform molecular profiling of 36 malignant phyllodes cases.
| Case (site) | PD-L1 protein (IHC) | EGFR protein (IHC) | EGFR (ISH) | EGFR (mutations) | NGS and Sanger (other mutations) |
|---|---|---|---|---|---|
| Case#1 (P) | Negative | Positive | Negative | Wild type | TP53 (G245S) PIK3CA (H1047R) PIK3CA (VUS G106_R108del) |
| Case#2 (P) | n/a | Positive | Negative | Wild type | None |
| Case#3 (P) | Negative | Positive | Negative | Wild type | PIK3CA (H1047R) PIK3CA (E545K) |
| Case#4 (P) | Negative | Positive | Negative | Wild type | None |
| Case#5 (P) | Negative | Positive | Negative | Wild type | TP53 (R248Q) |
| Case#6 (P) | Negative | Positive | Negative | Wild type | RET (VUS S653T) |
| Case#7 (P) | Negative | Positive | Negative | EGFR (V774M) | None |
| Case#8 (P) | Negative | Positive | Negative | Wild type | PIK3CA (H1047L) |
| Case#9 (P) | Negative | Positive | Amplified | Wild type | n/a |
| Case#10 (P) | Positive | Positive | Negative | Wild type | BRCA1 (M17751) BRCA2 (A371T, VUS) |
| Case#11 (P) | Negative | Positive | Amplified | Wild type | n/a |
| Case#12 (R) | Negative | Positive | n/a | Wild type | n/a |
| Case#13 (P) | Negative | Positive | n/a | Wild type | n/a |
| Case#14 (P) | Positive | Negative | Negative | Wild type | n/a |
| Case#15 (P) | Positive | Positive | Amplified | Wild type | TP53 (R248W) CDH1 (Q422K VUS) |
|
Case#16 P M |
Negative Positive |
Positive Positive |
Negative Negative |
Wild type Wild type |
PIK3CA (H1047L), KRAS (G12D) PIK3CA (H1047L), KRAS (G12D) |
|
Case#17 P M |
Negative Positive |
Positive Positive |
Negative Negative |
Wild type Wild type |
RB1 (P347fs) RB1 (P347fs) |
| Case#18 (R)* | n/a | n/a | n/a | n/a | n/a |
| Case#19 (M)* | n/a | n/a | Amplified | Wild type | n/a |
| Case#20 (P)* | n/a | Positive | n/a | n/a | TP53 (T140fs) (Y234C) |
| Case#21 (P)* | n/a | n/a | negative | Wild type | None |
| Case#22 (M)* | n/a | n/a | Amplified | Wild type | TP53 (R282W) |
| Case#23 (R)* | n/a | Positive | negative | Wild type | TP53 (G245S) |
| Case#24 (P)* | n/a | Positive | Amplified | EGFRvIII | n/a |
| Case#25 (P)* | n/a | Positive | Amplified | Wild type | TP53 (D281V) |
| Case#26 (M)* | n/a | Positive | Negative | Wild type | TP53 (R175H) |
| Case#27 (M) | n/a | Positive | Negative | Wild type | TP53 (V157F) |
| Case#28 (P) | n/a | Positive | n/a | Wild type | None |
| Case#29 (P) | n/a | Positive | n/a | Wild type | None |
| Case#30 (R) | n/a | n/a | n/a | n/a | None |
| Case#31 (P) | n/a | Positive | n/a | Wild type | TP53 (S99fs) |
| Case#32 (M) | n/a | n/a | Negative | Wild type | TP53 (R175H) |
| Case#33 (P) | Positive | n/a | n/a | Wild type | MLH1 |
| Case#34 (P) | Positive | Positive | n/a | Wild type | TP53 (L194R) ATM (T616I) |
| Case#35 (P) | Positive | Positive | Amplified | Wild type | TP53 (c.560–23_561del) TP53 (R273H) |
| Case#36 (M) | Negative | Positive | n/a | Wild type | n/a |
Gene expression profiling
IHC – immunohistochemistry; ISH – in situ hybridization; NGS – next-generation sequencing
P – Primary; R – recurrent; M – metastatic
n/a – not available
VUS – variant of unknown significance
Tumor characteristics
EGFR status
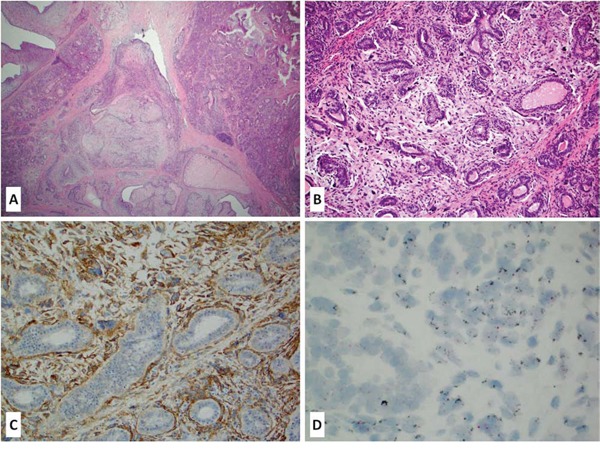
EGFR protein overexpression (H-score ≥ 20) was observed in 26 of 27 tested cases (96%) while amplification of the EGFR gene was observed in 8 of 24 tested cases (33%); all EGFR amplified cases strongly overexpressed EGFR protein (H-score > 200) (Figure 1A–1D). EGFRvIII mutant variant was unequivocally detected in one out of 29 tested cases (∼3%); an additional case exhibited a borderline MLPA score (0.8). Both cases were primary phyllodes tumors overexpressing EGFR protein by IHC. Unequivocal EGFRvIII mutant case also harbored EGFR gene amplification. Activating EGFR gene mutation was observed in one primary phyllodes tumor (presumed pathogenic V774 mutation). This case also overexpressed EGFR protein without EGFR gene amplification or any other potentially targetable mutations.
Figure 1. A-D: Primary malignant phyllodes tumor of the breast (A-B H&E stain, 10–20x magnification) with a strong membranous EGFR protein overexpression (C – IHC stain) accompanied by EGFR gene amplification (D – CISH).
Immune check-point proteins (PD-1 and PD-L1)
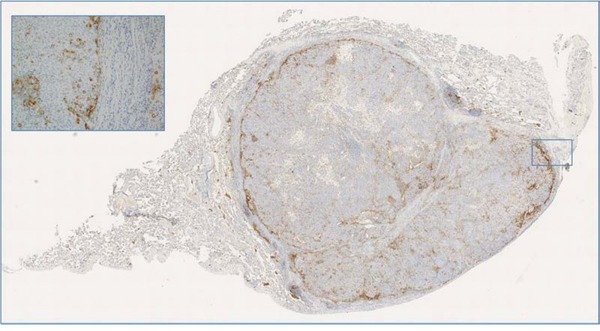
PD-1 and its ligand PD-L1 were evaluated in 22 phyllodes cases. Eight cases (36%) exhibited scattered, below threshold PD-L1 positivity on neoplastic cells while 3 cases including one metastatic phyllodes tumor showed positivity above the 5% threshold. In some cases PD-L1 expression was also detected on mono-nuclear cells within and surrounding the tumor (Figure 2). PD-1 positive TILs were completely absent in 12 cases, while in other cases varied in density from 1/10 hpf to > 100/10 hpf.
Figure 2. A case of metastatic phyllodes tumor to the lung with peripheral PD-L1 expression adjacent to the inflammatory cells and normal lung parenchyma; of note this case harbored RB1 gene mutation in both primary and metastatic tumor.
Mutational profile
NGS and/or Sanger sequencing assays were successful in 27 cases (Table 1). Apart from the above described EGFR mutations, several other genes were mutated including: TP53 and, PIK3CA (affecting 50% and 15% of cases respectively), and BRCA1, BRCA2, RET, CDH1, MLH1, ATM, KRAS, and RB1 (all affecting single phyllodes cases; Table 1). Of note, two cases with matching primary and metastatic (lung) samples harbored identical mutations in both sites: case#1 affecting a 56-year-old female had PIK3CA (H1047L) and KRAS (G12D) while case#2 (43-year-old female) harbored RB1 (P347fs) gene mutation. Of note, no mutations in KDR (VEGFR2) were detected (n = 27).
Whole genome expression profiling
Differential expression analysis of the mRNA as measured by the Illumina array platform was carried out by comparing the normalized expression of the transcripts to the expression of the genes in the normal breast tissue. At the two fold cutoff, 247 probes representing 243 genes were found to be up regulated and 443 probes representing 422 genes were found to be down regulated (Figure 3, Supplementary Table 1). List of upregulated genes includes the 57 probes (57 genes) and 19 probes (18 genes) that were upregulated by 5 fold. The list of significantly enriched Gene Ontology (GO) terms included terms associated with GTPase activity, cytokine binding, and several cell division terms.
Figure 3. Gene expression signature of six phyllodes cases along with the normal breast tissue.
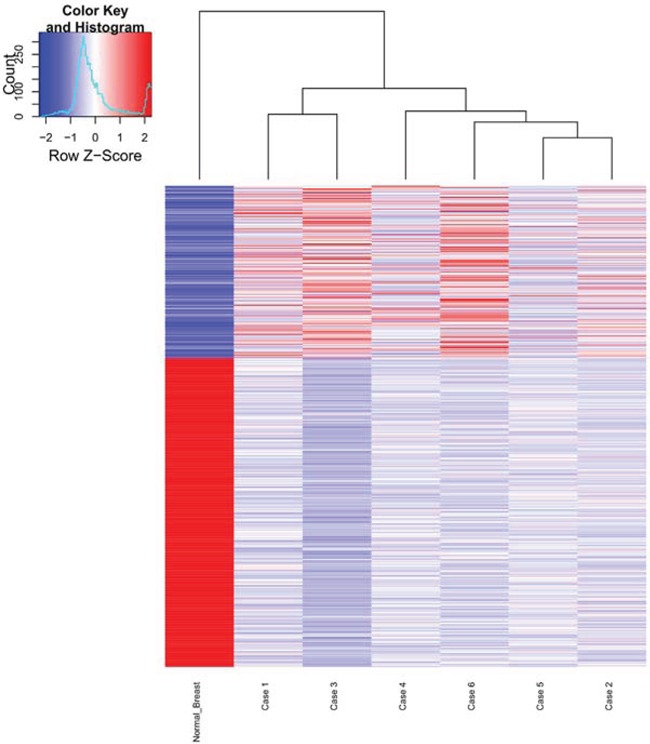
In the heatmap, rows represent genes and columns represent samples. “Upregulated” (depicted in red) is defined as a transcript with transcript level that is > 2 fold relative to normal breast control and down regulated (depicted in blue) is defined as a transcript with transcript level that is < 2 fold relative to control. The expression of the normal breast is shown in the far left column.
We found that 6 angiogenic markers (VEGFA, Angiopoietin-2, VCAM1, PDGFRA, PTTG1, and CYP3A5) were differentially expressed by at least 2 fold in phyllodes patients when compared with control (Figure 4, Supplementary Table 1). Moreover, our list significantly overlapped with the “angiome” list as published by Rivera et al. [31] (17 common genes, hypergeometric test p-value = 0.005).
Figure 4. Bar plots for 6 angiogenesis markers found to be differentially regulated in Phyllodes cases when compared to normal breast tissue.
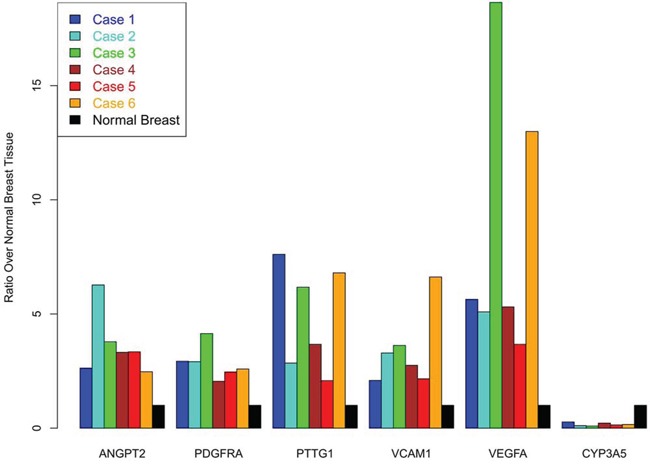
The height represents the ratio of expression for the gene in the phyllodes case over the expression in the normal breast. For CYP3A5, three phyllodes cases had no detectable expression of the transcript and the values are depicted as 0 ratio. Normal breast expression ratio is set to ‘1’ for all 6 biomarkers and is depicted as the ‘black’ bar for all 6 genes.
DISCUSSION
Malignant phyllodes tumor of the breast is a rare malignancy (<1%) with a substantial potential for both local relapse and distant metastasis (∼20%) [1]. Apart from surgery, no optimal treatment modalities with impact on overall survival are available at present [32]. Several recent studies indicated that molecular profiling of such “orphan tumors” may identify actionable targets with a successful outcome, even in metastatic setting [13, 22, 33–36].
The present study using comprehensive, multiplatform molecular approach revealed several potentially targetable pathways/biomarkers in these neoplasms. We found upregulation of several angiogenesis-related genes including VEGFA, Angiopoietin-2, VCAM1, PDGFRA, and PTTG1 [16–19]. These findings may be clinically relevant as the upregulation of proangiogenic genes may predict response to the targeted therapy (e.g. angiogenesis inhibitors). We recently reported on a recurrent mammary angiosarcoma that was successfully treated with anti-angiogenic drug sunitinib, identified as a potentially beneficial therapy option using the whole genome array analysis [34]. In addition, anti-angiogenic targeted therapies (e.g. tyrosine kinase inhibitors, monoclonal antibodies) are promising therapeutics for the treatment of a wide spectrum of soft tissue tumors [37]. We also found overexpression of EGFR protein (with or without EGFR gene amplification or mutations), which could lead to the use of dual VEGFR/EGFR inhibitors (e.g. Vandatenib) or a combination of anti-VEGFR (e.g. bevacizumab) and anti-EGFR (e.g. erlotinib/gefitinib/cetuximab) therapies [38, 39]. Overexpression of PDGFRA would suggest the potential study of pazopanib, a multikinase inhibitor, with c-KIT, FGFR, PDGFR and VEGFR being amongst the inhibited enzymes. Of note, we report here for the first time that EGFRvIII (a mutant form of EGFR with deletion of exons 2–7 of the gene) may play a role in a small subset of malignant phyllodes tumors, adding this cancer to the growing list of malignancies with this type of mutation [40], and potentially eligible for tumor directed immunotherapy.
The selective mutational profiling (TSACP) of malignant phyllodes tumors revealed that TP53 and PIK3CA gene mutations are common. These results are in line with previous data (COSMIC database, accessed on July 21, 2015; [41]). In contrast to the study of Tsang et al. [2] who also sequenced two matched phyllodes cases without recurrent mutations (SMAD4, IDH1, PIK3CA, RET, TP53), we found identical mutational profile in both primary and metastatic phyllodes cases (PIK3CA/KRAS and RB1 gene mutations, respectively). PIK3CA and RB1 alterations have been already described in phyllodes tumors [2, 13, 42] while Tsang et al. [2] found HRAS and Jardim et al. [33] NRAS in a borderline, and metastatic phyllodes tumors, respectively. The frequency of PIK3CA gene mutations in our study (15%) is the highest in comparison with the previous data [2] and this finding may imply a potential therapeutic benefit of the treatment with mTOR inhibitors.
Recently reported expression of the two immune check point targetable proteins PD-1 and PD-L1 in a variety of solid tumors including some sarcomas [20–26], led us to investigate them in this cohort of PT. Here we found that overexpression of PD-L1 characterizes a small subset of malignant phyllodes tumors including some metastatic cases, which may lead to the targeted immunotherapy for these patients. Numerous studies have reported remarkable benefits from PD-1/PD-L1 blockade in patients with other malignancies over-expressing PD-L1 (e.g. renal cell carcinoma, NSCLC, malignant melanoma, [21, 43–44]).
Limitations of our study are related to its retrospective design and the small number of cases tested by the Illumina microarray assay. Also, further prospective studies should confirm the clinical relevance of profiling-identified biomarkers.
In conclusion, this study provides additional support for comprehensive profiling in PT, which can identify several potentially targetable pathways including EGFR, angiogenesis, and immunotherapy for patients with locally advanced or metastatic tumors.
MATERIALS AND METHODS
Tissue samples
Thirty-six formalin-fixed paraffin-embedded (FFPE) samples of malignant phyllodes tumors were profiled at Caris Life Sciences, Phoenix, AZ (Molecular Intelligence Service™). All samples were previously diagnosed by referring pathologists and histologic diagnosis was confirmed centrally by a board certified pathologist.
Immunohistochemistry (IHC)
Expression of EGFR, PD-1 and PD-L1 was evaluated immunohistochemically using commercially available antibodies and detection kits [EGFR (Invitrogen); PD-1, (NAT1 antibody, Cell Marque); anti-PD-L1 (SP142, Spring Bioscience)]. The extent of EGFR expression was evaluated using H-score (intensity of staining graded on a subjective scale of 0–3 and percentage of cells with given intensity were multiplied and added up to provide an H-score, which ranged from 0–300). Tumor infiltrating lymphocytes (TIL), which expressed PD-1 on their plasma membrane, were evaluated and their density (number of PD-1+ TILs per high power field) was recorded. Membranous expression of PD-L1 in more than 5% of tumor cells was considered positive [20, 22–23, 27]. All IHC assays included positive and negative controls to support the validity of results.
EGFR gene alterations
Copy number changes: In-situ hybridization (fluorescent and chromogenic ISH)
FISH [EGFR/CEP7 probe] (Abbott Molecular/Vysis) and CISH (dual EGFR DNP/CEP 7 DIG probes, Ventana, Tucson, AZ) assays were used for evaluation of the EGFR gene status. EGFR gene was considered amplified if EGFR/CEP7 ratio > 2, or > 15 EGFR gene copies per cell were observed in > 10% of analyzed cells [28].
EGFRvIII mutation: fragment analysis (FA) sequencing and multiplex ligation-dependent probe amplification (MLPA)
Mutation analysis for EGFRvIII was performed on RNA extracted from FFPE tissue in 18 cases. Two sets of FAM linked primers were used to PCR amplify both the wild type and mutant EGFR alleles and PCR products were visualized using an ABI 3500xl. Signal generated from the wild type allele was used as an amplification control and samples were considered positive if EGFRvIII was detected at a level that is 5x typical background observed. Samples with EGFRvIII signals between 1–5 x standard background were considered indeterminate and < 1x standard background was considered a negative result. This assay requires samples to have at least 50% tumor nuclei [29].
Eleven PTs were evaluated for EGFRvIII status using a multiplex ligation-dependent probe amplification (MLPA) assay (SALSA MLPA KIT P315-A1 EGFR, MRC-Holland kit, MRC-Holland, Amsterdam, Netherlands). Values between 0.8 - 1.5 were considered normal, while values < 0.8 as a loss, > 1.5 as a gain and values > 2 as an amplification.
Sequencing analysis (NGS and sanger sequencing)
NGS was performed on genomic DNA isolated from FFPE samples using the Illumina MiSeq platform. Specific regions of the genome were amplified using the Illumina TruSeq Amplicon - Cancer Panel (TSACP). The NGS panel included 46 genes (available here: http://www.carismolecularintelligence.com/next-generation-sequencing-profile). All variants were detected with > 99% confidence based on the frequency of the mutation present and the amplicon coverage using a mutation frequency threshold of 10% [23, 30]. All regions that were sequenced achieved a minimum of 100x coverage and overall samples had an average coverage of > 500x; most samples achieving 1000–2000x average coverage. Sanger Sequencing for selected regions of BRAF, KRAS, c-KIT, EGFR, and PIK3CA was also used.
Microarray assays
For seven cases the whole-genome expression was analyzed using Illumina cDNA-mediated annealing, selection, extension and ligation (DASL) process with the HumanHT-12 v4 beadChip (Illumina Inc., San Diego, CA). Of the seven cases run on this platform one case was excluded from analysis due to overall low scanning intensity. The 6 remaining phyllodes cases were normalized using mean expression normalization where each array data was adjusted to have the same mean as the control breast microarray. The ratios of expression for each gene were calculated by dividing the normalized expression of the gene to the control breast tissue. The detection p-value of 0.001 was used to assess if a gene is expressed or not. The detection p-value is an output of the Genome Studio software (Illumina) and represents the confidence that a given transcript is expressed above the background defined by negative control probes on the array. All the data analysis for gene expression was carried out using the Genome Studio software and the R Software downloaded from CRAN website (http://cran.r-project.org).
SUPPLEMENTARY TABLE
Acknowledgments
The preliminary results of this study were presented at the European Congress of Medical Oncology (ESMO) in Madrid, Spain, September 26–30, 2014.
Footnotes
CONFLICTS OF INTEREST
Zoran Gatalica, Anatole Ghazalpour, Joanne Xiu, Ryan P. Bender, Erin Disciano, Aaron Schlum, and Sandeep Reddy are employees of Caris Life Sciences. Other authors have no conflict of interest to declare.
Editorial note
This paper has been accepted based in part on peer-review conducted by another journal and the authors’ response and revisions as well as expedited peer-review in Oncotarget.
REFERENCES
- 1.Spitaleri G, Toesca A, Botteri E, Bottiglieri L, Rotmensz N, Boselli S, Sangalli C, Catania C, Toffalorio F, Noberasco C, Delmonte A, Luini A, Veronesi P, et al. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol. 2013;88:427–436. doi: 10.1016/j.critrevonc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Tsang JY, Go EM, Tse GM. Identification of clinically relevant alterations in phyllodes tumor of the breast by amplicon-based next-generation sequencing. Breast Cancer Res Treat. 2015;151:717–719. doi: 10.1007/s10549-015-3396-1. [DOI] [PubMed] [Google Scholar]
- 3.Feakins RM, Mulcahy HE, Nickols CD, Wells CA. p53 expression in phyllodes tumours is associated with histological features of malignancy but does not predict outcome. Histopathology. 1999;35:162–169. doi: 10.1046/j.1365-2559.1999.00682.x. [DOI] [PubMed] [Google Scholar]
- 4.Millar EK, Beretov J, Marr P, Sarris M, Clarke RA, Kearsley JH, Lee CS. Malignant phyllodes tumours of the breast display increased stromal p53 protein expression. Histopathology. 1999;34:491–496. doi: 10.1111/j.1365-2559.1999.00666.x. [DOI] [PubMed] [Google Scholar]
- 5.Gatalica Z, Finkelstein S, Lucio E, Tawfik O, Palazzo J, Hightower B, Eyzaguirre E. p53 protein expression and gene mutation in phyllodes tumors of the breast. Pathol Res Pract. 2001;197:183–187. doi: 10.1078/0344-0338-00031. [DOI] [PubMed] [Google Scholar]
- 6.Jones AM, Mitter R, Springall R, Graham T, Winter E, Gillett C, Hanby AM, Tomlinson IP, Sawyer EJ, Phyllodes Tumour Consortium A comprehensive genetic profile of phyllodes tumours of the breast detects important mutations, intra-tumoral genetic heterogeneity and new genetic changes on recurrence. J Pathol. 2008;214:533–544. doi: 10.1002/path.2320. [DOI] [PubMed] [Google Scholar]
- 7.Vidal M, Peg V, Galván P, Tres A, Cortés J, Ramón y Cajal S, Rubio IT, Prat A. Gene expression-based classifications of fibroadenomas and phyllodes tumours of the breast. Mol Oncol. 2015;9:1081–1090. doi: 10.1016/j.molonc.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho SK, Thike AA, Cheok PY, Tse GM, Tan PH. Phyllodes tumours of the breast: the role of CD34, vascular endothelial growth factor and β-catenin in histological grading and clinical outcome. Histopathology. 2013;63:393–406. doi: 10.1111/his.12177. [DOI] [PubMed] [Google Scholar]
- 9.Kuijper A, van der Groep P, van der Wall E, van Diest PJ. Expression of hypoxia-inducible factor 1 alpha and its downstream targets in fibroepithelial tumors of the breast. Breast Cancer Res. 2005;7:R808–818. doi: 10.1186/bcr1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tse GM, Lui PC, Lee CS, Kung FY, Scolyer RA, Law BK, Lau TS, Karim R, Putti TC. Stromal expression of vascular endothelial growth factor correlates with tumor grade and microvessel density in mammary phyllodes tumors: a multicenter study of 185 cases. Hum Pathol. 2004;35:1053–1057. doi: 10.1016/j.humpath.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Lin JJ, Huang CS, Yu J, Liao GS, Lien HC, Hung JT, Lin RJ, Chou FP, Yeh KT, Yu AL. Malignant phyllodes tumors display mesenchymal stem cell features and aldehyde dehydrogenase/disialoganglioside identify their tumor stem cells. Breast Cancer Res. 2014;16:R29. doi: 10.1186/bcr3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tse GM, Lui PC, Scolyer RA, Putti TC, Kung FY, Law BK, Lau TS, Lee CS. Tumour angiogenesis and p53 protein expression in mammary phyllodes tumors. Mod Pathol. 2003;16:1007–1013. doi: 10.1097/01.MP.0000089907.67419.42. [DOI] [PubMed] [Google Scholar]
- 13.Cani AK, Hovelson DH, McDaniel AS, Sadis S, Haller MJ, Yadati V, Amin AM, Bratley J, Bandla S, Williams PD, Rhodes K, Liu CJ, Quist MJ, et al. Next-Gen Sequencing Exposes Frequent MED12 Mutations and Actionable Therapeutic Targets in Phyllodes Tumors. Mol Cancer Res. 2015;13:613–619. doi: 10.1158/1541-7786.MCR-14-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng CC, Tan J, Ong CK, Lim WK, Rajasegaran V, Nasir ND, Lim JC, Thike AA, Salahuddin SA, Iqbal J, Busmanis I, Chong AP, Teh BT, Tan PH. MED12 is frequently mutated in breast phyllodes tumours: a study of 112 cases. J Clin Pathol. 2015;68:685–691. doi: 10.1136/jclinpath-2015-202896. [DOI] [PubMed] [Google Scholar]
- 15.Nagasawa S, Maeda I, Fukuda T, Wu W, Hayami R, Kojima Y, Tsugawa K, Ohta T. MED12 exon 2 mutations in phyllodes tumors of the breast. Cancer Med. 2015;4:1117–1121. doi: 10.1002/cam4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kersting C, Kuijper A, Schmidt H, Packeisen J, Liedtke C, Tidow N, Gustmann C, Hinrichs B, Wülfing P, Tio J, Boecker W, van Diest P, Brandt B, Buerger H. Amplifications of the epidermal growth factor receptor gene (egfr) are common in phyllodes tumors of the breast and are associated with tumor progression. Lab Invest. 2006;86:54–61. doi: 10.1038/labinvest.3700358. [DOI] [PubMed] [Google Scholar]
- 17.Agelopoulos K, Kersting C, Korsching E, Schmidt H, Kuijper A, August C, Wülfing P, Tio J, Boecker W, van Diest PJ, Brandt B, Buerger H. Egfr amplification specific gene expression in phyllodes tumours of the breast. Cell Oncol. 2007;29:443–451. doi: 10.1155/2007/754712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan WJ, Lai JC, Thike AA, Lim JC, Tan SY, Koh VC, Lim TH, Bay BH, Tan MH, Tan PH. Novel genetic aberrations in breast phyllodes tumours: comparison between prognostically distinct groups. Breast Cancer Res Treat. 2014;145:635–645. doi: 10.1007/s10549-014-2982-y. [DOI] [PubMed] [Google Scholar]
- 19.Tse GM, Lui PC, Vong JS, Lau KM, Putti TC, Karim R, Scolyer RA, Lee CS, Yu AM, Ng DC, Tse AK, Tan PH. Increased epidermal growth factor receptor (EGFR) expression in malignant mammary phyllodes tumors. Breast Cancer Res Treat. 2009;114:441–448. doi: 10.1007/s10549-008-0030-5. [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response toanti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, Overberg P, Rose I, Basu GD, Vranic S, Lynch HT, Von Hoff DD, Hamid O. Programmed cell death (PD-1) and its ligand (PD-L1) expression in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965–2970. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 23.Gatalica Z, Bilalovic N, Palazzo JP, Bender RP, Swensen J, Millis SZ, Vranic S, Von Hoff D, Arceci RJ. Disseminated histiocytoses biomarkers beyond BRAFV600E: frequent expression of PD-L1. Oncotarget. 2015;6:19819–19825. doi: 10.18632/oncotarget.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Movva S, Wen W, Chen W, Millis SZ, Gatalica Z, Reddy S, von Mehren M, Van Tine BA. Multi-platform profiling of over 2000 sarcomas: identification of biomarkers and novel therapeutic targets. Oncotarget. 2015;6:12234–12447. doi: 10.18632/oncotarget.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA, Rodig SJ. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess M, Tawbi H. Immunotherapeutic approaches to sarcoma. Curr Treat Options Oncol. 2015;16:26. doi: 10.1007/s11864-015-0345-5. [DOI] [PubMed] [Google Scholar]
- 27.Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Caliò A, Cuppone F, Sperduti I, Giannarelli D, Chilosi M, Bronte V, Scarpa A, Bria E, Tortora G. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One. 2015;10:e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millis SZ, Bryant D, Basu G, Bender R, Vranic S, Gatalica Z, Vogelzang NJ. Molecular Profiling of Infiltrating Urothelial Carcinoma of Bladder and Nonbladder Origin. Clin Genitourin Cancer. 2015;13:e37–49. doi: 10.1016/j.clgc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Jeuken J, Sijben A, Alenda C, Rijntjes J, Dekkers M, Boots-Sprenger S, McLendon R, Wesseling P. Robust detection of EGFR copy number changes and EGFR variant III: technical aspects and relevance for glioma diagnostics. Brain Pathol. 2009;19:661–671. doi: 10.1111/j.1750-3639.2009.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millis SZ, Gatalica Z, Winkler J, Vranic S, Kimbrough J, Reddy S, O'Shaughnessy JA. Predictive Biomarker Profiling of > 6000 Breast Cancer Patients Shows Heterogeneity in TNBC, With Treatment Implications. Clin Breast Cancer. 2015 Apr 28; doi: 10.1016/j.clbc.2015.04.008. pii: S1526-8209(15)00098-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Rivera CG, Mellberg S, Claesson-Welsh L, Bader JS, Popel AS. Analysis of VEGF—a regulated gene expression in endothelial cells to identify genes linked to angiogenesis. PLoS One. 2011;6:e24887. doi: 10.1371/journal.pone.0024887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng S, Zhang X, Yang D, Wang X, Ren G. Effects of adjuvant radiotherapy on borderline and malignant phyllodes tumors: A systematic review and meta-analysis. Mol Clin Oncol. 2015;3:663–671. doi: 10.3892/mco.2015.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jardim DL, Conley A, Subbiah V. Comprehensive characterization of malignant phyllodes tumor by whole genomic and proteomic analysis: biological implications for targeted therapy opportunities. Orphanet J Rare Dis. 2013;8:112. doi: 10.1186/1750-1172-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva E, Gatalica Z, Vranic S, Basu G, Sandeep KR, Voss A. Refractory angiosarcoma of the breast with VEGFR2 upregulation successfully treated with Sunitinib: A case report and review of 16 additional cases with molecular profiling. Breast J. 2015;21:205–207. doi: 10.1111/tbj.12380. [DOI] [PubMed] [Google Scholar]
- 35.Myers CE, Gatalica Z, Spinelli A, Castro M, Linden E, Sartor O, Sargent M. Metastatic Cancer of Cowper's Gland: A Rare Cancer Managed Successfully by Molecular Profiling. Case Rep Oncol. 2014;7:52–57. doi: 10.1159/000357972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Von Hoff DD, Stephenson JJ, Jr., Rosen P, Loesch DM, Borad MJ, Anthony S, Jameson G, Brown S, Cantafio N, Richards DA, Fitch TR, Wasserman E, Fernandez C, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- 37.Versleijen-Jonkers YM, Vlenterie M, van de Luijtgaarden AC, van der Graaf WT. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit Rev Oncol Hematol. 2014;91:172–185. doi: 10.1016/j.critrevonc.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Morabito A, Piccirillo MC, Falasconi F, De Feo G, Del Giudice A, Bryce J, Di Maio M, De Maio E, Normanno N, Perrone F. Vandetanib (ZD6474), a dual inhibitor of vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) tyrosine kinases: current status and future directions. Oncologist. 2009;14:378–390. doi: 10.1634/theoncologist.2008-0261. [DOI] [PubMed] [Google Scholar]
- 39.Pennell NA, Lynch TJ., Jr. Combined inhibition of the VEGFR and EGFR signaling pathways in the treatment of NSCLC. Oncologist. 2009;14:399–411. doi: 10.1634/theoncologist.2008-0276. [DOI] [PubMed] [Google Scholar]
- 40.Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280:5350–5370. doi: 10.1111/febs.12393. [DOI] [PubMed] [Google Scholar]
- 41.Korcheva VB, Levine J, Beadling C, Warrick A, Countryman G, Olson NR, Heinrich MC, Corless CL, Troxell ML. Immunohistochemical and molecular markers in breast phyllodes tumors. Appl Immunohistochem Mol Morphol. 2011;19:119–125. doi: 10.1097/PAI.0b013e3181f5349a. [DOI] [PubMed] [Google Scholar]
- 42.Cimino-Mathews A, Hicks JL, Sharma R, Vang R, Illei PB, De Marzo A, Emens LA, Argani P. A subset of malignant phyllodes tumors harbors alterations in the Rb/p16 pathway. Hum Pathol. 2013;44:2494–2500. doi: 10.1016/j.humpath.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 44.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


