Abstract
Nicotinamide adenine dinucleotide (NAD) is a cofactor in redox reactions and a substrate for NAD-consuming enzymes, such as PARPs and sirtuins. As cancer cells have increased NAD requirements, the main NAD salvage enzymes in humans, nicotinamide phosphoribosyltransferase (NAMPT) and nicotinate phosphoribosyltransferase (NAPRT), are involved in the development of novel anti-cancer therapies. Knowledge of the expression patterns of both genes in tissues and tumors is critical for the use of nicotinic acid (NA) as cytoprotective in therapies using NAMPT inhibitors. Herein, we provide a comprehensive study of NAPRT and NAMPT expression across human tissues and tumor cell lines. We show that both genes are widely expressed under normal conditions and describe the occurrence of novel NAPRT transcripts. Also, we explore some of the NAPRT gene expression mechanisms. Our findings underline that the efficiency of NA in treatments with NAMPT inhibitors is dependent on the knowledge of the expression profiles and regulation of both NAMPT and NAPRT.
Keywords: human NAD salvage, NAPRT, NAMPT, alternative transcripts, anti-cancer therapies
INTRODUCTION
Nicotinamide adenine dinucleotide (NAD) is a coenzyme in oxidation-reduction reactions that release energy in the form of ATP. Additionally, it is a substrate for NAD-consuming enzymes such as poly(ADP-ribose) polymerases (PARPs) and sirtuins [1].
NAD-consuming enzymes are important in several cell functions, from gene silencing to DNA repair, cell signaling and cell survival, which has raised interest in the biosynthesis of NAD. In humans, NAD can be synthesized from different precursors, namely tryptophan in the de novo pathway and nicotinamide (Nam), nicotinamide riboside (NR) and nicotinic acid (NA) through distinct salvage pathways [2, 3].
NAD synthesis from Nam is catalyzed by nicotinamide phosphoribosyltransferase (NAMPT), which is the first and rate-limiting enzyme of this pathway, and has been associated with several diseases, including inflammation, metabolic disorders and cancer [4]. Cancer cells have high energetic requirements and a high rate of NAD turnover, as well as increased demands for DNA repair activity [4-6]. As NAMPT overexpression has been reported in various tumor types, such as colorectal, ovarian, gastric, prostate, lung and glioblastoma [7-12], NAMPT inhibitors have been developed as potential anticancer agents [13-17].
In this context, nicotinate phosphoribosyltransferase (NAPRT) is also being studied [17-20]. NAPRT is responsible for the first step of the conversion of NA to NAD. Although Nam is the main NAD salvageable precursor in human cells, most likely because this molecule is itself produced in NAD-consuming reactions [2], NA is more efficient in increasing NAD levels, thus, some tissues could preferentially use the NAD salvage pathway from NA [3, 21]. Lack of NAPRT expression in some tumors, such as neuroblastoma or glioblastoma [17], and lymphomas [22], places NAPRT as a biomarker for the use of NA as a chemoprotectant agent in the treatment with NAMPT inhibitors [19]. In NAPRT-negative tumors, inhibiting NAMPT provides a novel synthetic lethal therapeutic approach by inducing metabolic stress, while normal cells are rescued by NA via activation of the NAPRT pathway [17, 18, 23].
In order to predict the usefulness of NA-therapeutics, knowledge of the expression patterns of both human NAPRT and NAMPT is mandatory [6], but few studies have focused on the characterization of human NAPRT [24]. Most data concerning NAPRT expression refers to mouse and rat tissues [21] or human tumors [17, 19], but a thorough survey of NAPRT expression patterns in humans is missing. Here, we describe our results of a global study of NAPRT and NAMPT expression across human tissues and tumor cell lines. We show that both genes are widely expressed and that NAPRT is subject to extensive gene regulation, and discuss the implications of these findings for NA-protective therapeutics.
RESULTS
NAPRT and NAMPT expression
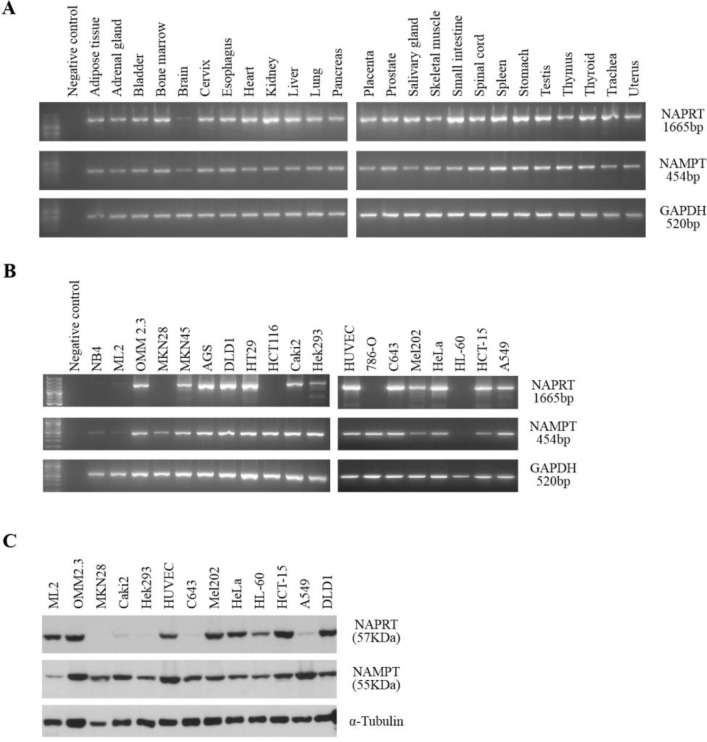
The initial step in this study was to evaluate NAPRT and NAMPT expression in a set of normal human tissues. RT-PCR results showed a widespread expression for both genes (Figure 1A). Curiously, the brain tissue appeared to express less NAMPT and NAPRT. Bioinformatics analyses using expressed sequence tag (EST) data available in the UniGene database [25] from NCBI showed that NAMPT is broadly expressed, whereas several tissues had no reported expression of NAPRT (Supplementary Figure 1). Data from the Human Protein Atlas database [26] showed that NAPRT and NAMPT mRNA were detected in all tissues, however, protein levels were highly diverse and not detected in several cases (Supplementary Figure 1).
Figure 1. NAPRT and NAMPT expression in normal human tissues and in tumor cell lines.
A. RT-PCR analysis of RNA from several human tissues shows that both genes are widely expressed in all tissues tested. Similar results were obtained from independent cDNA synthesis and with tissues from different sources. GAPDH is included as a control. B. RT-PCR analysis of RNA from human tumor cell lines shows some variability of both NAPRT and NAMPT among the tumor types tested. GAPDH is included as a control. C. Western-blot analysis reveals distinct patterns of NAPRT and NAMPT protein expression. α-tubulin is shown as a loading control.
To assess NAPRT and NAMPT expression in tumors, we studied a set of cell lines, which included renal, thyroid, cervix, lung, gastric and colorectal carcinomas, uveal melanoma, and leukemia, by RT-PCR and western blot (Figure 1B and 1C). We observed that NAMPT is expressed in all tumor types tested, although the leukemia cell lines (NB4, ML2 and HL-60) showed weaker expression (Figure 1B). Also, we found that the NAPRT gene is differentially expressed between cell lines (Figure 1B), with a marked decrease in expression in carcinoma cell lines 786-O (renal), MKN28 (gastric), HCT116 (colorectal) and in all leukemia cell lines tested (HL-60, NB4 and ML2). The western blot analysis confirmed that among the cell lines tested, Caki-2 and HEK293 (renal), C643 (thyroid) and A549 (lung) had weak NAPRT expression, and in MKN28 (gastric) the protein was undetectable (Figure 1C). NAMPT protein was detected in all cell lines analyzed.
As NAPRT-negative tumors can be targeted by NA rescue with NAMPT inhibitors, we further explored gene expression regulatory mechanisms as described below.
NAPRT gene expression regulation
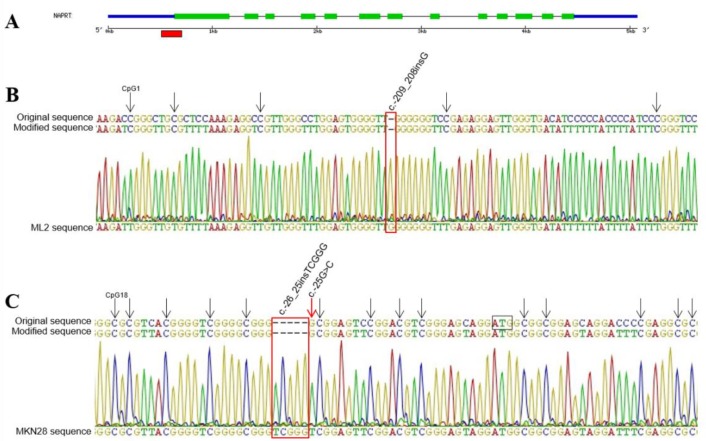
Given the absence of NAPRT expression in some tumor cell lines, we considered the occurrence of DNA cytosine methylation in the promoter region as previously suggested [19]. We analyzed a fragment, which included a total of 37 CpG sites, in selected cell lines with different mRNA expression (A549, C643, 786-O, HT29, HCT116, MKN28, AGS, HL60, ML2 and NB4) and observed that only the MKN28 cell line presented all cytosines methylated (Figure 2), which explains its silencing.
Figure 2. Methylation status of NAPRT promoter region.
A. Schematic representation of the gene structure, with the red box marking the sequenced region within the CpG island. B. Section of the unmethylated ML2 cell line sequencing electropherogram, showing that all cytosines including those in CpG sites (arrows) were converted to thyimines. C. Sequence showing the region surrounding the start codon (ATG) in MKN28 cell line, where all cytosines in GpG sites indicated by arrows are methylated including the additional CpG resultant from rs146163552 insertion. All mutations are indicated in red.
Also, we screened the promoter region for mutations in 13 tumor cell lines, by sequencing 436bp upstream the start codon. In this region, we detected seven variants (Supplementary Table 1). Two of these (rs146163552 and rs2305495) were found only in cell line MKN28, and one previously undescribed substitution (c.-256C>A) appeared only in HL60 cell line. The remaining variants were found in almost all analyzed cell lines.
To evaluate the prevalence of alternative transcripts in NAPRT, we focused on the sequence and tissue of origin of NAPRT ESTs deposited in UniGene (Supplementary Figure 2). Considering that known alternative protein coding NAPRT transcripts differ in the 3′ end sequence (Figure 3), we selected a small set of tissues, namely brain, liver, skeletal muscle and small intestine to be analyzed by 3′-RACE. This choice took into account both the expression levels and the diversity of transcripts of the NAPRT gene. We observed the existence of two fragments in the same tissue (Supplementary Figure 3A), although the shorter transcript was undetectable in the liver. Both transcripts share an identical 3′ UTR sequence and lack a recognizable polyadenylation signal (Supplementary Figure 3B).
Figure 3. Alternative NAPRT transcripts predicted in Ensembl Genome Browser.
Alignment of the predicted NAPRT transcripts from Ensembl, showing the differences to the reference sequence. The transcript NAPRT-002 corresponds to the reference sequence (NM_145201.5). Transcripts NAPRT-003 and -004 are protein coding. NAPRT1-003 has a deletion of 39 base pairs corresponding to 13 amino acids in exon 11, while the remaining sequence is maintained. NAPRT1-004 retains part of intron 11 resulting in a premature STOP codon. Transcripts NAPRT-001 and -010, classified as nonstop mediated decay, retain intron 12 and the consequent alteration of the reading frame leads to the readthrough of the STOP codon. Transcripts NAPRT-013 and -014, classified as nonsense mediated decay, lack exon 5, yet their sequence is incomplete. The remaining transcripts have several retained introns and no complete sequence is described.
Sequencing analysis of both fragments revealed that the most abundant one (A) is equivalent to the reference NAPRT transcript (NAPRT-002, Figure 3), and the shorter sequence (S) is a novel transcript that lacks exons 11 and 12. Two ESTs lacking exons 11 and 12 were detected in testis tissue and in squamous cell carcinoma of the skin (UniGene database, EST ID: GenBank entries HY025556.1 and BG676963.1, respectively), yet, a complete sequence is missing.
Novel NAPRT transcripts
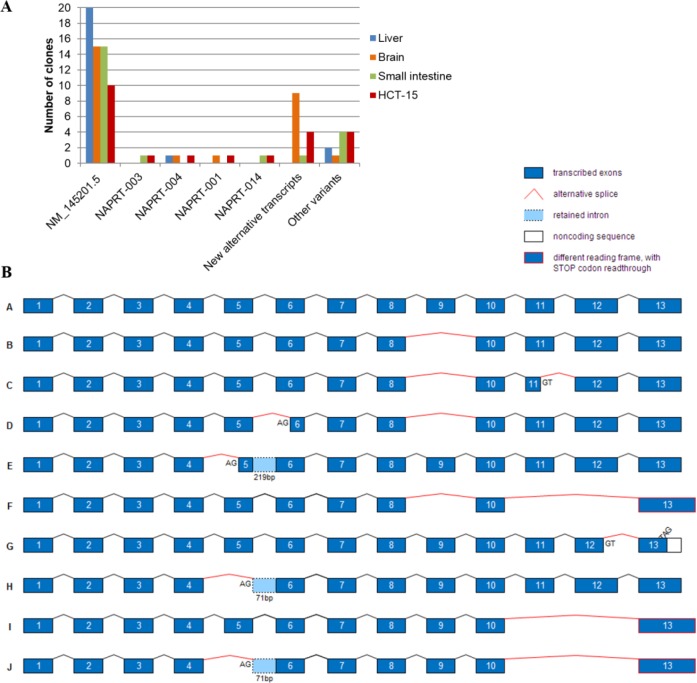
To obtain the full-length sequence of the novel NAPRT alternatively spliced transcripts, we amplified the whole coding sequence of three representative human normal tissues, liver, brain and small intestine, and a colorectal carcinoma cell line (HCT-15) and cloned the resulting RT-PCR products. The 94 clones sequenced revealed high diversity in NAPRT sequence (Figure 4A). In addition to NAPRT-003, -004, -001, -014 and -005 (Figure 3), 9 novel alternative transcripts were detected and are schematically represented in Figure 4B.
Figure 4. Novel alternative NAPRT transcripts.
Alternative spliced sequences were considered when donor and acceptor splice sites were identified, while a total of 10 variants presenting several exon deletions with no recognizable splice sites are referred to as other variants. A. Tissue distribution of NAPRT transcripts detected by cloning of the full-length cDNA from normal human tissues (liver, brain, small intestine) and HCT-15 tumor cell line. B. Schematic representation of NAPRT alternative transcript sequences (not in scale). Alternative splice sites (AG or GT) other than intron location, as well as premature STOP codons (TAG) are identified in each sequence. Sequence A, NAPRT RefSeq NM_145201.5; sequences B-E, found in brain; sequence F, found in small intestine; sequences G-J, found in HCT-15 cell line.
The most frequent of these new transcripts was found exclusively in the brain and lacks exon 9 (Figure 4B, sequence B). The removal of this exon could suppress NAPRT enzyme activity, as critical amino acids are located in exon 9 [24, 27].
The skipping of exons 11 and 12 (Figure 4B, sequences F, I and J) was identified in small intestine and in HCT-15 cell line. This results in STOP codon readthrough and may tag the mRNA to a mechanism of nonstop mediated decay, similarly to NAPRT-001 and -010 transcripts (Figure 3).
Exon 5 skipping appeared more than once, as well as the total or partial retention of intron 5 (Figure 4B, sequences E, H, J). These may decrease NAPRT activity, due to the location of several amino acids that bind ATP, PRPP and nicotinic acid [24, 27].
Skipping of exons 5, 9 and 11-12 were detected alone or in combination. Other alternative splicing events occurred in the middle of exons, leading to partial deletion of exon 5, exon 6, exon 11 or exon 12 (Figure 4B).
NAPRT and NAMPT mutations
We have previously identified NAPRT variants in predicted splicing sites [28], thus, we further screened several tumor cell lines for mutations. Selected regions from NAPRT and NAMPT were amplified and sequenced to detect the presence of potentially damaging mutations, such as nonsense or frameshift in coding sequences. Most alterations found were silent or intronic variants already described in existing databases, and detailed information of these results is depicted in Supplementary Table 2.
Of note, the NAPRT rs2015562 and rs2305496 variants that are predicted as splicing or gene expression modulators were found in the MKN28 cell line [28, 29]. In NAMPT, one missense variant was identified in the pulmonary tumor A549 cell line (g.23531A>G, Lys339Glu). To evaluate the effect of this alteration, we predicted the structure of the NAMPT protein with the Lys339Glu replacement and observed that all polar contacts of this residue are lost in the 3D model (Supplementary Figure 4). Although the Lys339Glu substitution was predicted as benign by PolyPhen (http://genetics.bwh.harvard.edu/pph2/), this and several other NAMPT missense mutations have been found in lung and upper aerodigestive tract tumors, as reported in The Catalogue of Somatic Mutations in Cancer (COSMIC) [30].
Globally, these variants should not be disregarded without further data, as some are predicted to alter splicing sites or gene expression levels, and the effect of coding silent mutations in protein expression or function is increasingly recognized in the context of human disease as well [31-33].
DISCUSSION
NAD salvage enzymes are essential in the regulation of cellular metabolism and are associated with human disease [34]. Among them, NAMPT has been extensively characterized and is a prospective therapeutic target in human cancer [13, 16, 35, 36]. NAPRT, on the other hand, has been scarcely studied, despite being a potential biomarker for the use of nicotinic acid in treatments with NAMPT inhibitors [6, 19, 20]. This strategy will be most beneficial in patients with tumors that overexpress NAMPT and/or downregulate NAPRT, while expressing normal NAPRT levels in healthy cells, which drove us to perform a global study on the expression patterns of both NAMPT and NAPRT genes in normal human tissues and tumor cell lines.
We observed that both genes are ubiquitously expressed at the mRNA level in all normal human tissues tested. While definitive conclusions on the expression of both genes would benefit from a quantitative approach, it is interesting to note the weaker expression of NAPRT and NAMPT that was observed in the brain, both in mRNA (Figure 1A) and protein expression (not shown). These results are in agreement with previous findings that the main routes to NAD in neurons are the de novo biosynthesis from tryptophan and the NAD salvage from nicotinamide riboside [3, 37, 38].
In tumor cell lines, we found that the NAPRT gene is more widely expressed than expected, at the mRNA level, considering that several cell lines are negative for the protein (Figure 1B and 1C). Even while expressing NAPRT transcripts, thyroid and lung carcinoma cell lines do not show protein expression, suggesting that these are good targets for NA therapy [39-41]. On the other hand, in some cell lines such as leukemia ML-2 and HL-60, the full-length transcript was barely detected, although expression of the NAPRT protein was found. These results imply that both transcriptional and post-transcriptional mechanisms control the expression of the NAPRT gene in tumor cell lines, thus, we searched for the presence of alternative transcripts and sequence alterations both in coding and regulatory regions.
Our results support the presence of more than one NAPRT transcript in most tissues (Supplementary Figure 3 and data not shown), all with identical 3′ end sequence. This indicates that NAPRT is subjected to alternative splicing but not to alternative polyadenylation. Additionally, the transcripts have no recognizable polyadenylation signal within the 10-30 nucleotide interval upstream of the cleavage site. As 20 to 30% of human genes yield transcripts with no detectable polyadenylation signal in their 3′ UTRs [42], this seems to be the case of NAPRT. We also described the sequences of novel NAPRT transcripts resulting from alternative splicing (Figure 4). These have different functional consequences, for example, transcripts with premature STOP codons or STOP codon readthrough may be eliminated by nonsense or nonstop mediated decay. In others, the loss of amino acids involved in enzyme activity or in establishing the dimer interface may result in NAPRT inactivation (Supplementary Table 3 and Supplementary Figure 5).
Some NAPRT transcripts appear to be tissue-specific. In our study, brain showed the highest number of alternative transcripts, which is supported by previous observations in brain cells, showing over 40% prevalence of alternative splicing [43, 44]. Considering that the reference transcript is also less expressed in brain, a regulatory role for these alternative NAPRT transcripts described here should be further considered.
As RNA binding proteins (RBPs) mediate post-transcriptional regulation, we searched for putative RBPs binding sites in the NAPRT sequence using the DoRiNA database (available at http://dorina.mdc-berlin.de) [45]. We found six predicted binding sites for Argonaute proteins, which are known transcriptional silencers as part of the RNA-induced silencing complex [46], and have been recently linked to alternative splicing [47]. In NAPRT sequence, two of the Argonaute binding sites are localized in regions lost in two alternative transcripts found in brain (Figure 4B, sequences C and E). Also, and although most of the splicing factors are ubiquitously expressed, some have been described as tissue-specific [48]. Among them, is the neuro-oncologic ventral antigen 1 (NOVA-1) protein, a brain specific RBP implicated in neurological disorders [49]. NOVA proteins control alternative splicing events by specifically recognizing pre-mRNA containing YCAY motifs [50]. If the YCAY motif is located within the exon, it would be removed from the mature mRNA [51]. Considering that NAPRT has two putative NOVA1 binding sites in exon 9, namely, the UCAU and UCAC motifs in positions 17 and 47 (according to SFmap web server available at http://sfmap.technion.ac.il/ [52]), NOVA binding could be a possible mechanism for the alternative splicing of NAPRT exon 9 in the brain.
Beyond alternative transcripts, we identified seven variants within the 500bp upstream the NAPRT start codon. We searched for potential human transcription factor binding sites (TFBS) applying the online tool TFSEARCH (Searching Transcription Factor Binding Sites, http://www.rwcp.or.jp/papia/), which is based on the TRANSFAC database [53], to evaluate the effect of such alterations on the prediction of TFBS. We observed loss of cAMP Response Element-Binding protein binding site (CREB) for rs3214817, and gain of Acute Myeloid Leukemia 1 protein binding site (AML1a) for rs896949, which were found in most of the cell lines studied. The TCGGG insertion (rs146163552), which was found only in the NAPRT-negative cell line MKN28, was predicted to result in the loss of one Specificity Protein 1 (Sp1) binding site and decrease of the score for another Sp1 binding site. Sp1 binds with high affinity to GC-rich motifs [54], which are present in the NAPRT promoter.
Additionally, because DNA methylation was recently established as a mechanism to suppress NAPRT gene expression [19], we evaluated the methylation status of the NAPRT promoter region in tumor cell lines. The methylation detected in the MKN28 cell line promoter sequence supports the hypothesis of epigenetic inactivation of NAPRT, since this was the only case for which no mRNA nor protein expression was found. In the other cell lines with weak NAPRT expression no methylation was found, strengthening the idea of tissue/cancer type specificity of expression and regulation mechanisms.
Overall, we demonstrate that NAMPT and NAPRT are ubiquitously expressed in normal human tissues, and we have detected novel NAPRT transcripts, in normal tissues and in tumor cell lines. Furthermore, we identified several modulators of the NAPRT gene expression, from mutations in transcription factor binding sites, to promoter methylation and alternative splicing. These mechanisms should be further investigated to understand the influence of the NAPRT gene in NAD metabolism in different tissues and disease conditions. In particular, evidence on how is NAPRT expression regulated in tumor cells could spark developments to expand the use of NA cytoprotective therapies in treatments using NAMPT inhibitors.
MATERIALS AND METHODS
Bioinformatics analysis
Expressed sequence tags (ESTs) of human NAPRT (NM_145201.4-5) and NAMPT (NM_005746.2) genes were extracted from the UniGene database [25] at NCBI (http://www.ncbi.nlm.nih.gov/unigene/), as counts per million transcripts. A heat map was created with the collected EST data displayed as Log2 transcripts per million using the web interface matrix2png v1.2.1 [55], following a previously described methodology [56]. Gene and protein expression data from both NAPRT and NAMPT was retrieved from Human Protein Atlas database, version 13, Nov2014 (www.proteinatlas.org) [26], and was classified into four categories of expression according to database annotations.
UniGene sequences representing the NAPRT gene were assembled and aligned against the mRNA RefSeq NM_145201.4-5 using the MUSCLE software [57] implemented in Geneious v5.5.6 [58]. A total of 196 sequences was analyzed and matched to one of the NAPRT transcripts described in Ensembl release 80, May 2015 (http://www.ensembl.org/) [59]. Sixty ESTs too small (< 300bp) to match any of the 14 described transcripts or those identified in our work, or containing different alterations were excluded from this approach. Additionally, the tissue distribution of all analyzed ESTs was determined.
Samples and cell lines
Total RNAs from human tissues consisted in the FirstChoice® Human Total RNA Survey Panel, from Ambion (Austin, TX, USA), the Human Total RNA Master Panel II from Clontech (Mountain View, CA, USA), and Pancreas and Adrenal Gland Total RNA from AMSBio (Oxon, UK). Tumor cell lines from ATCC - American Type Culture Collection (MD, USA) - included renal (786-O and Caki-2), cervix (HeLa), colorectal (HCT-15, DLD1, HCT116 and HT-29), gastric (AGS, MKN28 and MKN45) and lung (A549) carcinomas, leukemia (HL-60, NB4 and ML2), and embryonic cell lines HUVEC and HEK293. Thyroid carcinoma cell line C643 was obtained from Prof. Marc Mareel (Laboratory of Experimental Cancerology, Ghent University Hospital, Ghent, Belgium). Uveal melanoma cell lines Mel202 and OMM2.3 were established by Dr. B.R. Ksander (Schepens Eye Research Institute, Boston, USA) [60, 61] and were kindly provided by Dr. M. J. Jager (LUMC, Leiden, The Netherlands).
DNA, RNA and protein extraction
DNA, total RNA and protein were extracted from the cell lines using the Illustra TriplePrep Kit (GE Healthcare, Buckinghamshire, UK), according to the manufacturer's instructions. In all cases, genomic DNA was removed from RNA preparations using RNase-free DNase I, (Fermentas, Thermo Fisher Scientific Inc., Waltham, MA, USA) as follows: one microgram of RNA was incubated at 37°C for 30 min with 1 unit of DNase I, using the 1x reaction buffer containing MgCl2. Then, 1 μL of 50 mM EDTA was added and incubated at 65°C for 10 min to inactivate the enzyme. The prepared RNA was used as a template for reverse transcriptase. Protein concentrations were determined by the Quick Start Bradford Assay (Bio-Rad Laboratories, Hercules, CA, USA).
Polymerase chain reaction (PCR) and reverse transcription PCR (RT-PCR)
Complementary DNA (cDNA) was synthesized from 1 μg of RNA using the RETROscrip® First Strand Synthesis Kit (Ambion) with oligo-dT primers according to the manufacturer's instructions.
Amplification reactions from 1 μL of either DNA or the synthesized cDNA were prepared in a 10 μL final volume reaction, using 5 μL of HotStarTaq® Master Mix Kit (Qiagen, Germantown, MD, USA). Each primer final concentration was 0.2 μM and Q solution (Qiagen) was included in the reaction (10%). The complete list of primers, regions amplified and PCR conditions is shown in Supplementary Table 4. Expression of the glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH, reference transcript NM_002046) was used as a control in RT-PCR reactions.
All amplification products were visualized on 1.5% agarose gels and confirmed by sequencing. Gel image acquisition was processed with Quantity-One 1-D Analysis Software Version 4.6.8 (Bio-Rad, Hercules, CA, USA).
3′ Rapid amplification of cDNA ends (RACE) analysis
3′ RACE cDNA was synthesized using the SMARTer™ RACE cDNA Amplification Kit (Clontech), as previously described [62]. The 3′ RACE PCR was performed with a reaction mixture containing 12.5 μL of 2x QIAGEN multiplex PCR master mix (Qiagen), 8.5 μL of water, 2.5 μL of 10x Universal Primer A Mix (Clontech), 0.5 μL of the gene specific primer (10 μM) and 1 μL of the 3′ RACE cDNA in a final volume of 25 μL. Amplification conditions were carried out as indicated in the SMARTer™ RACE Kit protocol. Following the first PCR reaction, nested PCR was performed using 1 μL of the 3′ RACE PCR product in 12.5 μL of 2x QIAGEN multiplex PCR master mix (Qiagen), 9.5 μL of water, 1 μL of Nested Universal Primer (Clontech), and 1 μL (10 μM) of the nested gene specific primer (Supplementary Table 4) in a final volume of 25 μL.
3′ RACE products were resolved by 1.5% agarose gel electrophoresis, which image was acquired with Quantity-One Software (Bio-Rad), each DNA band was extracted from gel, purified and sequenced with the gene specific primers.
Sequencing analysis
PCR products were purified with ExoSAP-IT (USB Corporation, Santa Clara, CA, USA) by incubation at 37°C for 15 min, followed by enzyme inactivation for 15 min at 85°C, according to the manufacturer's instructions. The resulting purified fragments were sequenced using the ABI Big Dye Terminator Cycle Sequencing Ready Reaction kit v3.1 (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA, USA) and analyzed in an ABI PRISM 3130xl (Applied Biosystems).
Transcript cloning
NAPRT full-length transcripts from the liver, brain, small intestine tissues and from HCT-15 cell line were cloned in a TA-cloning plasmid (pUK-TA, in-house developed). A total of 94 clones were selected, plasmid DNA was extracted and sequenced as described above using the TA-F and TA-R primers [63]. The DNA sequence of the different clones was aligned against the NAPRT reference mRNA sequence as described above.
Western-blot analysis
Total protein extracts from cell lines (15 μg each) were separated in a 10% SDS-PAGE and transferred into a nitrocellulose Amersham Hybond-ECL membrane (GE Healthcare). Rabbit polyclonal antibodies anti-NAPRT (HPA023739) from Sigma (St. Louis, MO, USA) and anti-PBEF (H-300) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) were used at a 1:1000 dilution in 1% non-fat milk PBS Tween-20. Mouse monoclonal anti-α-tubulin antibody (Sigma) was used at a dilution of 1:40000 to assess protein loading. Goat anti-rabbit and goat anti-mouse horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) were used at 1:5000, and developed with the ECL-Plus detection kit (GE Healthcare).
DNA bisulfite treatment and sequencing
The presence of promoter CpG islands in the NAPRT sequence region ranging from 1000 bp upstream to 500 bp downstream the transcription start site was evaluated using the online CpG Island Searcher software (http://cpgislands.usc.edu/) with default settings.
DNA samples from selected cell lines were bisulfite modified with the EpiTect® Fast Bisulfite Conversion kit (Qiagen), according to manufacturer's instructions. Primer sequences specifically designed to amplify a 356bp fragment (from −281 to +75) within the CpG island in modified DNA are indicated in Supplementary Table 2. PCR products were visualized in 9% polyacrylamide gels by silver staining and were subjected to direct sequencing as described above.
Protein modeling and structure visualization
The human NAMPT (PDB id: 3DKJ) [64] and NAPRT (PDB id: 4YUB) [27] structures were used as a template in MODELLER [65] to build structural models containing mutations. The structures were visualized using Pymol v1.1r1 software [66].
SUPPLEMENTARY MATERIAL FIGURES AND TABLES
Footnotes
GRANT SUPPORT
iBiMED is supported by FEDER through the “Programa Operacional Fatores de Competitividade - COMPETE” and through “FCT - Fundação para a Ciência e Tecnologia” (UID/BIM/04501/2013). This work was co-financed by FCT/MEC national funds and FEDER through the grants PTDC/BIA-PRO/099888/2008 (POFC/QREN COMPETE FCOMP-01-0124-FEDER-009029) and Ciência2007-IPATIMUP to RMS, and Ciência2008-ICAAM and POCTI/MGI/41854/2001 to LTC.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Rongvaux A, Andris F, Van Gool F, Leo O. Reconstructing eukaryotic NAD metabolism. Bioessays. 2003;25:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- 2.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004;61:19–34. doi: 10.1007/s00018-003-3161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 4.Galli M, Van Gool F, Rongvaux A, Andris F, Leo O. The nicotinamide phosphoribosyltransferase: a molecular link between metabolism, inflammation, and cancer. Cancer Res. 2010;70:8–11. doi: 10.1158/0008-5472.CAN-09-2465. [DOI] [PubMed] [Google Scholar]
- 5.Bowlby SC, Thomas MJ, D'Agostino RB., Jr and Kridel SJ. Nicotinamide phosphoribosyl transferase (nampt) is required for de novo lipogenesis in tumor cells. PLoS One. 2012;7:e40195. doi: 10.1371/journal.pone.0040195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiarugi A, Dolle C, Felici R, Ziegler M. The NAD metabolome - a key determinant of cancer cell biology. Nat Rev Cancer. 2012;12:741–752. doi: 10.1038/nrc3340. [DOI] [PubMed] [Google Scholar]
- 7.Reddy PS, Umesh S, Thota B, Tandon A, Pandey P, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V, Rao MR, Kondaiah P, Somasundaram K. PBEF1/NAmPRTase/Visfatin: a potential malignant astrocytoma/glioblastoma serum marker with prognostic value. Cancer Biol Ther. 2008;7:663–668. doi: 10.4161/cbt.7.5.5663. [DOI] [PubMed] [Google Scholar]
- 8.Zhang LQ, Heruth DP, Ye SQ. Nicotinamide Phosphoribosyltransferase in Human Diseases. J Bioanal Biomed. 2011;3:13–25. doi: 10.4172/1948-593X.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi TQ, Che XM, Liao XH, Zhang DJ, Long HL, Li HJ, Zhao W. Overexpression of Nampt in gastric cancer and chemopotentiating effects of the Nampt inhibitor FK866 in combination with fluorouracil. Oncol Rep. 2011;26:1251–1257. doi: 10.3892/or.2011.1378. [DOI] [PubMed] [Google Scholar]
- 10.Shackelford RE, Bui MM, Coppola D, Hakam A. Over-expression of nicotinamide phosphoribosyltransferase in ovarian cancers. Int J Clin Exp Pathol. 2010;3:522–527. [PMC free article] [PubMed] [Google Scholar]
- 11.Van Beijnum JR, Moerkerk PT, Gerbers AJ, De Bruine AP, Arends JW, Hoogenboom HR, Hufton SE. Target validation for genomics using peptide-specific phage antibodies: a study of five gene products overexpressed in colorectal cancer. Int J Cancer. 2002;101:118–127. doi: 10.1002/ijc.10584. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Hasan MK, Alvarado E, Yuan H, Wu H, Chen WY. NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene. 2011;30:907–921. doi: 10.1038/onc.2010.468. [DOI] [PubMed] [Google Scholar]
- 13.Khan JA, Tao X, Tong L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat Struct Mol Biol. 2006;13:582–588. doi: 10.1038/nsmb1105. [DOI] [PubMed] [Google Scholar]
- 14.Nahimana A, Attinger A, Aubry D, Greaney P, Ireson C, Thougaard AV, Tjornelund J, Dawson KM, Dupuis M, Duchosal MA. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood. 2009;113:3276–3286. doi: 10.1182/blood-2008-08-173369. [DOI] [PubMed] [Google Scholar]
- 15.Pittelli M, Formentini L, Faraco G, Lapucci A, Rapizzi E, Cialdai F, Romano G, Moneti G, Moroni F, Chiarugi A. Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J Biol Chem. 2010;285:34106–34114. doi: 10.1074/jbc.M110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olesen UH, Petersen JG, Garten A, Kiess W, Yoshino J, Imai S, Christensen MK, Fristrup P, Thougaard AV, Bjorkling F, Jensen PB, Nielsen SJ, Sehested M. Target enzyme mutations are the molecular basis for resistance towards pharmacological inhibition of nicotinamide phosphoribosyltransferase. BMC Cancer. 2010;10:677. doi: 10.1186/1471-2407-10-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson M, Roulston A, Belec L, Billot X, Marcellus R, Bedard D, Bernier C, Branchaud S, Chan H, Dairi K, Gilbert K, Goulet D, Gratton MO, et al. The small molecule GMX1778 is a potent inhibitor of NAD+ biosynthesis: strategy for enhanced therapy in nicotinic acid phosphoribosyltransferase 1-deficient tumors. Mol Cell Biol. 2009;29:5872–5888. doi: 10.1128/MCB.00112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olesen UH, Thougaard AV, Jensen PB, Sehested M. A preclinical study on the rescue of normal tissue by nicotinic acid in high-dose treatment with APO866, a specific nicotinamide phosphoribosyltransferase inhibitor. Mol Cancer Ther. 2010;9:1609–1617. doi: 10.1158/1535-7163.MCT-09-1130. [DOI] [PubMed] [Google Scholar]
- 19.Shames DS, Elkins K, Walter K, Holcomb T, Du P, Mohl D, Xiao Y, Pham T, Haverty PM, Liederer B, Liang X, Yauch RL, O'Brien T, et al. Loss of NAPRT1 Expression by Tumor-Specific Promoter Methylation Provides a Novel Predictive Biomarker for NAMPT Inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6912–6923. doi: 10.1158/1078-0432.CCR-13-1186. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien T, Oeh J, Xiao Y, Liang X, Vanderbilt A, Qin A, Yang L, Lee LB, Ly J, Cosino E, LaCap JA, Ogasawara A, Williams S, et al. Supplementation of nicotinic acid with NAMPT inhibitors results in loss of in vivo efficacy in NAPRT1-deficient tumor models. Neoplasia. 2013;15:1314–1329. doi: 10.1593/neo.131718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282:24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- 22.Olesen UH, Hastrup N, Sehested M. Expression patterns of nicotinamide phosphoribosyltransferase and nicotinic acid phosphoribosyltransferase in human malignant lymphomas. APMIS. 2011;119:296–303. doi: 10.1111/j.1600-0463.2011.02733.x. [DOI] [PubMed] [Google Scholar]
- 23.Cerna D, Li H, Flaherty S, Takebe N, Coleman CN, Yoo SS. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) activity by small molecule GMX1778 regulates reactive oxygen species (ROS)-mediated cytotoxicity in a p53- and nicotinic acid phosphoribosyltransferase1 (NAPRT1)-dependent manner. J Biol Chem. 2012;287:22408–22417. doi: 10.1074/jbc.M112.357301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galassi L, Di Stefano M, Brunetti L, Orsomando G, Amici A, Ruggieri S, Magni G. Characterization of human nicotinate phosphoribosyltransferase: Kinetic studies, structure prediction and functional analysis by site-directed mutagenesis. Biochimie. 2012;94:300–309. doi: 10.1016/j.biochi.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, Dicuccio M, Edgar R, Federhen S, Feolo M, Geer LY, Helmberg W, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2008;36:D13–21. doi: 10.1093/nar/gkm1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 27.Marletta AS, Massarotti A, Orsomando G, Magni G, Rizzi M, Garavaglia S. Crystal structure of human nicotinic acid phosphoribosyltransferase. FEBS Open Bio. 2015;5:419–428. doi: 10.1016/j.fob.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duarte-Pereira S, Silva SS, Azevedo L, Castro L, Amorim A, Silva RM. NAMPT and NAPRT1: novel polymorphisms and distribution of variants between normal tissues and tumor samples. Scientific reports. 2014;4:6311. doi: 10.1038/srep06311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, Cherry JM, Snyder M. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, Kok CY, Jia M, De T, et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2014;43:D805–811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen R, Davydov EV, Sirota M, Butte AJ. Non-synonymous and synonymous coding SNPs show similar likelihood and effect size of human disease association. PLoS One. 2010;5:e13574. doi: 10.1371/journal.pone.0013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauna ZE, Kimchi-Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12:683–691. doi: 10.1038/nrg3051. [DOI] [PubMed] [Google Scholar]
- 33.Rice GI, Reijns MA, Coffin SR, Forte GM, Anderson BH, Szynkiewicz M, Gornall H, Gent D, Leitch A, Botella MP, Fazzi E, Gener B, Lagae L, et al. Synonymous Mutations in RNASEH2A Create Cryptic Splice Sites Impairing RNase H2 Enzyme Function in Aicardi-Goutieres Syndrome. Human mutation. 2013;34:1066–70. doi: 10.1002/humu.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Matheny CJ, Wei MC, Bassik MC, Donnelly AJ, Kampmann M, Iwasaki M, Piloto O, Solow-Cordero DE, Bouley DM, Rau R, Brown P, McManus MT, Weissman JS, et al. Next-generation NAMPT inhibitors identified by sequential high-throughput phenotypic chemical and functional genomic screens. Chemistry & biology. 2013;20:1352–1363. doi: 10.1016/j.chembiol.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan B, Young DA, Lu ZH, Wang T, Meier TI, Shepard RL, Roth K, Zhai Y, Huss K, Kuo MS, Gillig J, Parthasarathy S, Burkholder TP, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells: metabolic basis and potential clinical implications. J Biol Chem. 2013;288:3500–3511. doi: 10.1074/jbc.M112.394510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roulston A, Shore GC. New Strategies to Maximize Therapeutic Opportunities for NAMPT Inhibitors in Oncology. Molecular & Cellular Oncology. 2015:00–00. doi: 10.1080/23723556.2015.1052180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shackelford R, Hirsh S, Henry K, Abdel-Mageed A, Kandil E, Coppola D. Nicotinamide phosphoribosyltransferase and SIRT3 expression are increased in well-differentiated thyroid carcinomas. Anticancer research. 2013;33:3047–3052. [PubMed] [Google Scholar]
- 41.Xiao Y, Elkins K, Durieux JK, Lee L, Oeh J, Yang LX, Liang X, DelNagro C, Tremayne J, Kwong M, Liederer BM, Jackson PK, Belmont LD, et al. Dependence of tumor cell lines and patient-derived tumors on the NAD salvage pathway renders them sensitive to NAMPT inhibition with GNE-618. Neoplasia. 2013;15:1151–1160. doi: 10.1593/neo.131304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaudoing E, Freier S, Wyatt JR, Claverie JM, Gautheret D. Patterns of variant polyadenylation signal usage in human genes. Genome Res. 2000;10:1001–1010. doi: 10.1101/gr.10.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Q, Modrek B, Lee C. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res. 2002;30:3754–3766. doi: 10.1093/nar/gkf492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo G, Holste D, Kreiman G, Burge CB. Variation in alternative splicing across human tissues. Genome Biol. 2004:5. doi: 10.1186/gb-2004-5-10-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anders G, Mackowiak SD, Jens M, Maaskola J, Kuntzagk A, Rajewsky N, Landthaler M, Dieterich C. doRiNA: a database of RNA interactions in post-transcriptional regulation. Nucleic Acids Res. 2012;40:D180–186. doi: 10.1093/nar/gkr1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol Cell. 2007;26:611–623. doi: 10.1016/j.molcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Ameyar-Zazoua M, Rachez C, Souidi M, Robin P, Fritsch L, Young R, Morozova N, Fenouil R, Descostes N, Andrau JC, Mathieu J, Hamiche A, Ait-Si-Ali S, et al. Argonaute proteins couple chromatin silencing to alternative splicing. Nat Struct Mol Biol. 2012;19:998–1004. doi: 10.1038/nsmb.2373. [DOI] [PubMed] [Google Scholar]
- 48.Grosso AR, Gomes AQ, Barbosa-Morais NL, Caldeira S, Thorne NP, Grech G, von Lindern M, Carmo-Fonseca M. Tissue-specific splicing factor gene expression signatures. Nucleic Acids Res. 2008;36:4823–4832. doi: 10.1093/nar/gkn463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckanovich RJ, Yang YY, Darnell RB. The onconeural antigen Nova-1 is a neuron-specific RNA-binding protein, the activity of which is inhibited by paraneoplastic antibodies. J Neurosci. 1996;16:1114–1122. doi: 10.1523/JNEUROSCI.16-03-01114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valverde R, Edwards L, Regan L. Structure and function of KH domains. FEBS J. 2008;275:2712–2726. doi: 10.1111/j.1742-4658.2008.06411.x. [DOI] [PubMed] [Google Scholar]
- 51.Teplova M, Malinina L, Darnell JC, Song J, Lu M, Abagyan R, Musunuru K, Teplov A, Burley SK, Darnell RB, Patel DJ. Protein-RNA and protein-protein recognition by dual KH1/2 domains of the neuronal splicing factor Nova-1. Structure. 2011;19:930–944. doi: 10.1016/j.str.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paz I, Akerman M, Dror I, Kosti I, Mandel-Gutfreund Y. SFmap: a web server for motif analysis and prediction of splicing factor binding sites. Nucleic Acids Res. 2010;38:W281–285. doi: 10.1093/nar/gkq444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, Ignatieva EV, Ananko EA, Podkolodnaya OA, Kolpakov FA, Podkolodny NL, Kolchanov NA. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26:362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koutsodontis G, Moustakas A, Kardassis D. The role of Sp1 family members, the proximal GC-rich motifs, and the upstream enhancer region in the regulation of the human cell cycle inhibitor p21WAF-1/Cip1 gene promoter. Biochemistry. 2002;41:12771–12784. doi: 10.1021/bi026141q. [DOI] [PubMed] [Google Scholar]
- 55.Pavlidis P, Noble WS. Matrix2png: a utility for visualizing matrix data. Bioinformatics. 2003;19:295–296. doi: 10.1093/bioinformatics/19.2.295. [DOI] [PubMed] [Google Scholar]
- 56.Castro LF, Lopes-Marques M, Wilson JM, Rocha E, Reis-Henriques MA, Santos MM, Cunha I. A novel Acetyl-CoA synthetase short-chain subfamily member 1 (Acss1) gene indicates a dynamic history of paralogue retention and loss in vertebrates. Gene. 2012;497:249–255. doi: 10.1016/j.gene.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drummond A, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S, Moir R, Stones-Havas S, Sturrock S, et al. Geneious. 2011 http://www.geneious.com
- 59.Cunningham F, Amode MR, Barrell D, Beal K, Billis K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fitzgerald S, Gil L, Giron CG, Gordon L, et al. Ensembl 2015. Nucleic Acids Res. 2015;43:D662–669. doi: 10.1093/nar/gku1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen PW, Murray TG, Uno T, Salgaller ML, Reddy R, Ksander BR. Expression of MAGE genes in ocular melanoma during progression from primary to metastatic disease. Clin Exp Metastasis. 1997;15:509–518. doi: 10.1023/a:1018479011340. [DOI] [PubMed] [Google Scholar]
- 61.Ksander BR, Rubsamen PE, Olsen KR, Cousins SW, Streilein JW. Studies of tumor-infiltrating lymphocytes from a human choroidal melanoma. Invest Ophthalmol Vis Sci. 1991;32:3198–3208. [PubMed] [Google Scholar]
- 62.Pereira-Castro I, Quental R, da Costa LT, Amorim A, Azevedo L. Successful COG8 and PDF overlap is mediated by alterations in splicing and polyadenylation signals. Hum Genet. 2012;131:265–274. doi: 10.1007/s00439-011-1075-9. [DOI] [PubMed] [Google Scholar]
- 63.Pereira-Castro I, Costa LT, Amorim A, Azevedo L. Transcriptional regulation of the human mitochondrial peptide deformylase (PDF) Biochem Biophys Res Commun. 2012;421:825–831. doi: 10.1016/j.bbrc.2012.04.097. [DOI] [PubMed] [Google Scholar]
- 64.Burgos ES, Ho MC, Almo SC, Schramm VL. A phosphoenzyme mimic, overlapping catalytic sites and reaction coordinate motion for human NAMPT. Proc Natl Acad Sci U S A. 2009;106:13748–13753. doi: 10.1073/pnas.0903898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sali A, Potterton L, Yuan F, van Vlijmen H, Karplus M. Evaluation of comparative protein modeling by MODELLER. Proteins. 1995;23:318–326. doi: 10.1002/prot.340230306. [DOI] [PubMed] [Google Scholar]
- 66.Schrodinger, LLC The PyMOL Molecular Graphics System, Version 1.3r1. 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.