Abstract
Background
Influenza virus predictably causes an annual epidemic resulting in a considerable burden of illness in Australia. Children are disproportionately affected and can experience severe illness and complications, which occasionally result in death.
Methods
We conducted a retrospective descriptive study using data collated in the Australian and New Zealand Paediatric Intensive Care (ANZPIC) Registry of influenza-related intensive care unit (ICU) admissions over a 17-year period (1997–2013, inclusive) in children <16 years old. National laboratory-confirmed influenza notifications were used for comparison.
Results
Between 1997 and 2013, a total of 704 influenza-related ICU admissions were recorded, at a rate of 6.2 per 1,000 all-cause ICU admissions. Age at admission ranged from 0 days and 15.9 years (median = 2.1 years), with 135 (19.2%) aged <6 months. Pneumonia/pneumonitis and bronchiolitis were the most common primary diagnoses among influenza-related admissions (21.9% and 13.6%, respectively). More than half of total cases (59.2%) were previously healthy (no co-morbidities recorded), and in the remainder, chronic lung disease (16.7%) and asthma (12.5%) were the most common co-morbidities recorded. Pathogen co-detection occurred in 24.7% of cases, most commonly with respiratory syncytial virus or a staphylococcal species. Median length of all ICU admissions was 3.2 days (range 2.0 hours– 107.4 days) and 361 (51.3%) admissions required invasive respiratory support for a median duration of 4.3 days (range 0.2 hours– 107.5 days). There were 27 deaths recorded, 14 (51.9%) in children without a recorded co-morbidity.
Conclusion
Influenza causes a substantial number of ICU admissions in Australian children each year with the majority occurring in previously healthy children.
Introduction
Influenza virus causes a significant burden of disease annually in Australia [1]. Influenza activity varies from year to year and is dependent on the circulating viruses and prevailing population immunity. More severe seasons, such as during 2009, are often associated with novel viruses to which there is little or no prior immunity at the population level [2, 3].
Clinically, influenza infection spans a wide severity spectrum, from asymptomatic infection or mild illness, through to severe disease and death [2]. Severe illness occurs most commonly among children, the elderly, and individuals with pre-existing chronic medical conditions [2]. In infants and children, influenza can cause complications such as croup, bronchiolitis, exacerbation of asthma, pneumonia, and seizures, all of which are associated with hospitalisation and intensive care unit (ICU) admission [2, 4, 5]. In Australia, the age-specific burden of disease is U-shaped, with the highest rates of notifications and hospitalisations occurring in children aged under 5 years and in adults aged 65 years or greater [1].
Annual influenza immunisation is currently recommended for all individuals aged ≥6 months for whom it is desired to reduce the likelihood of becoming ill with influenza [6]. However Government funding for free vaccination under the Australian National Immunisation Program is currently limited to adults ≥65 years old, Aboriginal and Torres Strait Islander peoples aged ≥6 months to <5 years and ≥15 years, pregnant women, and individuals ≥6 months old at risk of severe illness due to medical conditions [6, 7].
Influenza activity is monitored primarily through reporting of laboratory confirmed cases under public health legislation [8], which does not routinely capture details around the severity of illness. Investigation of severe paediatric influenza-related illness, focussing only on hospitalisations and ICU admissions, has primarily been reported at the Australian state level or nationally only during peak years (such as the 2009 influenza pandemic) [9–13]. The aim of this study is to describe the epidemiology of severe paediatric influenza-related admissions to all major Australian paediatric ICUs over a 17 year period.
Methods
We conducted a retrospective descriptive study using data collated in the Australian and New Zealand Paediatric Intensive Care (ANZPIC) Registry. The ANZPIC Registry, established in 1997, collects paediatric intensive care patient episode information from contributing specialist paediatric ICUs (PICUs) as well as general ICUs across Australia and New Zealand [14]. In 1997, eight PICUs from five Australian states (NSW, QLD, SA, VIC, WA) contributed data to the ANZPIC Registry [14]. By 2013, one additional PICU and 11 ICUs (representing all states/territories) were participating. The original eight PICUs, of which seven have contributed data continuously since commencement, accounted for an annual average of 93% of admissions recorded in the ANZPIC Registry between 1997 and 2013 (1997 value: 100%, 2013 value: 84%).
Participating ICUs collect data in real-time either on a Microsoft Access database (Microsoft Corp, Redmond, WA, USA) supplied by the ANZPIC Registry or onto their local clinical information system (amended to include required ANZPIC Registry fields). Data are submitted to the centrally administered ANZPIC Registry electronically every six months [14].
A single record is created for every admission to a participating ICU and includes demographic data, physiological variables measured at the time of first face-to-face contact between the patient-ICU doctor, the ICU/hospital outcomes and length of stay, as well as the type and duration of respiratory support [14]. Admissions are defined using ANZPIC Registry-specific standardised diagnosis codes [15] in up to 9 diagnostic fields including ‘principal diagnosis’ (the diagnosis most directly responsible for the ICU admission), ‘underlying diagnosis’ (the principal underlying diagnosis contributing to the need for ICU admission), and up to 7 ‘associated diagnoses’. Associated diagnoses are conditions additional to the principal and underlying reasons that contributed to the ICU admission, and can include other syndromes, diseases, abnormalities or diagnoses identified on or during ICU admission. ANZPIC Registry coding requires infection diagnosis codes (700–799) to be used only in the ‘underlying’ or ‘associated’ diagnosis fields and not in the ‘principal diagnosis’ field. The treating clinician is responsible for selecting the appropriate diagnostic codes for each admission, and coding may be based on clinical symptoms, diagnostic test results, or a combination of these. Results of any diagnostic tests performed, including respiratory virus/bacteria detection or influenza type/subtype analysis, are not captured within the ANZPIC Registry. Immunisation history is also not collected.
For this study, we extracted ANZPIC Registry data for all Australian paediatric (aged 0 to <16 years) ICU admissions (New Zealand admissions not included) between 01 January 1997 and 31 December 2013 with a diagnosis code of “715 –Influenza Virus” occurring in any of the diagnostic fields. The de-identified line-listed data extract included patient demographic variables (age, sex, ethnicity, state), and hospital and ICU admission details—admission/discharge dates, diagnoses (principal, underlying, and associated), discharge/outcome, and respiratory support variables (nature and duration of respiratory support). Respiratory support was defined as any intervention to support respiratory function and includes both non-invasive and invasive methods. Some patients may have required combinations of both invasive and non-invasive support during their ICU admission. Non-invasive respiratory support includes continuous positive airway pressure, biphasic positive airway pressure, negative pressure ventilation, and high flow nasal cannula, while invasive respiratory support is mechanical ventilation delivered by endotracheal intubation or tracheostomy.
Data were aggregated during analysis, including grouping by year and age. Admissions were classified has having co-morbidities if they had a diagnosis code (in any diagnostic field) for any co-morbid condition defined in the Australian Immunisation Handbook as predisposing individuals to severe influenza, and therefore allowing publicly-funded vaccine use, including cardiac disease or congenital cardiovascular conditions, asthma, chronic respiratory disease, cystic fibrosis, immunodeficiency or immunosuppression, Down syndrome, chronic neurological conditions, diabetes, chronic renal failure, haemoglobinopathies, and chronic inherited metabolic disorders [6]. Admissions were classified as having co-detection if they had any other respiratory infection diagnosis code in any diagnostic field (in addition to an influenza code).
In Australia, diagnostic testing for influenza is funded under the Medical Benefits Scheme when required for medical management of a patient. As laboratory confirmed influenza has been a nationally notifiable condition since 2001, where testing is performed, all cases that meet the case definition are required to be reported to the appropriate State or Territory Health Department in accordance with public health legislation [8]. The National Notifiable Diseases Surveillance System (NNDSS) compiles information collected by State and Territory Health Departments into one passive national system. National influenza notification data for children <16 years old were obtained from the NNDSS for this study. Using mid-year Australian Bureau of Statistics population estimate data, we calculated the influenza notification rate in children <16 years per 100,000 population, and compared this with national influenza-related paediatric ICU admissions per 1,000 all-cause paediatric ICU admissions, as well as per 100,000 population.
Stata version 12 (StataCorp, College Station, TX, USA) was used for data analysis. Descriptive statistics were calculated, including means, medians, and standard deviations (SD). The association between patient characteristics and outcomes was investigated using Student’s t-test. The correlation between influenza-related ICU admissions and influenza notifications was calculated in Microsoft Excel (Microsoft, Redmond, WA, USA).
The Children’s Health Services Queensland Human Research Ethics Committee granted ethics approval for this study, including a waiver of individual consent due to the data being de-identified.
Results
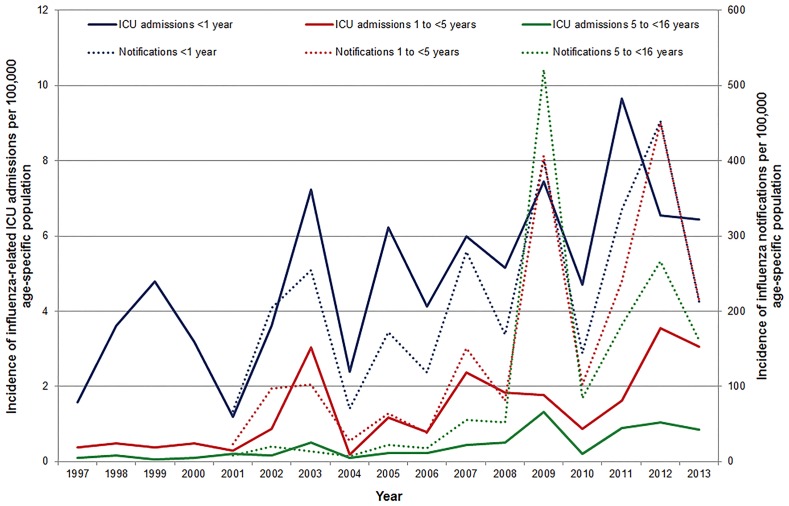
Between 01 January 1997 and 31 December 2013, 704 influenza-related ICU admissions were identified in the ANZPIC Registry of a total 113,197 paediatric ICU admissions from the same ICUs during the period (Table 1). This gives an average proportion for the entire study period of 6.2 influenza-related ICU admissions per 1,000 all-cause ICU admissions (95% CI: 5.8 to 6.7), however this proportion varied between years, peaking in 2003, 2009, and 2012 at 10.3, 11.4, and 11.3 per 1,000 ICU admissions, respectively (Fig 1). Annual influenza notifications also peaked in 2009 and 2012 at 484.2 and 326.2 per 100,000 population, respectively. Both influenza ICU admissions and notifications in children <16 years generally increased over the study period and the two datasets were correlated (correlation coefficient = 0.68).
Table 1. Demographics and admission characteristics of influenza-related ICU admissions, 1997–2013.
| Total * | |
|---|---|
| Number of participating ICUs † | Between 7 and 21 |
| Influenza-related admissions | 704 |
| Influenza-related admissions per 1000 all-cause ICU admissions (total ICU admissions) | 6.2 (113,197) |
| Sex | |
| MALE—Number (percent) | 400 (56.8%) |
| Ethnicity | |
| Aboriginal and Torres Strait Islander peoples—Number of recorded (percent) | 19 of 282 (6.7%) |
| Age | |
| Aged <6 months | 135 (19.2%) |
| Aged 6 months to <5 years | 353 (50.1%) |
| Aged 5 years to <16 years | 216 (30.7%) |
| Median (range) | 2.1 years |
| (0 days—15.9 years) | |
| Location (state/territory) ‡ | |
| Number (percent of total influenza admissions); total all-cause ICU admissions | |
| Australian Capital Territory | 9 (1.3%); 369 |
| New South Wales | 273 (39.1%); 32,950 |
| Northern Territory | 11 (1.6%); 548 |
| Queensland | 171 (24.5%); 28,902 |
| South Australia | 38 (5.4%); 9,514 |
| Tasmania | 14 (2.0%); 1,631 |
| Victoria | 137 (19.6%); 26,337 |
| Western Australia | 45 (6.5%); 12,946 |
| Patients with co-morbidities | |
| Number (percent) | 287 (40.8%) |
| Patients with infectious agent co-detection | |
| Number (percent) | 174 (24.7%) |
| Length of hospitalisation§ | 9.6 days |
| Median (range) | (6.2 hours—248.6 days) |
| Length of ICU stay | 3.2 days |
| Median (range) | (2.0 hours—107.5 days) |
| Any respiratory support during 1st hour of ICU admission** †† | |
| Number (percent) | 355 (52.2%) |
| Any respiratory support during ICU admission** †† | |
| Number (percent) | 418 (66.8%) |
| Invasive respiratory support during ICU admission‡‡ | |
| Number (percent) | 361 (51.3%) |
| Length of invasive respiratory support | 4.3 days |
| Median (range) | (0.2 hours—107.5 days) |
| Deaths | |
| Number with co-morbidities (percent of cases with co-morbidities) | 13 (4.5%) |
| Number without co-morbidities (percent of cases without co-morbidities) | 14 (3.4%) |
* For analysis by year see Supporting Information (S1 Table. Demographics and admission characteristics of influenza-related ICU admissions, 1997–2013)
† The number of participating ICUs varied over time.
‡ Three ICU admissions had no residential state recorded, and three were classified as residing overseas
§ Hospitalisation duration data missing for 98 cases
** Respiratory support is defined as any intervention to support respiratory function and includes both non-invasive and invasive methods. Some patients may have required combinations of both invasive and non-invasive support during their ICU admission. Non-invasive respiratory support includes: continuous positive airway pressure, biphasic positive airway pressure, negative pressure ventilation, and high flow nasal cannula.
†† Respiratory support in 1st hour data missing for 24 cases; Respiratory support during admission data missing for 78 cases.
‡‡ Invasive respiratory support is mechanical ventilation delivered by endotracheal intubation or tracheostomy.
Fig 1. Incidence of influenza-related ICU admissions (primary axis) and influenza notifications* (secondary axis), per 100,000 age-specific population, Australia, 1997–2013*, by age group and year.
* Influenza became nationally notifiable in 2001 –notification data were not nationally collected prior to 1 January 2001.
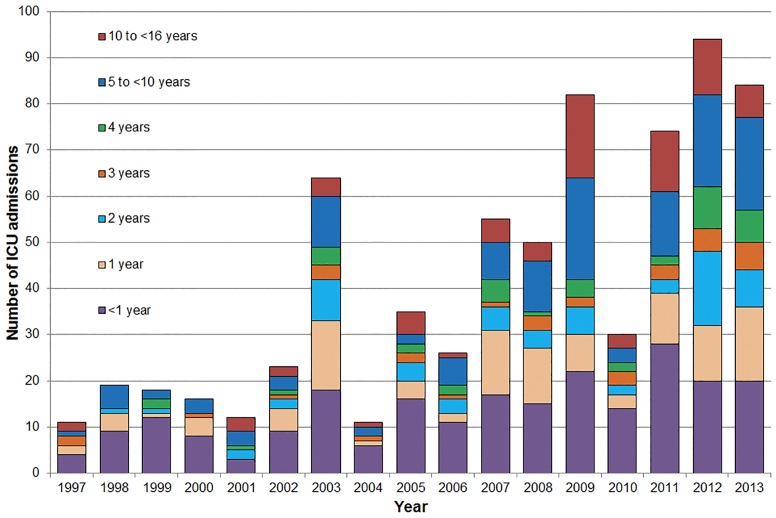
Over the study period, a higher proportion of ICU admissions were male (56.8%), and 6.7% of cases where ethnicity data were available (n = 282) were identified as Aboriginal or Torres Strait Islander people (Table 1). The median age of children admitted with influenza was 2.1 years (range: 0 days to 15.9 years), however there was some variation over the study period (S1 Table). Compared with the 1997–2008 period, the mean age of ICU admissions was significantly higher after 01 January 2009, (mean (SD) age: 3.3 (3.8) years vs 4.5 (4.4) years, mean difference (MD) = 1.2 years, 95%CI: 0.6 to 1.8, p = 0.0001). Across all years, admission counts were highest for children in their first year of life (Fig 2). Overall, children <1 year old accounted for 11.1% of influenza notifications (aged <16 years) and 33.0% of paediatric influenza-related ICU admissions.
Fig 2. Paediatric (0 to <16 years) influenza-related ICU admissions, Australia, 1997–2013, by year and age at admission.
Of the 704 admissions, two (0.3%) had influenza coded in the principal diagnosis field, 87 (12.4%) in the underlying diagnosis field, and 617 (87.6%) in one of the associated diagnosis fields (two cases had the code included in both the underlying and an associated diagnosis field). The most common principal diagnoses for influenza ICU admissions were: pneumonia/pneumonitis (n = 154, 21.9%), bronchiolitis (n = 96, 13.6%), seizures (n = 70, 9.9%), croup (n = 43, 6.1%), and respiratory failure (n = 40, 5.7%). Of 232 cases aged <1 year, 50 (21.6%) had prematurity as an underlying or associated diagnosis code, of which 26 (52.0%) were aged <6 months and 24 (48.0%) were aged between 6 months and <1 year.
Immunisation Handbook defined co-morbidities [6] were coded in 40.8% (n = 287) of cases, most commonly chronic lung disease (includes bronchopulmonary dysplasia) (n = 48, 16.7%) and asthma (n = 36, 12.5%). Relatively few of the 135 infants aged ≤6 months had co-morbidities (30.4%), with the proportion of admissions with co-morbidities increasing with age (37.1% of 6 month to <5 year olds; 53.2% of 5 to <16 year olds). Compared to previously healthy children, cases with co-morbidities were significantly older, stayed in ICU longer, and, when needed, required respiratory support for a longer period (Table 2).
Table 2. Comparisons between groups with/without co-morbidities or with/without co-detection, influenza-related ICU admissions, 1997–2013.
| Comparison of interest | WITH | WITHOUT | Mean difference | Odds Ratio | 95% CI | P value | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||||
| Co-morbidities | Age | 4.7 (4.3) years | 3.3 (4.0) years | 1.4 years | - | 0.8 to 2.0 years | <0.0001 |
| Length of ICU stay | 7.1 (9.7) days | 5.0 (7.5) days | 2.1 days | - | 0.8 to 3.3 days | 0.002 | |
| Need for respiratory support | - | - | - | 1.1 | 0.8 to 1.6 | 0.55 | |
| Duration of any respiratory support (mean length)* | 5.2 (9.2) days | 3.4 (7.4) days | 1.9 days | - | 0.5 to 3.2 days | 0.006 | |
| Duration of invasive respiratory support (mean length)† | 3.4 (6.3) days | 3.1 (7.1) days | 0.4 days | - | -0.6 to 1.4 days | 0.45 | |
| Death | - | - | - | 1.4 | 0.6 to 3.2 | 0.43 | |
| Co-detection | Age | 3.7 (4.3) years | 4.0 (4.1) years | 0.3 years | - | 0.4 to 1.0 years | 0.46 |
| Length of ICU stay | 8.5 (9.0) days | 5.0 (8.1) days | 3.5 days | - | 2.1 to 5.0 days | <0.0001 | |
| Need for respiratory support | - | - | - | 1.8 | 1.2 to 2.8 | 0.005 | |
| Duration of any respiratory support (mean length)* | 8.1 (8.0) days | 5.4 (9.8) days | 2.6 days | - | 0.7 to 4.6 days | 0.009 | |
| Duration of invasive respiratory support (mean length)† | 8.2 (7.5) days | 5.5 (8.7) days | 2.8 days | - | 0.9 to 4.7 days | 0.005 | |
| Death | - | - | - | 0.5 | 0.2 to 1.5 | 0.26 |
* Respiratory support is defined as any intervention to support respiratory function and includes both non-invasive and invasive methods. Some patients may have required combinations of both invasive and non-invasive support during their ICU admission. Non-invasive respiratory support includes: continuous positive airway pressure, biphasic positive airway pressure, negative pressure ventilation, and high flow nasal cannula.
† Invasive respiratory support is mechanical ventilation delivered by endotracheal intubation or tracheostomy.
Co-detection (having a second infection diagnosis code), was common, with 24.7% (n = 174) of influenza-related ICU admissions. Respiratory syncytial virus (RSV) was most frequent among co-infected influenza cases (n = 46, 26.5%). Bacterial co-detection was recorded among 12.8% of cases (n = 22), with 26.7% (n = 6) of these coded as a staphylococcal species. There was no difference in the age of co-infected and influenza-only cases (p = 0.46), however co-infected cases had a significantly longer length of ICU stay compared to non-co-infected cases (MD = 3.5 days, 95%CI: 2.1 to 5.0 days, p<0.0001, Table 2). Co-detection was also associated with significant differences in the need for and mean length of respiratory support, both any and invasive, compared to cases only diagnosed with influenza (p = 0.009 and p = 0.005, respectively).
Overall, influenza cases stayed in ICU for a median of 3.2 days (range: 2.0 hours to 107.5 days) and in hospital for a median of 9.6 days (range: 6.2 hours to 248.6 days) (Table 1). There was no significant variation in the length of ICU or hospital stay over time (ICU p = 0.26, hospital p = 0.09) or by age (ICU p = 0.39, hospital p = 0.26) (see S1 Table). Most cases (78.5%) spent ≤7 days in ICU and another 13.2% were discharged from ICU in the subsequent 7 days.
Overall, 66.8% of cases required some form of respiratory support during their ICU admission, for a median length of 3.5 days (range: 0.2 hours to 107.5 days), with 52.2% receiving support during the first hour of ICU admission (Table 1). Approximately half of the admissions (51.3%) required invasive respiratory support, for a median length of 4.3 days (range: 0.2 hours to 107.5 days). There was no significant variation in the length of any respiratory support or length of invasive respiratory support by year (p = 0.76 and p = 0.60, respectively) or by age (p = 0.94 and p = 0.67, respectively).
A total of 27 deaths were recorded over the study period, which equated to 3.8% of all influenza-related ICU admissions (Table 1). The median age of children who died was 4.7 years (range: 48 days to 14.5 years). The highest number of deaths occurred in 2003 (n = 7, 10.9%). Among cases without co-morbidities (n = 417) there were 14 deaths (3.4%), compared with 13 deaths among cases with co-morbidities (n = 287, 4.5%). Chronic encephalopathy was the most common co-morbidity coded for deceased cases (n = 7), followed by chronic lung disease (n = 3). Among deceased cases, four children (14.8%) had co-detection of a second respiratory infection, of which two were RSV and two were Pseudomonas.
Discussion
Over half the 704 influenza-related ICU admissions recorded in the ANZPIC Registry between 1997 and 2013 were previously healthy children with no co-morbidity. All children with influenza-related ICU admissions required long hospitalisations and ICU stays, and many required invasive respiratory support for considerable lengths of time, regardless of whether they had co-morbid conditions. Infants aged <1 year were more frequently represented among influenza-related ICU admissions than among influenza notifications, suggesting that younger infants are more vulnerable to severe disease and is consistent with findings from previous studies [9, 11]. We also identified that infants who had been born prematurely were over-represented among influenza-related ICU admissions (1 in 5) compared to the Australian population incidence of prematurity (approximately 1 in 10 live births) [16].
Severe paediatric influenza-related illness occurs in Australia, even in years with low influenza activity. In this study we have only focused on ICU admissions, however it is estimated that between 10–30% of influenza-related hospitalisations result in an ICU admission, depending on the season [12, 17–22]. Consistent with other reports of Australian paediatric influenza-related ICU admissions [9–13], we found a median length of stay of approximately 3 days and that half of the admissions required invasive respiratory support. The number of ICU admissions, as well as the high level of support required, highlights the substantial burden of influenza-related illness in children on the Australian healthcare system.
Findings from our study are a likely underestimate of disease burden. During the early years of our study period, only influenza-related cases admitted to participating ICUs were included, therefore any admissions at non-participating ICUs would have been missed. By 2013, the ANZPIC Registry captured 92.4% of all paediatric ICU admissions in Australia and New Zealand [14], and ANZPIC Registry data had become increasingly representative of total Australian ICU admissions as the number of participating ICUs increased over the study period. Additionally, as our study used data from an existing database and did not include a review of medical charts or diagnostic testing results, we were reliant on the accuracy of existing coding. Any admissions due to, but not coded as ‘influenza’, and those not coded with existing co-detections and co-morbidities, will have been misclassified. Identifying influenza and recording of the related diagnostic code is dependent on clinician awareness and testing, as well as the perceived importance of the illness/diagnosis, which could all vary between seasons [23, 24]. Improvements in diagnostic testing, with the widespread use of PCR from 2007 onwards [25], and high media coverage in some seasons (e.g. 2007 due to several influenza-related child deaths [26], influenza pandemic in 2009) may have influenced influenza testing and increased awareness among clinicians, primarily during the latter part of our study period. We cannot know whether the demographic profile and severity of missing cases was different from those included in our analysis, and the number of missing admissions, if any, cannot be quantified. Furthermore, due to the unavailability of typing results within the ANZPIC Registry, we were unable to investigate seasonal trends by influenza type.
As previously healthy children and those with co-morbidities were equally represented among influenza-related ICU admissions and deaths, more emphasis should be placed on annual influenza vaccination for all children. This has already been recognised in the United States and United Kingdom, with influenza vaccination universally recommended for all children [27, 28]. It has been estimated that fully vaccinated children are between 74–86% less likely to have an influenza-related hospital or ICU admission compared to unvaccinated children [10, 29, 30] and immunisation has been shown to reduce the burden of influenza in the community [13, 31, 32]. However influenza vaccination coverage among children in Australia remains low, even among individuals eligible for government-funded vaccination [6, 11, 12, 29]. Vaccination history is not collected in the ANZPIC Registry, however given the low influenza vaccine coverage among Australian children [29], it is unlikely that many (if any) of our ICU cohort were vaccinated. Australian primary care providers and paediatricians could improve coverage by discussing influenza vaccination with the parents of all their paediatric patients, including those that are otherwise healthy. Such discussions would be assisted by firming up the language used in the Australian Immunisation Handbook to be more aligned to that used in the United States: “Routine annual influenza vaccination is recommended for all persons aged ≥6 months who do not have contraindications”[27] and by expanding funding to provide free vaccine to all children aged <5 years, if not all children aged <17 years like in the United Kingdom [28].
Additionally, as approximately 20% of all influenza-related ICU admissions (including 50% of those coded as preterm infants) occurred in infants too young to be vaccinated (<6 months old), maternal vaccination should also be emphasised. Influenza vaccination during pregnancy has been shown to be safe and in addition to protecting the mother from severe infection, it also appears to reduce the likelihood of premature birth and subsequent influenza infection in the infant [33, 34]. Maternal vaccination has been recommended in Australia since March 2000, with funded vaccine available through the National Immunisation Program from January 2010 [35]. Although national coverage estimates are not available [36], state-based cross-sectional surveys have estimated that approximately 25–30% of women receive the vaccine during pregnancy [34, 37, 38]. Education, provided by a health care professional, about the possible severity of influenza infection, as well as the efficacy and safety of influenza vaccination, could improve uptake among children and pregnant women [33, 34, 37, 38]. As coverage in Australia increases, the impact on paediatric influenza-related hospitalisations and ICU admissions should be monitored.
Conclusion
In Australia, influenza causes a substantial number of ICU admissions just over half of which occur in previously healthy children without documented co-morbidities. Lack of co-morbidities was also identified in half of all deaths. In the clinical setting, consideration should be given to recommending influenza vaccination to all children and pregnant women to prevent the severe outcomes associated with influenza infection, including hospitalisations and ICU admissions, among children.
Supporting Information
(PDF)
Acknowledgments
We thank the intensivists, data managers and other staff in the participating ICUs for contributions to the ANZPIC Registry, and Jan Alexander for her assistance with the data request. The ANZPIC Registry is supported by the Australian and New Zealand Intensive Care Society, the Ministry of Health (New Zealand), and State and Territory Health Departments through the Australian Health Ministers’ Advisory Council.
Ms Kaczmarek is the recipient of a Sidney Myer Health Scholarship and receives student support from the Queensland Children’s Medical Research Institute and The University of Queensland School of Public Health. A/Prof Lambert is supported by an Early Career Fellowship from the Australian Government National Health and Medical Research Council and a people support grant from the Queensland Children’s Hospital Foundation.
Data Availability
All relevant aggregate data are within the paper and its Supporting Information files. Although authors are prohibited from supplying any data to third parties under the ANZPIC Registry and NNDSS data release policies, underlying raw data can be requested directly from the ANZPIC Registry (j.alexander@uq.edu.au) and NNDSS (epi@health.gov.au) data custodians. Information about the ANZPIC Registry is available here: http://www.anzics.com.au/pages/CORE/ANZPICR-registry.aspx. Information about the NNDSS is available here: http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndssnndssintro.htm.
Funding Statement
The authors received no specific funding for this work. Ms Kaczmarek is the recipient of a Sidney Myer Health Scholarship and receives student support from the Queensland Children’s Medical Research Institute and The University of Queensland School of Public Health. A/Prof Lambert is supported by an Early Career Fellowship from the Australian Government National Health and Medical Research Council and a people support grant from the Queensland Children’s Hospital Foundation.
References
- 1.Kaczmarek M, Owen R, Barr IG. Annual report of the National Influenza Surveillance Scheme, 2008. Commun Dis Intell Q Rep. 2010;34(1):8–22. [DOI] [PubMed] [Google Scholar]
- 2.Fraaij PL, Heikkinen T. Seasonal influenza: the burden of disease in children. Vaccine. 2011;29(43):7524–8. 10.1016/j.vaccine.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Chiu SS, Lo JY, Chan KH, Chan EL, So LY, Wu P, et al. Population-based hospitalization burden of influenza a virus subtypes and antigenic drift variants in children in Hong Kong (2004–2011). PLoS One. 2014;9(4):e92914 10.1371/journal.pone.0092914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruf BR, Knuf M. The burden of seasonal and pandemic influenza in infants and children. Eur J Pediatr. 2014;173(3):265–76. 10.1007/s00431-013-2023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iskander M, Booy R, Lambert S. The burden of influenza in children. Curr Opin Infect Dis. 2007;20(3):259–63. [DOI] [PubMed] [Google Scholar]
- 6.Australian Government Department of Health. The Australian Immunisation Handbook. 10th Edition ed. Canberra, Australia: Commonwealth of Australia; 2013. [Google Scholar]
- 7.Australian Government Department of Health. Public Summary Document—July 2014 PBAC Meeting: Trivalent Influenza Vaccine. 2014; Available from: Available from: http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2014-07/trivalent-vaccine-psd-07-2014.pdf [Accessed Jan 2015].
- 8.Australian Government Department of Health. Australian national notifiable diseases case definitions: Influenza case definition. 2008; Available from: Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndss-casedefs-cd_flu.htm [Accessed Sep 2014].
- 9.Daley AJ, Nallusamy R, Isaacs D. Comparison of influenza A and influenza B virus infection in hospitalized children. J Paediatr Child Health. 2000;36(4):332–5. [DOI] [PubMed] [Google Scholar]
- 10.Dixon GA, Moore HC, Kelly H, Jacoby P, Carcione D, Williams S, et al. Lessons from the first year of the WAIVE study investigating the protective effect of influenza vaccine against laboratory-confirmed influenza in hospitalised children aged 6–59 months. Influenza Other Respir Viruses. 2010;4(4):231–4. 10.1111/j.1750-2659.2010.00141.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Onise K, Raupach JC. The burden of influenza in healthy children in South Australia. Med J Aust. 2008;188(9):510–3. [DOI] [PubMed] [Google Scholar]
- 12.Khandaker G, Zurynski Y, Ridley G, Buttery J, Marshall H, Richmond PC, et al. Clinical epidemiology and predictors of outcome in children hospitalised with influenza A(H1N1)pdm09 in 2009: a prospective national study. Influenza Other Respir Viruses. 2014;8(6):636–45. 10.1111/irv.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lester-Smith D, Zurynski YA, Booy R, Festa MS, Kesson AM, Elliott EJ. The burden of childhood influenza in a tertiary paediatric setting. Commun Dis Intell Q Rep. 2009;33(2):209–15. [DOI] [PubMed] [Google Scholar]
- 14.Alexander J, Millar J, Slater A, Woosley J. Report of the Australian and New Zealand Paediatric Intensive Care Registry, 2013. Australian and New Zealand Intensive Care Society (ANZICS); 2014; Available from: Available from: http://www.anzics.com.au/Downloads/2013%20ANZPICR%20Annual%20Report.pdf [Accessed Jul 2015].
- 15.Slater A, Shann F, McEniery J, Group AS. The ANZPIC registry diagnostic codes: a system for coding reasons for admitting children to intensive care. Intensive Care Med. 2003;29(2):271–7. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Zeki R, Hilder L, Sullivan E. Australia’s mothers and babies 2011 Perinatal statistics series no. 28. Canberra: AIHW National Perinatal Epidemiology and Statistics Unit; 2013. [Google Scholar]
- 17.Bagdure D, Curtis DJ, Dobyns E, Glode MP, Dominguez SR. Hospitalized children with 2009 pandemic influenza A (H1N1): comparison to seasonal influenza and risk factors for admission to the ICU. PLoS One. 2010;5(12):e15173 10.1371/journal.pone.0015173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris SK, Parkin P, Science M, Subbarao P, Yau Y, O'Riordan S, et al. A retrospective cross-sectional study of risk factors and clinical spectrum of children admitted to hospital with pandemic H1N1 influenza as compared to influenza A. BMJ Open. 2012;2(2):e000310 10.1136/bmjopen-2011-000310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrag SJ, Shay DK, Gershman K, Thomas A, Craig AS, Schaffner W, et al. Multistate surveillance for laboratory-confirmed, influenza-associated hospitalizations in children: 2003–2004. Pediatr Infect Dis J. 2006;25(5):395–400. [DOI] [PubMed] [Google Scholar]
- 20.Dawood FS, Chaves SS, Perez A, Reingold A, Meek J, Farley MM, et al. Complications and associated bacterial coinfections among children hospitalized with seasonal or pandemic influenza, United States, 2003–2010. J Infect Dis. 2014;209(5):686–94. 10.1093/infdis/jit473 [DOI] [PubMed] [Google Scholar]
- 21.Song X, DeBiasi RL, Campos JM, Fagbuyi DB, Jacobs BR, Singh N. Comparison of pandemic and seasonal influenza A infections in pediatric patients: were they different? Influenza Other Respir Viruses. 2012;6(1):25–7. 10.1111/j.1750-2659.2011.00258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawood FS, Fiore A, Kamimoto L, Bramley A, Reingold A, Gershman K, et al. Burden of seasonal influenza hospitalization in children, United States, 2003 to 2008. J Pediatr. 2010;157(5):808–14. 10.1016/j.jpeds.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 23.Streng A, Grote V, Liese JG. Severe influenza cases in paediatric intensive care units in Germany during the pre-pandemic seasons 2005 to 2008. BMC Infect Dis. 2011;11:233 10.1186/1471-2334-11-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heininger U, Baer G, Ryser AJ, Li Y. Comparative analysis of clinical characteristics of pandemic influenza a/h1n1 and seasonal influenza a infections in hospitalized children. Pediatr Infect Dis J. 2013;32(3):293–6. [DOI] [PubMed] [Google Scholar]
- 25.Kaczmarek MC, Valenti L, Kelly HA, Ware RS, Britt HC, Lambert SB. Sevenfold rise in likelihood of pertussis test requests in a stable set of Australian general practice encounters, 2000–2011. Med J Aust. 2013;198(11):624–8. [DOI] [PubMed] [Google Scholar]
- 26.Owen R, Barr IG, Pengilley A, Liu C, Paterson B, Kaczmarek M, et al. Annual report of the National Influenza Surveillance Scheme, 2007. Commun Dis Intell Q Rep. 2008;32(2):208–26. [DOI] [PubMed] [Google Scholar]
- 27.Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Public Health England. The national childhood flu immunisation programme 2015/16—Information for healthcare practitioners. 2015; Available from: Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/456626/PHE_Childhood_influenza_programme_2015_16.pdf [Accessed Oct 2015].
- 29.Blyth CC, Jacoby P, Effler PV, Kelly H, Smith DW, Robins C, et al. Effectiveness of trivalent flu vaccine in healthy young children. Pediatrics. 2014;133(5):e1218–25. 10.1542/peds.2013-3707 [DOI] [PubMed] [Google Scholar]
- 30.Ferdinands JM, Olsho LE, Agan AA, Bhat N, Sullivan RM, Hall M, et al. Effectiveness of influenza vaccine against life-threatening RT-PCR-confirmed influenza illness in US children, 2010–2012. J Infect Dis. 2014;210(5):674–83. 10.1093/infdis/jiu185 [DOI] [PubMed] [Google Scholar]
- 31.Pebody RG, Green HK, Andrews N, Zhao H, Boddington N, Bawa Z, et al. Uptake and impact of a new live attenuated influenza vaccine programme in England: early results of a pilot in primary school-age children, 2013/14 influenza season. Euro Surveill. 2014;19(22). [DOI] [PubMed] [Google Scholar]
- 32.Pebody RG, Green HK, Andrews N, Boddington N, Zhao H, Yonova I, et al. Uptake and impact of vaccinationg school age children against influenza during a season with circulation of drifted influenza A and B strains, England, 2014/15. Euro Surveill. 2015;20(39):1–11. [DOI] [PubMed] [Google Scholar]
- 33.Flood EM, Rousculp MD, Ryan KJ, Beusterien KM, Divino VM, Toback SL, et al. Parents' decision-making regarding vaccinating their children against influenza: A web-based survey. Clin Ther. 2010;32(8):1448–67. 10.1016/j.clinthera.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 34.Lu AB, Halim AA, Dendle C, Kotsanas D, Giles ML, Wallace EM, et al. Influenza vaccination uptake amongst pregnant women and maternal care providers is suboptimal. Vaccine. 2012;30(27):4055–9. 10.1016/j.vaccine.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 35.National Centre for Immunisation Research & Surveillance. Significant events in influenza vaccination practice in Australia. 2014 [updated March 2015]; Available from: Available from: http://ncirs.edu.au/immunisation/history/Influenza-history-March-2015.pdf [Accessed Aug 2015].
- 36.O'Grady KA, McHugh L, Nolan T, Richmond P, Wood N, Marshall HS, et al. FluMum: a prospective cohort study of mother-infant pairs assessing the effectiveness of maternal influenza vaccination in prevention of influenza in early infancy. BMJ Open. 2014;4(6):e005676 10.1136/bmjopen-2014-005676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiley KE, Massey PD, Cooper SC, Wood NJ, Ho J, Quinn HE, et al. Uptake of influenza vaccine by pregnant women: a cross-sectional survey. Med J Aust. 2013;198(7):373–5. [DOI] [PubMed] [Google Scholar]
- 38.Maher L, Hope K, Torvaldsen S, Lawrence G, Dawson A, Wiley K, et al. Influenza vaccination during pregnancy: coverage rates and influencing factors in two urban districts in Sydney. Vaccine. 2013;31(47):5557–64. 10.1016/j.vaccine.2013.08.081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant aggregate data are within the paper and its Supporting Information files. Although authors are prohibited from supplying any data to third parties under the ANZPIC Registry and NNDSS data release policies, underlying raw data can be requested directly from the ANZPIC Registry (j.alexander@uq.edu.au) and NNDSS (epi@health.gov.au) data custodians. Information about the ANZPIC Registry is available here: http://www.anzics.com.au/pages/CORE/ANZPICR-registry.aspx. Information about the NNDSS is available here: http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndssnndssintro.htm.