Abstract
BACKGROUND
Surgical site infection (SSI) is a costly complication leading to increased resource use and patient morbidity. We hypothesized that postdischarge SSI results in a high rate of preventable readmissions.
METHODS
We used our institutional American College of Surgeons National Surgical Quality Improvement Program database to identify patients undergoing general surgery procedures from 2006 to 2011.
RESULTS
SSIs developed in 10% of the 3,663 patients who underwent an inpatient general surgical procedure. SSI was diagnosed after discharge in 48% of patients. Patients with a diagnosis of SSI after discharge were less likely to have a history of smoking (15% vs 28%, P = .001), chronic obstructive pulmonary disease (3% vs 9%, P = .015), congestive heart failure (0% vs 3%, P = .03), or sepsis within 48 hours preoperatively (17% vs 32%, P = .001) compared with patients diagnosed before discharge. Over 50% of the patients diagnosed with SSI after discharge required readmission.
CONCLUSIONS
A diagnosis of SSI after discharge is associated with a high readmission rate despite occurring in healthier patients. We propose discharge teaching improvements and a wound surveillance clinic within the first week may result in a decreased readmission rate.
Keywords: Surgical site infection, Readmission, National Surgical Quality Improvement Program
Hospital-acquired infections account for a significant amount of morbidity associated with postoperative complications.1 Surgical site infections (SSIs) are costly and represent the second most common hospital-acquired infection.2 In October 2011, the Centers for Disease Control and Prevention Division of Healthcare Quality Promotion put forth the “National Action Plan to Prevent Healthcare-Associated Infections: Roadmap to Elimination,”3 which includes a 5-year national prevention target of a 25% reduction in admission and readmission SSIs. Beginning in 2013, according to the Hospital Readmission Reduction Program within the Patient Protection and Affordable Care Act, hospitals will be penalized by Medicare for a higher than expected readmission rate for acute myocardial infarction, congestive heart failure, and pneumonia. The list of conditions that will be monitored for readmission is expected to expand over the upcoming years. SSI is one postoperative complication associated with increased readmissions.4,5 Postdischarge complications constitute a large proportion of all complications, with SSI, urinary tract infections, pulmonary embolism, and deep vein thrombosis occurring frequently (34% to 66%) after discharge.6 The rate of SSIs has remained stable; however, with a decreasing length of stay, the rate of postdischarge diagnosis of SSI has increased.7,8 We sought to characterize patients diagnosed with SSI infection after discharge and readmission rates of this patient population with the intention of improving surveillance to prevent readmission and the associated complications.
Methods
This is a retrospective analysis of data collected for the National Surgical Quality Improvement Program (NSQIP). The data were collected from January 1, 2006, to June 30, 2011, for the University of Wisconsin by surgical clinical reviewers using an American College of Surgeons– validated 8-day sampling system according to NSQIP guidelines. The data set was filtered to include only elective or emergency inpatient procedures identified as general surgery for the surgical specialty category. Outcomes of interest included all patients who were identified as having at least 1 postoperative occurrence of a superficial, deep, or organ space SSI diagnosed in the postoperative period including up to 30 days after surgery. Wound disruption occurrences were omitted from this analysis. The definitions of SSIs were in accordance with NSQIP SSI variables and can be found online.9 Statistical analysis was performed on independent variables. The Wilcoxon rank sum test was used to compare continuous variables, and the Fisher exact test was used to compare categorical variables. All tests were considered significant at the P < .05 level, and P values were 2 tailed.
Results
The NSQIP database contained 3,633 patients who underwent inpatient general surgical procedures between 2006 and 2011. In this patient population, SSIs infections were identified in 9.9% (N = 359) of surgical procedures. These patients were compared using the NSQIP descriptors listed in Table 1; 55.1% of the patients who developed SSIs were male. There was an overall trend toward increased comorbidities in patients who developed SSIs, and statistically significant associations were noted for a variety of conditions. For example, when compared with patients without SSIs, patients with SSIs were more likely to have dyspnea, partial or total functional dependence, ventilator dependence before surgery, open wounds, bleeding disorders, or sepsis within 48 hours preoperatively. A higher percentage of those patients with severe, life-threatening, or moribund American Society of Anesthesiologists classification also developed SSIs. As expected, healthier patients were less likely to develop an SSI complication.
Table 1.
Descriptive table comparing SSI versus no SSI
| No SSI (%) | SSI (%) | P value | |
|---|---|---|---|
| Totals | 3,274 (90.12) | 359 (9.88) | |
| Characteristic | |||
| Age | 54.45 (SD = 16.9) | 56.35 (SD = 15.94) | .05 |
| BMI | 29.78 (SD = 8.95) | 30.79 (SD = 9.27) | .01 |
| Race | .0855 | ||
| White | 2,921 (89.22) | 327 (91.09) | |
| Black | 156 (4.76) | 20 (5.57) | |
| Other | 197 (6.02) | 12 (3.34) | |
| Female sex | 1,805 (55.13) | 161 (44.85) | .000226 |
| Diabetes | 226 (6.9) | 30 (8.36) | .32 |
| Smoker | 572 (17.47) | 80 (22.28) | .295 |
| Dyspnea | 324 (9.90) | 50 (13.93) | .05 |
| Partial/total functional dependence | 316 (9.65) | 57 (15.88) | .00046 |
| Ventilator | 76 (2.32) | 16 (4.46) | .02 |
| COPD | 138 (4.22) | 23 (6.41) | .059 |
| Ascites | 64 (1.96) | 12 (3.34) | .11 |
| CHF | 47 (1.44) | 6 (1.67) | .64 |
| HTN | 1,421 (43.4) | 173 (48.19) | .09 |
| ARF | 19 (.58) | 3 (.84) | .47 |
| Dialysis | 43 (1.31) | 5 (1.39) | .89 |
| Disseminated cancer | 156 (4.76) | 22 (6.13) | .24 |
| Open wound | 182 (5.56) | 39 (10.86) | .00025 |
| Steroids | 266 (8.12) | 19 (5.29) | .062 |
| Weight loss >10% 6 mo preoperatively | 165 (5.04) | 27 (7.52) | .0609 |
| Bleeding d/o | 208 (6.35) | 33 (9.19) | .0444 |
| Transfusions within 72 h preoperatively | 43 (1.31) | 6 (1.67) | .62 |
| Sepsis within 48 h preoperatively | 519 (15.93) | 91 (25.35) | .00015 |
| Wound class | 4.06E-13 | ||
| Clean | 879 (26.85) | 39 (10.86) | |
| Clean/contaminated | 1,541 (47.07) | 193 (53.76) | |
| Contaminated | 514 (15.7) | 60 (16.71) | |
| Dirty/infected | 340 (10.38) | 67 (18.66) | |
| ASA category | 6.26E-06 | ||
| No disturbance | 247 (7.55) | 12 (3.34) | |
| Mild | 1,766 (53.97) | 163 (45.4) | |
| Severe | 1,086 (33.19) | 156 (43.45) | |
| Life-threatening or moribund | 173 (5.29) | 28 (7.8) | |
| LOS days | 6.79 (SD = 10.35) | 12.88 (SD = 14.85) | <.0001 |
| Days before surgery | 1.09 (SD = 5.73) | 1.35 (SD = 4.99) | .0627 |
| Days after surgery | 5.71 (SD = 7.67) | 11.52 (SD = 12.90) | <.0001 |
ARF = acute renal failure; ASA = American Society of Anesthesiologists; BMI = body mass index; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disorder; d/o = disorder; HTN = hypertension; LOS = length of stay; SD = standard deviation; SSI = surgical site infection.
The timing of SSI diagnosis as a postoperative occurrence is tracked in NSQIP from the day of operation until 30 days after surgery. We compared patients whose diagnosis of an SSI occurred during hospitalization with those diagnosed after discharge and found a trend toward increased comorbidities in those patients who were diagnosed during their index hospitalization (Table 2). Cigarette smoking, partial or total functional dependence, ventilator dependence before surgery, chronic obstructive pulmonary disease, congestive heart failure, bleeding disorder, and sepsis within 48 hours preoperatively was statistically significantly increased in patients who were diagnosed while they were inpatients. The majority of procedures in our patient population were classified as clean/contaminated; however, a higher percentage of patients with SSI diagnosed during their hospitalization had wound classifications of contaminated or dirty/infected compared with patients with SSIs diagnosed after discharge. Patients with higher American Society of Anesthesiologists classifications were also more likely to have their SSI diagnosed while inpatients. Overall, SSIs occurred more often in patients with increased comorbidities, and the diagnosis occurred while they were inpatients. Increased length of stay was also associated with SSI diagnosis, and this trend continued in patients diagnosed as inpatients as would be expected. Our results are in agreement with previous studies showing that in general patients diagnosed with SSIs after discharge are healthier.8 This poses a problem in identifying the population of patients who will develop SSIs after discharge in order to institute preventative measures for readmission.
Table 2.
Descriptive table comparing diagnosis of SSI during index hospitalization versus diagnosis after discharge
| SSI diagnosis during index hospitalization (%) |
SSI diagnosis after discharge (%) |
P value | |
|---|---|---|---|
| Totals | 187 (52.09) | 172 (47.91) | |
| Characteristics | |||
| Age | 59.52 (SD = 15.47) | 52.91 (SD = 15.78) | <.0001 |
| BMI | 29.44 (SD = 6.83) | 32.26 (SD = 11.19) | .1611 |
| Race | .579 | ||
| White | 168 (89.84) | 159 (92.44) | |
| Black | 11 (5.88) | 9 (5.23) | |
| Other | 8 (4.28) | 4 (2.33) | |
| Female sex | 73 (39.04) | 88 (51.16) | .256 |
| Diabetes | 17 (9.09) | 13 (7.56) | .7 |
| Smoker | 54 (28.88) | 26 (15.12) | .0022 |
| Dyspnea | 32 (17.11) | 18 (10.46) | .19 |
| Partial/total functional dependence | 43 (22.99) | 14 (8.14) | .00013 |
| Ventilator | 14 (7.49) | 2 (1.16) | .004 |
| COPD | 18 (9.63) | 5 (2.91) | .01 |
| Ascites | 9 (4.81) | 3 (1.74) | .14 |
| CHF | 6 (3.21) | 0 | .03 |
| HTN | 97 (51.87) | 76 (44.19) | .16 |
| ARF | 3 (1.6) | 0 | .24 |
| Dialysis | 5 (2.67) | 0 | .06 |
| Disseminated cancer | 14 (7.49) | 8 (4.65) | .28 |
| Open wound | 23 (12.3) | 16 (9.3) | .39 |
| Steroids | 11 (5.88) | 8 (4.65) | .64 |
| Weight loss .10% 6 mo preoperatively | 17 (9.09) | 10 (5.81) | .31 |
| Bleeding d/o | 24 (12.83) | 9 (5.23) | .01 |
| Transfusions within 72 h preoperatively | 5 (2.67) | 1 (.58) | .21 |
| Sepsis within 48 h preoperatively | 61 (32.62) | 30 (17.44) | .001 |
| Wound class | .017 | ||
| Clean | 13 (6.95) | 26 (15.12) | |
| Clean/contaminated | 97 (51.87) | 96 (55.81) | |
| Contaminated | 34 (18.18) | 26 (15.12) | |
| Dirty/infected | 43 (22.99) | 24 (13.95) | |
| ASA category | .00084 | ||
| No disturbance | 6 (3.21) | 6 (3.49) | |
| Mild | 69 (36.9) | 94 (54.65) | |
| Severe | 90 (48.13) | 66 (38.37) | |
| Life-threatening or moribund | 22 (11.76) | 6 (3.49) | |
| LOS days | 18.87 (SD = 18.2) | 6.36 (SD = 4.13) | <.0001 |
| Days before surgery | 2.24 (SD = 6.71) | .38 (SD = 1.21) | <.0001 |
| Days after surgery | 16.63 (SD = 15.81) | 5.97 (SD = 3.73) | <.0001 |
ARF = acute renal failure; ASA = American Society of Anesthesiologists; BMI = body mass index; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disorder; d/o = disorder; HTN = hypertension; LOS = length of stay; SD = standard deviation; SSI = surgical site infection.
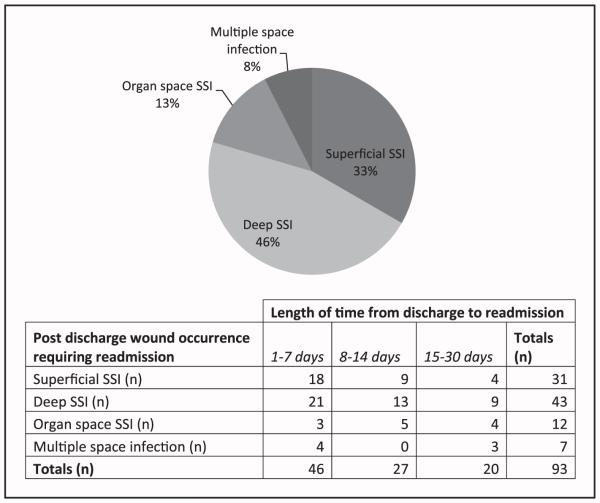
The majority of SSIs (58.1%) in patients diagnosed after discharge were superficial in nature compared with deep (29.6%), organ space (7.6%), and multiple space SSIs (4.7%). Readmission occurred in approximately 54% of patients who were diagnosed after discharge, and one third of those patients developed superficial SSIs. Table 3 compared the NSQIP variables for outpatient treatment versus readmission of patients diagnosed with any SSI after discharge. These patients were similar in their comorbidities overall, except patients with greater than 10% weight loss in the 6 months preceding their operation were more likely to be readmitted. This suggests that it is not entirely the health or comorbidities of the patient that dictates readmission. We additionally found that there was no difference in readmission rates when we compared payer status (ie, private, Medicare/Medicaid, or self-pay) or whether the patient identified our facility as their primary medical facility versus a referring institution. Fig. 1 shows the rates for various types of SSIs requiring readmission and the time from discharge to readmission. Thirty-one percent of all superficial SSIs diagnosed after discharge required readmission, accounting for 33% of the patients readmitted. Eighty-four percent of deep, 92% of organ space, and 87% of multiple space infections required readmission, accounting for 46%, 13%, and 7.5% of total patients, respectively, readmitted with a postdischarge diagnosis of SSI. Readmissions in all groups except organ space SSIs were more likely to occur in the first week after discharge. These findings suggest a critical period of time in which SSI surveillance on an outpatient basis could be beneficial in preventing readmissions.
Table 3.
Descriptive table comparing readmission versus outpatient treatment for SSI diagnosed after discharge
| SSI diagnosis after discharge, treated as outpatient |
SSI diagnosis after discharge, readmitted | P value | |
|---|---|---|---|
| Totals (%) | 79 (45.9) | 93 (54.1) | |
| Characteristics | |||
| Age | 52.9 (SD = 15.99) | 52.9 (SD = 15.68) | .97 |
| BMI | 33.44 (SD = 11.81) | 31.26 (SD = 10.60) | .19 |
| Race | .48 | ||
| White | 71 (89.97) | 88 (94.62) | |
| Black | 5 (6.33) | 4 (4.3) | |
| Other | 3 (3.8) | 1 (1.08) | |
| Female sex | 41 (51.9) | 47 (50.54) | .87 |
| Diabetes | 8 (10.13) | 5 (5.38) | .26 |
| Smoker | 9 (11.39) | 17 (18.28) | .28 |
| Dyspnea | 8 (10.13) | 10 (10.76) | .31 |
| Partial/total functional dependence | 6 (7.59) | 8 (8.6) | 1 |
| Ventilator | 1 (1.27) | 1 (1.08) | 1 |
| COPD | 4 (5.06) | 1 (1.08) | .18 |
| Ascites | 1 (1.27) | 2 (2.15) | 1 |
| CHF | 0 | 0 | |
| HTN | 38 (48.10 ) | 38 (40.86) | .3591 |
| ARF | 0 | 0 | |
| Dialysis | 0 | 0 | |
| Disseminated cancer | 3 (3.80) | 5 (5.38) | .72 |
| Open wound | 7 (8.86) | 9 (9.68) | 1 |
| Steroids | 4 (5.06) | 4 (4.3) | 1 |
| Weight loss .10% 6 mo preoperatively | 1 (1.27) | 9 (9.68) | .02 |
| Bleeding d/o | 4 (5.06) | 5 (5.38) | 1 |
| Transfusions within 72 h preoperatively | 1 (1.27) | 0 | .45 |
| Sepsis within 48 h preoperatively | 11 (13.92) | 19 (20.43) | .31 |
| Wound class | .26 | ||
| Clean | 15 (18.99) | 11 (11.83) | |
| Clean/contaminated | 44 (55.7) | 52 (55.91) | |
| Contaminated | 8 (10.13) | 18 (19.35) | |
| Dirty/infected | 12 (15.19) | 12 (12.9) | |
| ASA category | .3 | ||
| No disturbance | 5 (6.33) | 1 (1.08) | |
| Mild | 42 (53.16) | 52 (55.91) | |
| Severe | 30 (37.97) | 36 (38.71) | |
| Life-threatening or moribund | 2 (2.53) | 4 (4.3) |
ARF = acute renal failure; ASA = American Society of Anesthesiologists; BMI = body mass index; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disorder; d/o = disorder; HTN = hypertension; LOS = length of stay; SD = standard deviation; SSI = surgical site infection.
Figure 1.
Readmission by SSI type and table of length of time from discharge to readmission.
Overall, the readmission rate for our population regardless of complications was 8.9%. Two percent required readmission without any documented NSQIP complication, and 20.2% required readmission if they had an SSI with or without other complications. Over 20 postoperative occurrence codes exist in the NSQIP database. The occurrence of multiple complications in the same patient could influence the timing of diagnosis or the need for readmission in our patient population. For that reason, a subset of the population who had an SSI as the only postoperative complication was selected for analysis (Table 4). We found a 10.1% readmission rate in patients with an SSI as their only complication. Over half (58.7%) of the patients who were found to have SSI as their only postoperative complication were diagnosed after discharge from the hospitalization, and 15.6% required readmission. This is in contrast to readmission rates of 2.2% in patients with an inpatient diagnosis of SSI as the only postoperative complication. When patients in this population were seen in the emergency department (ED) for their SSI, 50% were readmitted compared with 6% when they presented to the clinic. Along the same lines, if patients were seen after 5 PM, 54.5% were readmitted compared with 7.5% who were seen between 8 AM and 5 PM. When SSI is the only complication, the readmission rate was lower than in patients with multiple complications. Importantly, there was a significant increase in readmissions if the diagnosis was delayed until after discharge despite being an isolated postoperative complication.
Table 4.
Readmission by SSI type and length of time from discharge to readmission
| Postoperative complication (SSI only) | Diagnosis (%) | Readmission (%) | |
|---|---|---|---|
| During hospitalization | 45 (41.3) | 1 (2.2) | |
| After discharge | 64 (58.7) | 10 (15.6) | P = .025 |
| Diagnosis after discharge | Location (%) | Readmission (%) | |
|
| |||
| ED | 14 (21.9) | 7 (50) | |
| Clinic | 50 (78.1) | 3 (6) | P = .00047 |
|
| |||
| Time of day (%) | Readmission (%) | ||
|
| |||
| 8 AM–5 PM | 53 (82.8) | 4 (7.5) | |
| 5 PM–8 AM | 11 (17.2) | 6 (54.5) | P = .0009 |
ED = emergency department; SSI = surgical site infection.
Comments
Readmissions for preventable postoperative complications are receiving more scrutiny than ever before.10,11 The identification of preventable readmissions is increasingly important because payment for these hospitalizations in facilities with a higher than expected readmission rate may be eliminated.12 Studies have shown that a diagnosis of SSI after discharge is associated with readmission.2 Currently available surveillance methods are variable in their ability to accurately identify these patients; therefore, the actual number of patients with SSIs diagnosed after discharge is likely higher than reported.6,13 This study sought to characterize the population of patients with an after discharge diagnosis of SSI who are at risk for potentially preventable readmission.
Our results are in agreement with previous studies8 that suggest an after discharge diagnosis of SSI usually occurs in healthy people, making it difficult to identify risk factors for readmission. Once discharged, the patient or his/her family assumes the responsibility for monitoring wound complications. Although patients receive discharge instructions, it is often an overwhelming experience as fears about care outside of the confines of the hospital become apparent. Educational barriers and/or a lack of a support system may interfere with the timely recognition and treatment of an infection.14 However, social support system and education level variables are not currently available in the NSQIP database for analysis, and they are likely too complex to directly infer causality of readmissions. We did find that neither payer status nor location of medical home affected readmissions in patients with SSIs with or without other complications. This is in contrast to a report in June 2011 that revealed that Medicaid recipients were readmitted for all causes within 30 days nearly twice as frequently as privately insured and uninsured patients.15 Our study limited the analysis to readmissions after SSI complications with or without other complications, suggesting that the differences between payer groups observed in other studies when all readmissions are included may be caused by complications not related to SSIs. In patients with SSIs, it appears that payer status and medical home are not feasible identifiable characteristics to use for surveillance efforts.
We found that the majority of readmissions related to SSIs diagnosed after discharge occurred in the first week after discharge. This was in agreement with a recent retrospective review of the NSQIP database from 2005 to 2010 that found that 75% of the postdischarge complications occurred within 14 days after discharge and SSIs were among the most common complications.16 Patients are often discharged home much earlier in their hospitalization than in the past, leaving little opportunity for education and surveillance of SSIs. Evaluation of the discharge process from a systems level has shown that work environment of those discharging the patient (ie, nursing staff) impacts the readmission rate.17 Checklists have been shown to be effective for increasing safety and reducing complications, which, in turn, could reduce readmissions.18,19 Bundling discharge materials with a checklist for the nursing staff to review with patients before discharge may improve the consistency and delivery of information. An early telephone follow-up with dedicated health professionals could potentially intervene in an SSI complication before it becomes necessary for readmission. Our data suggest that efforts toward prevention should target the first and second week after discharge in order to capture the majority of patients.
The use of EDs as urgent care facilities places a heavy burden on these facilities and represents poor resource use.20 Two percent of all ED visits are patients discharged within the past 7 days.21 We found that readmissions were much more likely in those patients who were evaluated for their SSI in the ED compared with the clinic. Our population was too small to detect any difference in the comorbidities of those seen in the ED to determine if they were less healthy. Additionally, we did not access information regarding social support or financial resources that may have led to an ED visit rather than a clinic appointment, both of which likely play a role in a patient’s decision to seek emergency care. Although social support and financial resources may not be easily controlled, the development of a protocol in which ED physicians/residents unfamiliar with the patient could better direct care after hours when the operating surgeon is unavailable to consult may prevent a reflexive automatic readmission. Effective discharge processes that provide a plan for patients during normal clinic hours will prevent a significant number of unnecessary readmissions as well as lessen the ever-growing burden on the ED.20
Telemedicine is still in its infancy; however, it has the potential to have a substantial role in providing wound assessments in those patients without the resources available for a clinic visit. Ultimately, this could translate into decreased ED visits and preventable readmissions. Early pilot studies have shown promise with postoperative patient management and decreasing anxiety in vascular surgery patients.22 Telemedicine is suited for the surveillance of wound care, and studies have shown an improvement in patient satisfaction with telemedicine.23 The electronic medical record enables sharing of these telemedicine visits for documentation and transmission to the surgeon for decision making. An adjustment of discharge surveillance is necessary to capture the superficial SSI early enough to enable outpatient treatment.
Limitations of our study include the following: it was retrospective, it was performed in a single institution, and it solely used NSQIP variables for the determination of comorbidities and the identification of risk factors. Additionally, 30-day occurrences and readmission surveillance may miss a proportion of the population, especially in cases in which implants are present (ie, mastectomy with immediate reconstruction) and a longer period of surveillance is necessary.24 Other methods of data collection such as National Healthcare Safety Network (NHSN) or Electronic Medical Record (EMR) surveillance may provide more information to help identify this patient population.13 Although our reported rate of SSIs was higher than the often-quoted 2% overall rate of SSIs, the rate varies widely depending on the wound classification of the procedure, type of case, length of surveillance, and method of data collection. A population-based retrospective cohort study in 2010 including over 600,000 surgical cases from multiple specialties reported an overall SSI rate of 13.5%.8 A prospective cohort study of 1,506 patients on a general surgery service measuring inpatient and postdischarge SSIs found that 14.8% developed an SSI.25 An analysis of SSIs using an American College of Surgery NSQIP data set containing over 600,000 general and vascular surgery cases reported varying rates of SSIs according to wound classification including 2.58% in clean, 6.67% in clean/contaminated, 8.61% in contaminated, and 11.80% in dirty wounds.26 These studies show the variability of reported SSI rates, and our rate of SSIs was within the range of published data.
In conclusion, after discharge, diagnosis of SSI is a source of potentially preventable readmissions. Given that these patients are often healthy and without clear identifiable risk factors, a generalizable method for all patients being discharged should be implemented. Identifying these patients in the early discharge period and providing interventions in the outpatient setting could mean the difference between a preventable and a necessary readmission. Focusing efforts on the development of strategies for readmission prevention are necessary not only for the health of the patient but also the economics of health care in general.
Acknowledgments
We would like to acknowledge the University of Wisconsin NSQIP personnel Deborah Armstrong and Barbara Braunger for their assistance with data collection and Victoria Rajamanickam, PhD, for providing statistical assistance with this study.
Footnotes
The authors declare no conflict of interest.
References
- 1.Klevens RM, Edwards JR, Richards CL, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National action plan to prevent healthcare-associated infections: roadmap to elimination. Available at: http://www.hhs.gov/ash/initiatives/hai/actionplan/. Accessed April 4, 2013.
- 4.Wick EC, Shore AD, Hirose K, et al. Readmission rates and cost following colorectal surgery. Dis Colon Rectum. 2011;54:1475–9. doi: 10.1097/DCR.0b013e31822ff8f0. [DOI] [PubMed] [Google Scholar]
- 5.Cafardi SG. Readmissions due to hospital-acquired conditions (HACs): multivariate modeling and under-coding analyses. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Pay ment/HospitalAcqCond/Downloads/Final-Report-Readmissions.pdf. Accessed April 4, 2013.
- 6.Bilimoria KY, Cohen ME, Ingraham AM, et al. Effect of postdischarge morbidity and mortality on comparisons of hospital surgical quality. Ann Surg. 2010;252:183–90. doi: 10.1097/SLA.0b013e3181e4846e. [DOI] [PubMed] [Google Scholar]
- 7.Prospero E, Cavicchi A, Bacelli S, et al. Surveillance for surgical site infection after hospital discharge: a surgical procedure-specific perspective. Infect Control Hosp Epidemiol. 2006;27:1313–7. doi: 10.1086/509838. [DOI] [PubMed] [Google Scholar]
- 8.Daneman N, Lu H, Redelmeier DA. Discharge after discharge: predicting surgical site infections after patients leave hospital. J Hosp Infect. 2010;75:188–94. doi: 10.1016/j.jhin.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 9.ACS NSQIPdclassic variables and definitions. Available at: http://nsqip.healthsoftonline.com/lib/Documents/Ch_4_Variables_Definitions_ 062810.pdf. Accessed April 4, 2013.
- 10.Hospitals must reduce readmissions as CMS moves to cut reimbursement Hosp Case Manag. 2010;18:129–34. 139. [PubMed] [Google Scholar]
- 11.Berenson RA, Paulus RA, Kalman NS. Medicare’s readmissions-reduction program–a positive alternative. N Engl J Med. 2012;366:1364–6. doi: 10.1056/NEJMp1201268. [DOI] [PubMed] [Google Scholar]
- 12.Coye MJ. CMS’ stealth health reform. Plan to reduce readmissions and boost the continuum of care. Hosp Health Netw. 2008;82:24. [PubMed] [Google Scholar]
- 13.Petherick ES, Dalton JE, Moore PJ, et al. Methods for identifying surgical wound infection after discharge from hospital: a systematic review. BMC Infect Dis. 2006;6:170. doi: 10.1186/1471-2334-6-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lattimer C. Better coordination of care reduces readmissions. Front Health Serv Manage. 2009;25:43–6. [PubMed] [Google Scholar]
- 15.All-cause readmissions by payer and age, 2008 . Statistical Brief #115. Agency for Healthcare Research and Quality; Rockville, MD: 2011. [PubMed] [Google Scholar]
- 16.Kazaure HS, Roman SA, Sosa JA. Association of postdischarge complications with reoperation and mortality in general surgery. Arch Surg. 2012;147:1000–7. doi: 10.1001/2013.jamasurg.114. [DOI] [PubMed] [Google Scholar]
- 17.McHugh MD, Ma C. Hospital nursing and 30-day readmissions among Medicare patients with heart failure, acute myocardial infarction, and pneumonia. Med Care. 2013;51:52–9. doi: 10.1097/MLR.0b013e3182763284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–9. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 19.Robbins J. Hospital checklists. Transforming evidence-based care and patient safety protocols into routine practice. Crit Care Nurs Q. 2011;34:142–9. doi: 10.1097/CNQ.0b013e31820f7467. [DOI] [PubMed] [Google Scholar]
- 20.Schiff GD. System dynamics and dysfunctionalities: levers for overcoming emergency department overcrowding. Acad Emerg Med. 2011;18:1255–61. doi: 10.1111/j.1553-2712.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 21.Burt CW, McCaig LF, Simon AE. Emergency department visits by persons recently discharged from U.S. hospitals. Natl Health Stat Report. 2008;6:1–9. [PubMed] [Google Scholar]
- 22.Robaldo A, Rousas N, Pane B, et al. Telemedicine in vascular surgery: clinical experience in a single centre. J Telemed Telecare. 2010;16:374–7. doi: 10.1258/jtt.2010.091011. [DOI] [PubMed] [Google Scholar]
- 23.Dobke MK, Bhavsar D, Gosman A, et al. Pilot trial of telemedicine as a decision aid for patients with chronic wounds. Telemed J E Health. 2008;14:245–9. doi: 10.1089/tmj.2007.0038. [DOI] [PubMed] [Google Scholar]
- 24.Lankiewicz JD, Yokoe DS, Olsen MA, et al. Beyond 30 days: does limiting the duration of surgical site infection follow-up limit detection? Infect Control Hosp Epidemiol. 2012;33:202–4. doi: 10.1086/663715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delgado-Rodríguez M, Gómez-Ortega A, Sillero-Arenas M, et al. Epidemiology of surgical-site infections diagnosed after hospital discharge: a prospective cohort study. Infect Control Hosp Epidemiol. 2001;22:24–30. doi: 10.1086/501820. [DOI] [PubMed] [Google Scholar]
- 26.Ortega G, Rhee DS, Papandria DJ, et al. An evaluation of surgical site infections by wound classification system using the ACS-NSQIP. J Surg Res. 2012;174:33–8. doi: 10.1016/j.jss.2011.05.056. [DOI] [PubMed] [Google Scholar]