Cerebral malaria (CM) is a severe manifestation of Plasmodium falciparum infection, characterized by seizures and coma, respiratory distress, hypoglycemia, and acidosis. Intensely studied for decades, important advances in understanding this clinically complex syndrome have been achieved, but highly efficacious treatment remains elusive.1 Even with rapid delivery of antimalarial drugs on diagnosis and the best supportive care, many CM victims do not survive.1 An effective malaria vaccine to prevent CM and other severe malaria syndromes is not yet available, making continued efforts to identify novel therapies, particularly those that can serve as adjuncts to antimalarial drugs, essential.2 Defibrotide (DF), a mixture of single-stranded ≈50-mer DNA aptamers with a minor component of double stranded DNA that is derived from depolymerized mammalian genomic DNA,3–5 is an exciting potential new recruit to the ranks of such adjunctive treatments. This multipotent drug displays endothelial-protective, antiischemic, anti-inflammatory, and mild anticoagulant effects and has been successfully used to treat comatose children experiencing veno-occlusive disease.3–5
CM develops when mature intraerythrocytic stages of P. falciparum adhere to the brain microvasculature, interrupt normal blood flow, and promote endothelial activation, culminating in disruption of normal vascular function1 (Figure). Intense systemic and local inflammatory responses are believed to be important players in disease pathogenesis,6 and more recently, a role for dysregulated hemostasis has also been suggested.7,8 Francischetti et al identified a potential role for tissue factor (TF) in CM9 and argued that the TF-mediated coagulation-inflammation cycle underlies the pathogenesis of this disease.7,8 In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Francischetti et al10 advance our understanding of malarial parasite/host interactions in this context. They argue that effective treatment of CM will require control of multiple pathological targets and propose that DF may be a suitable candidate (Figure). In their article, Francischetti et al show that DF at a concentration achievable in plasma blocks induction of endothelial TF-mediated coagulation by parasitized red blood cells and suppresses elastase and cathepsin G activity.10 The latter observation is intriguing because cathepsin G induces platelet aggregation3–5 and can cleave factor X, thereby promoting coagulation.11 Elastase decreases TF pathway inhibitor levels12 and cleaves membrane-bound thrombomodulin.13 This is notable because brain thrombomodulin expression is already among the lowest within human tissues.6,14 Maintenance of thrombomodulin by DF with simultaneous suppression of TF-induced coagulation and platelet aggregation could therefore provide protection against malaria-induced coagulopathy in the brain. Prompted by the recent identification of dendritic cells (DCs) as prominent in the coagulation-inflammation cycle,15 Francischetti et al further show that at a concentration achievable in vivo, DF suppresses Toll-like receptors 4 and 2 agonist-driven proinflammatory cytokine production by DCs.10 Toll-like receptor 2 suppression is particularly noteworthy in this context because P. falciparum-derived glycosylphosphatidylinositols (PfGPIs) interact with this receptor,16 and Francischetti et al demonstrate that PfGPIs promote DC activation and inflammatory cytokine production.10 Moreover, PfGPIs initiate TF-mediated procoagulant activity on microvascular endothelial cells in a cytokine-independent manner. At drug concentrations higher than might be found in vivo, Francischetti et al further show that DF directly decreases platelet aggregation and blocks the alternative complement pathway.10 The latter is noteworthy because parasitized red blood cells enhance complement component 5 activation, and PfGPIs promote expression of the complement component 5a receptor on human monocytes. Together, complement component 5a and PfGPI induce inflammatory cytokine and chemokine production and may suppress angiogenesis,17 dysregulation of which is observed in CM.18 Thus, in both DCs and endothelial cells, DF suppresses inflammatory cytokine secretion, promotes production of anti-inflammatory factors, blocks TF-mediated coagulation, and supports inhibition of coagulation. Although the mechanistic basis for these effects remains to be established, Francischetti et al10 consider that DF may act through adenosine receptors.19 Consistent with this, they observe that DF-exposed DCs produce prostaglandin E2 and have enhanced lipopolysaccharide-induced IL-10 secretion.
Figure.
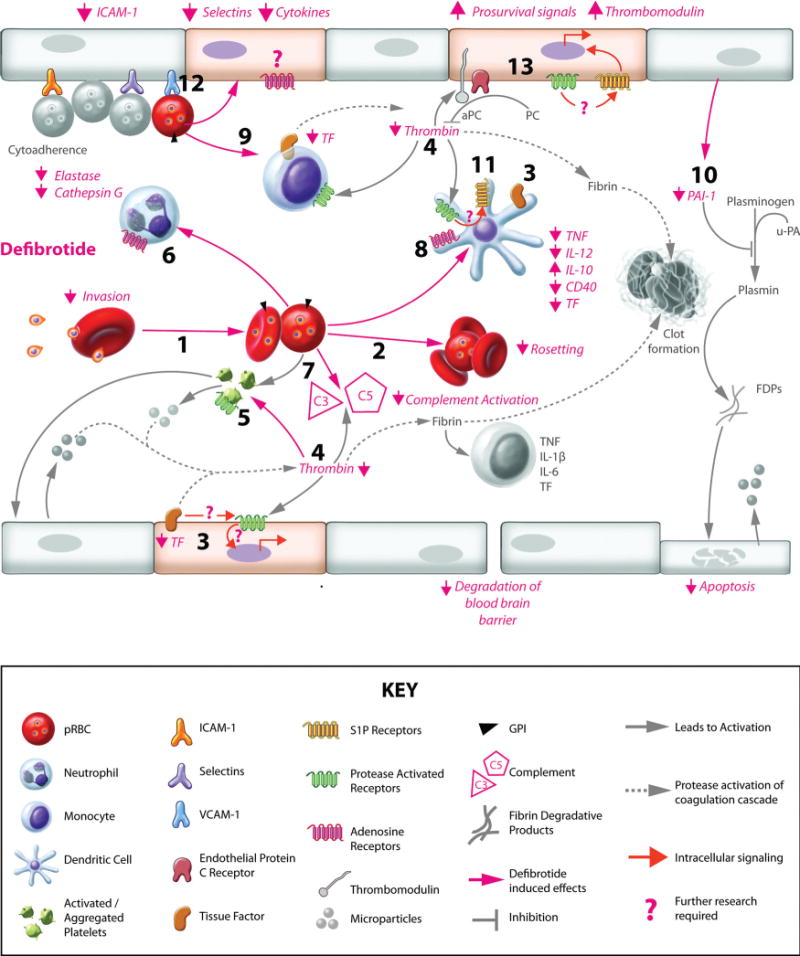
Defibrotide (DF) and the coagulation-inflammation cycle in cerebral malaria (CM). CM-induced dysregulation of coagulation and inflammation is precipitated by adherence of Plasmodium falciparum–infected red blood cells (pRBCs) to endothelial cells (ECs), pRBC/red blood cell (RBC) rosettes, inflammatory infiltrate and cytokine production, and fibrin clots, leading to occlusion of the brain microvasculature and degradation of the blood-brain barrier. As shown by Francischetti et al,10 DF may protect against CM by acting directly on P. falciparum. Specifically, DF (1) inhibits RBC reinvasion by merozoites and (2) decreases rosette formation. Additional DF benefits may be suppression of coagulation through (3) reduced tissue factor (TF) expression and activity, (4) reduced activation of thrombin, (5) decreased platelet activation and aggregation,25 and (6) diminished activity of neutrophil-derived elastase and cathepsin G. Also relevant to CM inflammatory pathogenesis, DF (7) suppresses complement activation and (8) modulates dendritic cell (DC) activation through adenosine receptors (ARs), decreasing CD40, tumor necrosis factor (TNF), and interleukin (IL)-12 expression and increasing immunoregulatory IL-10 expression. Other previously reported important effects of DF with relevance to CM include (9) reduced activation and downregulated proinflammatory/procoagulant status in monocytes26; (10) decreased plasminogen activator inhibitor (PAI)-1 and (3) TF expression, which reduces fibrin clot maintenance, coagulation initiation, and TF/protease-activated receptor (PAR) signaling,5,27 potentially involving (11) sphingosine-1 phosphate (S1P) receptors on DCs15; (12) decreased leukocyte and possibly pRBC recruitment by reducing the expression of EC adherence molecules,28 including intercellular adhesion molecule (ICAM)-1; (13) and increased expression of thrombomodulin29 and stabilization of ECs.30 u-PA indicates urokinase-type plasminogen activator; FDPs, fibrin degradation products; GPI, glycosylphosphatidylinositol; VCAM, vascular cell adhesion molecule. Areas that require further study (“?”) include the role of PARs in CM and the extent to which ARs on ECs and DCs are critical in the DF-induced protective response against CM. Paradoxically, S1P receptors are implicated in the coagulation-inflammation cycle,15 yet availability of S1P is important for protection against CM;31, therefore, the interactions between ARs, PARs, and S1P receptors, both in pathogenesis and in the context of DF treatment, should be investigated.
In addition to a potential for interrupting the coagulation-inflammation cycle, DF may act directly on the malarial parasite. Francischetti et al show that DF blocks parasite invasion of red blood cells and rosetting, the agglutination of parasitized red blood cells with other infected and uninfected red blood cells.10 Furthermore, inclusion of the drug in parasitized blood fed to mosquitoes decreases midgut parasite development. Finally, in a murine model for CM, treatment with DF thrice daily starting at day 1 of infection delays development of parasitemia and neurological signs and significantly reduces systemic interferon-γ, which is important in disease pathogenesis in this model20,21; there is also a trend toward extended time to death. Initiation of treatment after development of significant parasitemia (day 4) does not have these effects, suggesting that it may be less effective in controlling malaria-induced inflammation and coagulation once established. Although these in vivo data show modest drug efficacy, considered together with the in vitro work, these observations at minimum provide leads for critical future research (Figure). For example, detailed characterization of the role of adenosine receptors in the DF response in the context of malaria could help to optimize drug efficacy and identify specific components that are most stable in the face of degradative plasma exonucleases.3–5 Additionally, the impact of DF on protease-activated receptor activity should be explored. On cleavage by coagulation proteases, protease-activated receptors promote inflammatory responses and endothelial cell activation,22 and, indeed, are pivotal for the participation of DCs in the coagulation-inflammation cycle. To date, the role of protease-activated receptors has not been investigated in malaria, but they are clearly involved in the pathophysiology of sepsis and several other disease conditions.22 Moreover, there is intriguing evidence that protease-activated receptor and adenosine receptor signaling are coupled.23
In summary, Francischetti et al not only reemphasize the importance of the coagulation-inflammation cycle in the pathogenesis of CM but also introduce a potential new therapeutic approach for treatment of this deadly disease. Importantly, DF is a safe and efficacious treatment already in clinical use.3–5 Given its multipotent affects, DF or related compounds used in conjunction with effective antimalarial drugs could revolutionize clinical management of CM. It also deserves mention that CM patients may not be the only beneficiaries of new treatment approaches using DF/antimalarial drug combinations. Recent evidence suggests that placental malaria pathogenesis is also driven by the coagulation-inflammation cycle,24,32 making malaria-exposed pregnant women another group who might be considered for DF-containing adjunctive treatments.
Acknowledgments
We thank William “Kip” Carter for his indefatigable efforts in producing the artwork for this article.
Sources of Funding
J.M.M. is supported by National Institutes of Health grants R21 AI090439 and R01 HD046860. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.John CC, Kutamba E, Mugarura K, Opoka RO. Adjunctive therapy for cerebral malaria and other severe forms of Plasmodium falciparum malaria. Expert Rev Anti Infect Ther. 2010;8:997–1008. doi: 10.1586/eri.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins SJ, Kain KC, Liles WC. Immunopathogenesis of falciparum malaria: implications for adjunctive therapy in the management of severe and cerebral malaria. Expert Rev Anti Infect Ther. 2011;9:803–819. doi: 10.1586/eri.11.96. [DOI] [PubMed] [Google Scholar]
- 3.Kornblum N, Ayyanar K, Benimetskaya L, Richardson P, Iacobelli M, Stein CA. Defibrotide, a polydisperse mixture of single-stranded phosphodiester oligonucleotides with lifesaving activity in severe hepatic veno-occlusive disease: clinical outcomes and potential mechanisms of action. Oligonucleotides. 2006;16:105–114. doi: 10.1089/oli.2006.16.105. [DOI] [PubMed] [Google Scholar]
- 4.Larocca A, Cavallo F, Magarotto V, Rossi D, Patriarca F, Boccadoro M, Palumbo A. Defibrotide: a review on clinical use and future development. Expert Opin Biol Ther. 2008;8:1201–1212. doi: 10.1517/14712598.8.8.1201. [DOI] [PubMed] [Google Scholar]
- 5.Richardson P, Linden E, Revta C, Ho V. Use of defibrotide in the treatment and prevention of veno-occlusive disease. Expert Rev Hematol. 2009;2:365–376. doi: 10.1586/ehm.09.30. [DOI] [PubMed] [Google Scholar]
- 6.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malaria J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francischetti IM. Does activation of the blood coagulation cascade have a role in malaria pathogenesis? Trends Parasitol. 2008;24:258–263. doi: 10.1016/j.pt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15:81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francischetti IM, Seydel KB, Monteiro RQ, Whitten RO, Erexson CR, Noronha AL, Ostera GR, Kamiza SB, Molyneux ME, Ward JM, Taylor TE. Plasmodium falciparum-infected erythrocytes induce tissue factor expression in endothelial cells and support the assembly of multimolecular coagulation complexes. J Thromb Haemost. 2007;5:155–165. doi: 10.1111/j.1538-7836.2006.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francischetti IM, Oliveira CJ, Ostera GR, Yager SB, Debierre-Grockiego F, Carregaro V, Jaramillo-Gutierrez G, Hume JC, Jiang L, Moretz SE, Lin CK, Ribeiro JM, Long CA, Vickers BK, Schwarz RT, Seydel KB, Iacobelli M, Ackerman HC, Srinivasan P, Gomes RB, Wang X, Monteiro RQ, Kotsyfakis M, Sa-Nunes A, Waisberg M. Defibrotide interferes with several steps of the coagulation-inflammation cycle and exhibits therapeutic potential to treat severe malaria. Arterioscler Thromb Vasc Biol. 2011;32:786–798. doi: 10.1161/ATVBAHA.111.240291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugina TN, Kiseleva EV, Chistov IV, Umarova BA, Strukova SM. Receptors of the PAR family as a link between blood coagulation and inflammation. Biochemistry (Mosc) 2002;67:65–74. doi: 10.1023/a:1013952114485. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi DA, Wun TC, Likert KM, Broze GJ., Jr The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood. 1992;79:1712–1719. [PubMed] [Google Scholar]
- 13.Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj MS, Kuppuswamy MN, Manepalli AN, Bajaj SP. Transcriptional expression of tissue factor pathway inhibitor, thrombomodulin and von Willebrand factor in normal human tissues. Thromb Haemost. 1999;82:1047–1052. [PubMed] [Google Scholar]
- 15.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1–S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Krishnegowda G, Li G, Gowda DC. Proinflammatory responses by glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum are mainly mediated through the recognition of TLR2/TLR1. Exp Parasitol. 2011;128:205–211. doi: 10.1016/j.exppara.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conroy A, Serghides L, Finney C, Owino SO, Kumar S, Gowda DC, Liles WC, Moore JM, Kain KC. C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria. PLoS One. 2009;4:e4953. doi: 10.1371/journal.pone.0004953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conroy AL, Phiri H, Hawkes M, Glover S, Mallewa M, Seydel KB, Taylor TE, Molyneux ME, Kain KC. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case-control study. PLoS One. 2010;5:e15291. doi: 10.1371/journal.pone.0015291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi G, Barone D, Lanzarotti E, Tettamanti R, Porta R, Moltrasio D, Cedro A, Salvetti L, Mantovani M, Prino G. Defibrotide, a single-stranded polydeoxyribonucleotide acting as an adenosine receptor agonist. Eur J Pharmacol. 1993;238:327–334. doi: 10.1016/0014-2999(93)90864-e. [DOI] [PubMed] [Google Scholar]
- 20.Belnoue E, Potter SM, Rosa DS, Mauduit M, Gruner AC, Kayibanda M, Mitchell AJ, Hunt NH, Renia L. Control of pathogenic CD8+ T cell migration to the brain by IFN-γ during experimental cerebral malaria. Parasite Immunol. 2008;30:544–553. doi: 10.1111/j.1365-3024.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 21.Grau GE, Heremans H, Piguet PF, Pointaire P, Lambert PH, Billiau A, Vassalli P. Monoclonal antibody against interferon γ can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Poll T, de Boer JD, Levi M. The effect of inflammation on coagulation and vice versa. Curr Opin Infect Dis. 2011;24:273–278. doi: 10.1097/QCO.0b013e328344c078. [DOI] [PubMed] [Google Scholar]
- 23.Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE. Inhibiting protease-activated receptor 4 limits myocardial ischemia/reperfusion injury in rat hearts by unmasking adenosine signaling. J Pharmacol Exp Ther. 2008;324:1045–1054. doi: 10.1124/jpet.107.133595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poovassery JS, Sarr D, Smith G, Nagy T, Moore JM. Malaria-induced murine pregnancy failure: distinct roles for IFN-γ and TNF. J Immunol. 2009;183:5342–5349. doi: 10.4049/jimmunol.0901669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulutin ON, Balkuv-Ulutin S, Bezer-Goker B, Cizmeci G, Ferhanoglu B, Ozsoy Y, Ugur MS, Ulutin T, Yaman A, Yardimci T. Effect of defibrotide on platelet function. Semin Thromb Hemost. 1996;22(suppl 1):21–24. [PubMed] [Google Scholar]
- 26.Morabito F, Gentile M, Gay F, Bringhen S, Mazzone C, Vigna E, Musto P, Di Raimondo F, Palumbo A. Insights into defibrotide: an updated review. Expert Opin Biol Ther. 2009;9:763–772. doi: 10.1517/14712590903008507. [DOI] [PubMed] [Google Scholar]
- 27.Falanga A, Vignoli A, Marchetti M, Barbui T. Defibrotide reduces procoagulant activity and increases fibrinolytic properties of endothelial cells. Leukemia. 2003;17:1636–1642. doi: 10.1038/sj.leu.2403004. [DOI] [PubMed] [Google Scholar]
- 28.Scalia R, Kochilas L, Campbell B, Lefer AM. Effects of defibrotide on leukocyte-endothelial cell interaction in the rat mesenteric vascular bed: role of P-selectin. Methods Find Exp Clin Pharmacol. 1996;18:669–676. [PubMed] [Google Scholar]
- 29.Zhou Q, Chu X, Ruan C. Defibrotide stimulates expression of thrombomodulin in human endothelial cells. Thromb Haemost. 1994;71:507–510. [PubMed] [Google Scholar]
- 30.Koehl GE, Geissler EK, Iacobelli M, Frei C, Burger V, Haffner S, Holler E, Andreesen R, Schlitt HJ, Eissner G. Defibrotide: an endothelium protecting and stabilizing drug, has an anti-angiogenic potential in vitro and in vivo. Cancer Biol Ther. 2007;6:686–690. doi: 10.4161/cbt.6.5.3959. [DOI] [PubMed] [Google Scholar]
- 31.Finney CA, Hawkes CA, Kain DC, Dhabangi A, Musoke C, Cserti-Gazdewich C, Oravecz T, Liles WC, Kain KC. S1P is associated with protection in human and experimental cerebral malaria. Mol Med. 2011;17:717–725. doi: 10.2119/molmed.2010.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avery JW, Smith GM, Owino SO, Sarr D, Nagy T, Mwalimu S, Matthias J, Kelly LF, Poovassery JS, Middii J, Abramowsky C, Moore JM. Maternal malaria induces a procoagulant and antifibrinolytic state that is embryotoxic but responsive to anticoagulant therapy. PLoS One. doi: 10.1371/journal.pone.0031090. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


